Abstract
The effect of light and water on aromatic rice remain largely unclear. A pot experiment was conducted to investigate the influences of light-water treatments (CK: natural light and well-watered conditions, WS: natural light and water-stressed conditions, LL: low light and well-watered conditions, LL-WS: low light and water-stressed treatment) on yield and 2-acetyl-1-pyrroline (2AP) formation in aromatic rice. Compared with CK, the light-water treatments decreased grain yield (10.32–39.19%) due to reductions in the filled grain percentage and total dry weight, in the regulation of biomass distribution, and in the attributes of gas exchange and antioxidant response parameters. The 2AP content in grains increased in the LL treatment (5.08–16.32%) but decreased in the WS treatment compared with that in CK. The changes in 2AP were associated with changes in 2AP formation-related traits and element content. Low light and water stress led to yield declines in aromatic rice, but low light alleviated the decrease in 2AP content caused by water stress.
Similar content being viewed by others
Introduction
Rice is one of the most important food crops worldwide. Aromatic rice has a higher grain quality than non-aromatic rice, and consumers prefer aromatic rice due to its pleasant smell1,2. Aromatic rice plays a significant role in international rice markets3. The global demand for aromatic rice is increasing4.
Many volatile compounds have been detected in aromatic rice5,6,7, of these, 2-acetyl-1-proline (2AP) is a determinant of the aromatic properties of aromatic rice8,9. Previous studies have suggested that proline is an important precursor for 2AP formation10,11. In addition, the ornithine, glutamate, γ-aminobutyric acid (GABA), Δ1-pyrroline-5-carboxylate(P5C), Δ1pyrroline-5-carboxylate synthetase (P5CS), ornithine aminotransferase (OAT) and proline dehydrogenase (PDH) are highly related to the biosynthesis of 2AP12,13,14,15,16. Moreover, some studies have reported that micronutrients such as Mn and Zn contribute to the synthesis of 2AP in aromatic rice14,17.
In addition to the effects of genotype, environmental factors and cultivation practices affect the accumulation of 2AP in aromatic rice6. A previous study reported that the 2AP content was negatively correlated with sunshine hours18. Shading promoted the accumulation of 2AP in aromatic rice19,20. However, shading can lead to the inhibition of the transportation of photosynthetic products which ultimately causes yield decline21. In addition, shading resulted in changes in the antioxidant defence of rice plants22. In previous studies, comparative transcriptome profiling was performed, and certain genes in rice that are expressed under low light were identified23.
Irrigation is important for crop production. Water stress reduces the photosynthesis rate, growth, and biomass production and thereby decreases grain yield24,25,26. In addition, water stress leads to increases in the production of reactive oxygen species (ROS) and changes in antioxidant parameters27,28, and the expression of a series of genes in response to drought stress has been assessed29,30. However, the accumulation of 2AP in aromatic rice is affected by irrigation practices31. The 2AP content in aromatic rice can be increased with alternate wetting and drying conditions32. Drought stress during the grain filling stage can enhance the accumulation of 2AP in aromatic rice11.
A previous study reported that the synthesis of 2AP was highly related to abiotic stresses33. Low light or water stress could lead to improved 2AP accumulation. However, the effects of light-water on aromatic rice remain largely unknown. In this study, two elite Chinese aromatic rice varieties, Xiangyaxiangzhan and Yuxiangyouzhan, were grown under four light-water treatments to explore how light and water regulate yield and 2AP formation in aromatic rice.
Results
Effects of the light-water treatments on yield and yield-related traits
Compared with CK, LL and LL-WS significantly decreased the grain yield in Xiangyaxiangzhan by 39.19% and 34.64%, respectively. WS, LL, and LL-WS significantly decreased the grain yield in Yuxiangyouzhan by 25.44%, 30.79%, and 29.42%, respectively, when compared to those under CK. The light-water treatments (WS, LL, and LL-WS) decreased the filled grain percentage, and a significant decrease compared to CK was detected under LL and LL-WS. The light-water treatments had no notable effect on the effective panicles or the 1,000-grain weight in either variety (Table 1).
Effect of the light-water treatments on organ dry weight
Compared with CK, WS, LL, and LL-WS resulted in reductions in total dry weight due to reductions in the dry weight of the stem sheath, panicle, and leaf, except for the dry weight of the Xiangyaxiangzhan leaves (Table 2).
Effects of the light-water treatments on gas exchange parameters and SPAD value
Compared with CK, WS and LL-WS significantly decreased Pn in Xiangyaxiangzhan at AS (after shading). The LL and LL-WS significantly decreased Pn in Yuxiangyouzhan at AS. There was no significant difference in Pn among the treatments in either variety at MS (maturity stage). For Xiangyaxiangzhan, the Tr decreased substantially in response to light-water treatment at AS. For Yuxiangyouzhan, the WS and LL-WS caused significant reductions in the Tr at MS and AS, respectively. LL significantly improved the Tr in Yuxiangyouzhan at MS, and the Gs in Xiangyaxiangzhan was significantly decreased under the light-water treatments at AS. WS and LL-WS resulted in a marked reduction in Gs in Yuxiangyouzhan at AS and MS, while LL significantly increased Gs. For Xiangyaxiangzhan, the Ci showed a significant reduction under WS and LL-WS compared with that under CK at AS and MS, respectively. For Yuxiangyouzhan, LL and LL-WS significantly increased the Ci at AS compared with that under CK. WS and LL-WS substantially reduced the Ci, but LL significantly increased the Ci in Yuxiangyouzhan at MS. For Xiangyaxiangzhan, significant increase in the SPAD values at AS and MS compared with that under CK were observed in response to the light-water treatments. For Yuxiangyouzhan, WS significantly reduced the SPAD value at AS, while LL and LL-WS significantly increased the SPAD value at MS (Table 3).
Effect of the light-water treatments on antioxidant response and MDA content
Compared with CK, LL and LL-WS significantly increased SOD activity at AS, while the light-water treatments substantially decreased SOD activity at MS in Xiangyaxiangzhan. For Yuxiangyouzhan, WS and LL significantly increased SOD activity at AS. LL-WS significantly increased SOD activity, but LL significantly decreased SOD activity at MS. For Xiangyaxiangzhan, WS and LL-WS significantly reduced POD activity at AS and MS compared to that under CK. LL significantly decreased the POD activity at AS but significantly increased the POD activity at MS. Compared with CK, WS and LL-WS significantly increased the POD activity at AS, and the light-water treatments substantially increased the POD activity at MS in Yuxiangyouzhan. WS and LL-WS significantly increased the CAT activity at AS while LL and LL-WS significantly increased the CAT activity at MS in Xiangyaxiangzhan. For Yuxiangyouzhan, light-water treatments significantly increased the CAT activity at AS and MS. Compared with CK, LL and LL-WS significantly increased the MDA content at AS while WS and LL-WS significantly decreased the MDA content at MS in Xiangyaxiangzhan. For Yuxiangyouzhan, WS and LL significantly decreased the MDA content at AS. LL and LL-WS significantly increased the MDA content but WS significantly decreased the MDA content at MS compared to that under CK (Table 4).
Effect of the light-water treatments on the 2AP content
Higher 2AP content in the grains was observed under LL and LL-WS than under CK. LL and LL-WS significantly increased the 2AP content in Yuxiangyouzhan by 18.67% and 16.32%, respectively, compared with that under CK. The WS significantly decreased the 2AP content in grains of Xiangyaxiangzhan and Yuxiangyouzhan by 24.44% and 7.19%, respectively, compared with that under CK (Table 5).
Effects of the light-water treatments on P5C content, proline content, and GABA content
Compared with CK, WS significantly increased the P5C content in leaves at AS for Xiangyaxiangzhan and at MS for Yuxiangyouzhan. For Xiangyaxiangzhan, LL significantly decreased the P5C content in leaves at AS, and LL and LL-WS significantly increased the P5C content in leaves at MS. WS significantly decreased the P5C content in grains at AS for Xiangyaxiangzhan, but significantly increased the P5C content in grains at AS for Yuxiangyouzhan compared with those in the control. For Xiangyaxiangzhan, WS and LL-WS significantly increased the P5C content in grains at AS but significantly reduced the P5C content in grains at MS (Table 6).
The light-water treatments significantly increased the proline content in leaves at AS in Xiangyaxiangzhan compared with that in CK. WS and LL-WS significantly increased the proline content in leaves at MS in both varieties. LL-WS significantly increased the proline content in leaves at AS in Yuxiangyouzhan compared with that under CK. The light-water treatments significantly increased the proline content in grains at AS in Yuxiangyouzhan. The WS and LL-WS significantly increased the proline content in grains at MS in Xiangyaxiangzhan compared with that under CK (Table 6).
Compared with CK, WS and LL significantly reduced the GABA content in leaves, while LL significantly increased the GABA content in leaves at MS in Xiangyaxiangzhan. For Yuxiangyouzhan, LL-WS significantly increased the GABA content in leaves at AS but LL significantly decreased the GABA content in leaves at MS. The GABA content in Xiangyaxiangzhan and Yuxiangyouzhan grains at AS was noticeably increased by the light-water treatments. LL significantly increased the GABA content in grains at MS in Xiangyaxiangzhan compared with that under CK. WS and LL-WS significantly increased the GABA content in grains at MS in Yuxiangyouzhan compared with that under CK (Table 6).
Compared with CK, LL-WS and LL significantly increased the soluble protein content in leaves at AS and MS in Xiangyaxiangzhan, respectively. LL-WS significantly increased the soluble protein content in leaves at MS in Yuxiangyouzhan. The soluble protein content in grains was not noticeably affected by the light-water treatments at AS or MS. (Table 6).
Effect of the light-water treatments on P5CS, PDH, OAT, and DAO activity
Compared with CK, WS significantly increased the P5CS activity in leaves at AS and MS in Xiangyaxiangzhan and Yuxiangyouzhan. LL-WS resulted in a significant increase in P5CS activity in leaves at AS in Xiangyaxiangzhan and at MS in Yuxiangyouzhan. WS and LL-WS significantly increased the P5CS activity in grains at AS in Yuxiangyouzhan and at MS in Xiangyaxiangzhan. Compared with CK, LL and LL-WS significantly increased the P5CS activity in grains at MS in Yuxiangyouzhan (Table 7).
The PDH activity in leaves at AS in Xiangyaxiangzhan was significantly decreased under the light-water treatments compared to that under CK. LL significantly reduced the PDH activity in leaves at MS in Xiangyaxiangzhan. The PDH activity in leaves at AS and MS in Yuxiangyouzhan was not significantly affected by the light-water treatments. The WS significantly increased the PDH activity in grains at AS and MS in Xiangyaxiangzhan, while LL and LL-WS significantly increased the PDH activity in grains at MS in Yuxiangyouzhan compared with that under CK (Table 7).
Compared with CK, the light-water treatments significantly decreased the OAT activity in leaves at AS but significantly increased the OAT activity in leaves at MS in Xiangyaxiangzhan. For Yuxiangyouzhan, WS and LL-WS significantly reduced the OAT activity in leaves at AS but LL significantly increased the OAT activity in leaves at AS. The OAT activity in grains at MS was significantly increased under LL-WS in Xiangyaxiangzhan. The WS resulted in a significant increase in the OAT activity in grains at AS, but LL significantly decreased the OAT activity in grains at MS in Yuxiangyouzhan compared with that under CK (Table 7).
Compared with CK, LL-WS significantly increased the DAO activity in leaves at MS in Xiangyaxiangzhan. For Yuxiangyouzhan, WS and LL-WS significantly increased the DAO activity in leaves at AS, while LL significantly decreased the DAO activity in leaves at MS. The WS significantly increased the DAO activity in grains at AS, while LL significantly decreased the DAO activity in grains at AS in Yuxiangyouzhan compared with that under CK. The DAO activity in grains at AS and MS in Xiangyaxiangzhan was not significantly affected by the light-water treatments (Table 7).
Effects of the light-water treatments on Na, Mg, Mn, and Fe contents
Compared with CK, WS significantly reduced the Na content in leaves at AS while LL and LL-WS significantly increased the Na content in leaves at AS in Xiangyaxiangzhan. The Na content in Xiangyaxiangzhan leaves at MS was significantly increased under the light-water treatments. For Yuxiangyouzhan, WS and LL-WS significantly decreased the Na content in leaves at AS, while LL and LL-WS significantly increased the Na content in leaves at AS and MS, respectively. The Na content in grains at AS and MS was significantly reduced under WS and LL-WS in Xiangyaxiangzhan while LL-WS significantly reduced the Na content in grains at MS in Yuxiangyouzhan compared with that under CK (Table 8).
The Mg content in Xiangyaxiangzhan leaves at AS and MS was not significantly affected by the light-water treatments. For Yuxiangyouzhan, WS significantly increased the Mg content in leaves at MS compared to that under CK. WS significantly increased the Mg content in Xiangyaxiangzhan grains at AS. The light-water treatments did not significantly affect the Mg content in Yuxiangyouzhan grains (Table 8).
Compared with CK, WS and LL-WS significantly increased the Mn content in leaves and grains at AS and MS but LL significantly decreased the Mn content in leaves and grains at AS in Xiangyaxiangzhan. For Yuxiangyouzhan, the light-water treatments significantly increased the Mn content in leaves at AS and MS, and the Mn content in grains at AS was significantly decreased under the light-water treatments compared with that in the control. WS and LL-WS significantly increased the Mn content in grains at MS, but LL significantly decreased the Mn content in grains at AS (Table 8).
Compared with CK, for Xiangyaxiangzhan, LL and LL-WS significantly decreased the Fe content in leaves at AS, while WS and LL-WS significantly decreased the Fe content in leaves at MS. For Yuxiangyouzhan, LL and WS significantly increased the Fe content in leaves at AS and MS, respectively. LL and LL-WS significantly decreased the Fe content in Yuxiangyouzhan leaves at MS. For Xiangyaxiangzhan, the light-water treatments significantly decreased the Fe content in grains at AS. WS and LL significantly increased the Fe content in grains at MS in Xiangyaxiangzhan but significantly decreased the Fe content in grains at MS in Yuxiangyouzhan. WS and LL-WS significantly decreased the Fe content in grains at AS in Yuxiangyouzhan compared with that under CK (Table 8).
Correlation analysis
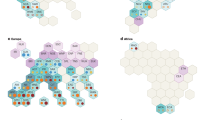
There was a significant positive correlation between the grain yield and the panicle dry weight and the total dry weight at AS and MS (Fig. 1). The 2AP content in grains was significantly negatively correlated with the P5C content in grains, P5CS activity in leaves at MS, and PDH activity in gains at AS. The Mn content in leaves at MS and in grains at AS and MS showed a significant positive correlation with the 2AP content in grains (Fig. 2).
Discussion
The effects of low light and water stress on grain yield in rice have been reported19,21,34,35. Shading and water stress have a negative significant effect on the total dry weight of rice19,26. In this study, we confirmed that low light reduced the yield of rice mainly by reducing the filled grain percentage and the total dry weight (Tables 1 and 2). This study found a significant positive correlation between grain yield and the dry weight of the panicle and total dry weight (Fig. 1). The light-water treatments had no significant effect on the panicle number or 1,000-grain weight (Table 1), this finding is consistent with a previous report in which the 1,000-grain weight was not affected by a shading treatment during early grain filling35 but is different from the result of another study due to the difference in the shading duration19. Shading resulted in a reduction in the number of effective panicles, and the extent of the reduction varies depending on the treatment period19,21,36. A significant reduction in effective panicles could be observed at the tillering stage36. Many studies have shown that water stress resulted in a significant reduction in the filled grain percentage towards the mid-tillering, booting and flowering stages37,38. In this study, the light-water stress treatment reduced the grain yield and the filled grain percentage (Table 1).
Studies have reported that shading significantly increased the total chlorophyll content of plants39,40. In this study, a significant increase in the SPAD value in response to light-water treatments was observed in Xiangyaxiangzhan. For Yuxiangyouzhan, WS significantly decreased the SPAD value, while LL and LL-WS significantly increased the SPAD value at MS (Table 3). Leaf gas exchange is important for plants in response to abiotic stress41. Studies have reported that low light and water deficits caused a change in Pn, Tr, Gs, and Ci22,42,43,44,45,46,47. In this study, the light-water treatments affected the gas exchange parameters after the shading treatment, and at the maturity stage, the effect varied between varieties (Table 3). The differences in the changes in SPAD and gas exchange parameters were mainly due to the time and degree of the shading and water stress treatments.
Shading and water stress both result in the accumulation of reactive oxygen species (ROS) and cause damage to proteins and lipids48,49,50. Superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) are key enzymes used for scavenging reactive oxygen species; MDA is the product of lipid peroxidation in cells and reflects the extent of cell membrane damage under stressful conditions48,51,52. Shading significantly reduced SOD activity and increased MDA content during the grain filling stage22. Shade tolerant varieties maintain a lower MDA content and higher SOD, POD, and CAT activity and soluble protein content50. Moreover, the MDA content was significantly increased and the activities of SOD and CAT were significantly reduced after a PEG treatment49, which may have been due to the drought-induced accumulation of H2O2 in the guard cells53. In this study, the low light treatments significantly increased the SOD activity and MDA content in both rice varieties. However, different changes in CAT activity and POD activity after shading were observed in the two varieties. At the maturity stage, the shading treatment resulted in a significant reduction in SOD activity and increased POD and CAT activity in Yuxiangyouzhan rice, while the MDA content was significantly increased (Table 4). The light-water treatments had a regulatory effect on the antioxidant response parameters. Further studies are needed to evaluate the molecular basis of the complex responses of rice plants to abiotic stress, i.e., light-water treatments54.
Many previous studies have reported that abiotic stresses increase the content of 2AP in grains19,25,26. Lower levels of water irrigation affected 2AP accumulation in aromatic rice25,26. The 2AP content in grains increased significantly after shading during the grain filling period19. In this study, low light treatments increased the 2AP content in grains of both varieties, but WS significantly decreased the content of 2AP in grains (Table 5). The responses of different genotypes to the levels of water stress may explain the difference in the changes in 2AP accumulation in this study and in previous studies25,26.
Shading significantly increased the GABA content in Yuxiangyouzhan and Nongxiang18 and increased the proline content in Yuxiangyouzhan grains19. The proline content in tomato was reduced in drought tolerant varieties55. Different water regimes coupled with nitrogen affect the biosynthesis of 2AP by regulating physiological and biochemical parameters such as the P5C, proline, and GABA content and the activity of P5CS, PDH, OAT, and DAO26. In this study, the light-water treatments regulated the P5C, proline, and GABA content in leaves and grains as well as the P5CS, PDH, OAT, and DAO activity in leaves and grains (Tables 6 and 7). The relationship of the 2AP content in grain to the studied physiological parameters was assessed (Fig. 2a–c). The relationship between the 2AP content and the 2AP-related physiological and biochemical parameters differed among experimental treatments and genotypes56,57,58. Moreover, the light-water treatments regulated the dynamics of the element content in leaves and grains (Table 8), and the relationship between the 2AP in grains and the element content was also assessed (Fig. 2d–f). Inconsistent results were obtained for the relationship between the 2AP content and the element content of different elements among different experimental treatments59. Moreover, element levels in plants and the deficits or excess elements such as iron in plants are related to oxidative stress in plants60,61. Therefore, element absorption regulated by the light-water treatments further influenced oxidative stress in the rice plants, which resulted in more complex changes in the metabolic physiology of the plants. Further studies on the molecular basis of 2AP biosynthesis regulation in aromatic rice under light-water treatments should be conducted.
Overall, light-water treatments during the early grain filling stage regulate yield and 2AP formation, which results from biomass accumulation, photosynthesis, antioxidant responses, 2AP formation related physiological attributes, and element absorption in the plant.
Conclusion
Light-water treatments during the early grain filling stage regulates yield by affecting the plant dry weight, gas exchange parameters, and antioxidant responses. However, these treatments also influence 2AP accumulation by regulating 2AP formation-related physiological parameters and elemental levels. Further study is needed to balance yield with 2AP accumulation under light-water treatments.
Methods
Experimentation and treatments
A pot experiment was conducted at the Experimental Farm, South China Agricultural University, Guangzhou, China during July–November 2017. This region is favourable for the growth of aromatic rice due to its humid subtropical monsoon climate. Two aromatic rice varieties, Yuxiangyouzhan and Xiangyaxiangzhan, were used in this study. The two varieties are popular aromatic rice cultivars in South China. The soil used for the experiment was collected from paddy fields19.
Two light levels (natural light and low light) were employed in this study. The low light treatment was implemented with a black netting layer and was equivalent to a 67% reduction in the full natural light level19. Two water treatments, well-watered and water-stressed, were conducted in this study (Fig. 3). The water stress treatment was conducted according to the method described in a previous study62. The well-watered treatment was flooded to a depth of 1–2 cm by manually adding tap water63. Four light-water treatments (CK: natural light and well-watered treatment, WS: natural light and water-stressed treatment, LL: low light and well-watered treatment, LL-WS: low light and water-stressed treatment) were conducted during the early grain filling stage. The treatments lasted for 15 days, from September 26th to October 10th.
Seeds of the two aromatic rice varieties were sown on July 15th and 15-day-old seedlings were transplanted into pots with four seedlings per hill and five hills per pot. A compound fertilizer (15:15:15) was applied basally in the amount of 5.5 g per pot. The rice plants were harvested on November 6th. Except during the water treatment period, the irrigation was carried out according to routine management practices: a 2–4 cm water layer was maintained from transplanting to 7 days before harvest, and then the soil was allowed to dry out naturally. Other managements practices were the same in all treatments and followed local recommendations.
Sampling and measurement
Determination of yield and yield-related traits and dry matter weight
The determination of yield and yield-related traits was performed according to a previously reported method64. At the maturity stage (MS), four pots were randomly harvested from each treatment. The grains were sun-dried to a moisture content of 14%. The effective panicles per pot were determined by counting the panicle numbers in four pots from each treatment. The grain number per panicle and the filled grain number were counted in the same four pots, and the filled grain percentage was calculated. The 1,000-grain weight was measured by weighing 1,000 grains from four random samples. Six representative plants were selected randomly and taken to the laboratory. The plants were separated into their panicles, leaves, and stem sheaths and then dried at 80 °C to a constant weight.
Determination of gas exchange parameters and SPAD value
The net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Cond) and intercellular CO2 concentration (Ci) of the leaf blades were determined with an LI-6400XT portable photosynthesis system (LI-COR, Inc., USA) after shading and at maturity from 9:00 am to 11:00 am on sunny days, and four measurements were taken for each treatment. Meanwhile, the SPAD value was measured by a SPAD meter ‘SPAD-502′ (Konica Minolta, Japan), with four replications for each treatment.
Determination of malondialdehyde (MDA) and antioxidant activities
The malondialdehyde (MDA) and antioxidant activities were measured as described method by Li et al.65. MDA was reacted with thiobarbituric acid (TBA), and the absorbance of the reaction solutions was recorded at 532 nm, 600 nm, and 450 nm. The MDA content was expressed as μmol g−1 FW. The superoxide (SOD, EC 1.15.1.1) activity was measured by using the nitro-blue tetrazolium (NBT) method. The reaction mixture contained 1.75 ml of sodium phosphate buffer (pH 7.8), 0.3 ml of 130 mM methionine buffer, 0.3 ml of 750 μmol NBT buffer, 0.3 ml of 100 μmol EDTA-Na2 buffer, 0.3 ml of 20 μmol lactoflavin and 0.05 ml of enzyme extract. After the reaction, the change in colour was measured at 560 nm. The SOD activity was expressed as U g−1 FW. For peroxidase (POD EC1.11.1.7) activity, the enzyme extract (50 μl) was added to the reaction solution containing 1 ml of 0.3% H2O2, 0.95 ml of 0.2% guaiacol, and 1 ml of 50 mM sodium phosphate buffer (pH 7.0). The absorbance was read at 470 nm. The POD activity was expressed as U g−1 FW. For the catalase (CAT, EC 1.11.1.6) activity, an aliquot of enzyme extract (50 μl) was added to the reaction solution containing 1 ml of 0.3% H2O2 and 1.95 ml of sodium phosphate buffer, and the absorbance was recorded at 240 nm. The CAT activity was expressed as U g−1 FW.
Determination of 2AP concentration
The 2AP concentration in the grains was measured using a previously described procedure19,66 that used the synchronization, distillation and extraction method (SDE) combined with GC–MS–QP 2010 Plus system (Shimadzu Corporation, Japan).
Determination of 2AP formation related to physiological traits
Fresh samples of grains and flag leaves were collected from each plot and immediately stored at − 80 °C until the determination of the 1-pyrroline-5-carboxylic acid (P5C) content, proline content, soluble protein content, γ-aminobutyric acid (GABA) content, proline dehydrogenase (PDH) activity, pyrroline-5-carboxylic acid syntheses (P5CS) activity, ornithine aminotransferase (OAT) activity, and diamine oxidase (DAO) activity.
The P5C concentration was determined according to a previously described method67. The reaction mixture consisted of 0.2 ml of enzyme extraction supernatant, 0.5 ml of 10% trichloroacetic acid (TCA), and 0.2 ml of 40 mM 2-aminobenzaldehyde. The absorbance was measured at 440 nm after the reaction, and the P5C concentration was expressed as μmol g−1 FW. The proline content was evaluated by using a previously reported method68. The proline content was expressed as μg g−1 FW. The soluble protein content was determined according to a previously reported method69 with G-250. The soluble protein content was expressed as μg g−1 FW. The GABA content was measured according to previously described methods70,71. Plant tissue (0.500 g) was homogenized with 60% ethanol (5 ml) and then oscillated for 4 h in an oscillations instrument (HZS-H, China) at 200 oscillations per minute. Then, the supernatant was centrifuged at 8,000 rpm for 3 min. The reaction mixture in a 10 ml test tube consisted of 1 ml of the supernatant, 0.6 ml of 0.2 M (pH 9.0) sodium tetraborate, 2 ml of 5% toluene and 1 ml of 7% sodium hypochlorite. The prepared mixture was heated in a boiling water bath for 5 min and then cooled. The absorbance of the reaction mixture was measured at 645 nm. The GABA content was expressed as μg g−1 FW.
The activity of PDH was measured by following a previously described method72. After the reaction, the absorbance was read at 440 nm, and the PDH activity was expressed as U g−1 FW. The P5CS activity was determined according to a reported method73. The reaction solutions contained 10 mM ATP, 20.0 mM MgCl2, 50 mM Tris–HCl buffer, 50 mM sodium glutamate, 100 mM hydroxamate-HCL and 0.5 ml of enzyme extract. The prepared mixture was kept in a 37℃ water bath for 5 min, and then the reaction was terminated by the addition of 0.5 ml of a stop buffer (2.5% FeCl3 and 6% TCA, dissolved in 100 ml of 2.5 M HCl). The P5CS activity was expressed as U g−1 FW. The activity of OAT was assayed by using a previously described method71. The absorbance of the supernatant fraction was read at 440 nm. The OAT activity was expressed as U g−1 FW. The DAO activity was measured according to previously reported methods74,75. The DAO activity was expressed as U g−1 FW.
Determination of the Na, Mg, Mn, and Fe contents in leaves and grains
Briefly, the plant tissue (leaves and grains) was oven-dried and ground into a fine powder. Then 0.30 g of the plant tissue sample was digested with a 10 ml diacidic mixture of HNO3:HClO4 (4:1 v/v), after which the resultant solutions were diluted to 25 ml. The Na, Mg, Mn, and Fe contents in leaves and grains were estimated by using an atomic absorption spectrophotometer (AA6300C, Shimadzu, Japan)59.
Statistics
Analysis of variance (ANOVA) and correlation coefficients were performed using Statistix version 8 (Analytical, Tallahassee, Florida, USA). The differences amongst means separated by using the least significant difference (LSD) test at 5% significance level.
Data availability
All data generated or analyzed during this study are included in the article.
References
Kovach, M. J. et al. The origin and evolution of fragrance in rice (Oryza sativa L.). Proc. Natl. Acad. Sci. 106, 14444–14449 (2009).
Mundo, A. M. D. & Juliano, B. O. Consumer preference and properties of raw and cooked milled rice. J. Text. Stud. 12, 107–120 (1981).
Hashemi, F. S. G. et al. The genetic and molecular origin of natural variation for the fragrance trait in an elite Malaysian aromatic rice through quantitative trait loci mapping using SSR and gene-based markers. Gene 555, 101–107 (2015).
Hashemi, F. G. et al. Biochemical, genetic and molecular advances of fragrance characteristics in rice. Crit. Rev. Plant Sci. 32, 445–457 (2013).
Buttery, R. G., Turnbaugh, J. G. & Ling, L. C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 36, 1006–1009 (1988).
Champagne, E. T. Rice aroma and flavor: a literature review. Cereal Chem. 85, 445–454 (2008).
Tsugita, T. Aroma of cooked rice. Food Rev. Int. 1, 497–520 (1985).
Buttery, R. G. et al. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 31, 823–826 (1983).
Jezussek, M., Juliano, B. O. & Schieberle, P. Comparison of key aroma compounds in cooked brown rice varieties based on aroma extract dilution analyses. J. Agric. Food Chem. 50, 1101–1105 (2002).
Huang, T. C. et al. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J. Agric. Food Chem. 56, 7399–7404 (2008).
Yoshihashi, T., Huong, N. T. T. & Inatomi, H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 50, 2001–2004 (2002).
Bradbury, L. M. et al. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 68, 439–449 (2008).
Chen, S. et al. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 20, 1850–1861 (2008).
Li, M. et al. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 103, 167–175 (2016).
Kaikavoosi, K. et al. 2-Acetyl-1-pyrroline augmentation in scented indica rice (Oryza sativa L.) varieties through Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene transformation. Appl. Biochem. Biotechnol. 177, 1466–1479 (2015).
Wakte, K. et al. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): a status review. J. Sci. Food Agric. 97, 384–395 (2017).
Mo, Z. W. et al. Supplementation of 2-AP, Zn and La improves 2-acetyl-1-pyrroline concentrations in detached aromatic rice panicles in vitro. PLoS ONE 11, e149523 (2016).
Yang, X. et al. Effects of sowing date on aroma formation, quality and yield of early season aromatic rice. Acta Agriculturae Boreali-Sinica 29, 128–135 (2014).
Mo, Z. W. et al. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 8, 9 (2015).
Tang, Y. J. et al. Effect of continuous shading treatment 15 days before harvest on grain yield, quality and aroma of aromatic rice. Southwest China J. Agric. Sci. 28, 939–943 (2015).
Zhu, P. et al. Effect of shading on the photosynthetic characteristics and yield at later growth stage of hybrid rice combination. Acta Agron. Sin. 34, 2003–2009 (2008).
Pan, S. G. et al. Effects of nitrogen and shading on root morphologies, nutrient accumulation, and photosynthetic parameters in different rice genotypes. Sci. Rep. 6, 32148 (2016).
Sekhar, S. et al. Comparative transcriptome profiling of low light tolerant and sensitive rice varieties induced by low light stress at active tillering stage. Sci. Rep. 9, 1 (2019).
Mostajeran, A. & Rahimi-Eichi, V. Effects of drought stress on growth and yield of rice (Oryza sativa L.) cultivars and accumulation of proline and soluble sugars in sheath and blades of their different ages leaves. Agric. Environ. Sci. 5, 264–272 (2009).
Mo, Z. W. et al. Regulations in 2-acetyl-1-pyrroline contents in fragrant rice are associated with water-nitrogen dynamics and plant nutrient contents. J. Cereal Sci. 88, 96–102 (2019).
Mo, Z. W. et al. Nitrogen application and different water regimes at booting stage improved yield and 2-acetyl-1-pyrroline (2AP) formation in fragrant rice. Rice 12, 1–16 (2019).
Gao, J. et al. Differential responses of lipid peroxidation and antioxidants in Alternanthera philoxeroides and Oryza sativa subjected to drought stress. Plant Growth Regul. 56, 89 (2008).
Kang, G. Z., Wang, Z. X. & Sun, G. C. Participation of H2O2 in enhancement of cold chilling by salicylic acid in banana seedlings. Acta Bot. Sin. 45, 567–573 (2003).
Shinozaki, K. & Yamaguchishinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 58, 221–227 (2006).
Rodriguez, M. et al. Identification of genes induced upon water-deficit stress in a drought-tolerant rice cultivar. J. Plant Physiol. 163, 577–584 (2006).
Wang, P. et al. Effects of different irrigation modes on aroma content of aromatic rice at booting stage. Guangdong Agric. Sci. 8, 1–3 (2013).
Bao, G. G. et al. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice. Plant Physiol. Biochem. 133, 149–157 (2018).
Niu, X. et al. RNAi-directed downregulation of OsBADH2 results in aroma (2-acetyl-1-pyrroline) production in rice (Oryza sativa L.). BMC Plant Biol. 8, 100 (2008).
Chen, W. et al. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 142, 67–76 (2011).
Kobata, T., Sugawara, M. & Takatu, S. Shading during the early grain filling period does not affect potential grain dry matter increase in rice. Agron. J. 92, 411–417 (2000).
Deng, F. et al. Effects of different-growing-stage shading on rice grain-filling and yield. J. Sichuan Agric. Univ. 27, 265–269 (2009).
Davatgar, N. et al. Physiological and morphological responses of rice (Oryza sativa L.) to varying water stress management strategies. Int. J. Plant Prod. 3, 19–32 (2012).
Sarvestani, Z. T. et al. Study of water stress effects in different growth stages on yield and yield components of different rice (Oryza sativa L.) cultivars. Pak. J. Biol. Sci. 11, 1303–1309 (2008).
Panda, D. et al. Impact of low light stress on physiological, biochemical and agronomic attributes of rice. J. Pharmacogn. Phytochem. 8, 1814–1821 (2019).
Viji, M. M., Thangaraj, M. & Jayapragasam, M. Effect of low light on photosynthetic pigments, photochemical efficiency and Hill reaction in rice (Oryza sativa L.). J. Agron. Crop Sci. 178, 193–196 (1997).
Zhang, Z. et al. Calcium is involved in exogenous NO-induced enhancement of photosynthesis in cucumber (Cucumis sativus L.) seedlings under low temperature. Sci. Hortic. 261, 108953 (2019).
Akram, H. M. et al. Impact of water deficit stress on various physiological and agronomic traits of three basmati rice (Oryza sativa L.) cultivars. J. Anim. Plant Sci. 23, 1415–1423 (2013).
Ding, L. et al. Effects of drought stress on photosynthesis and water status of rice leaves. Chin. J. Rice Sci. 28, 65–70 (2014).
Li, W. et al. Effect of shading on gas exchange and chlorophyll fluorescence of rice leaves in tillering stage. Southwest China J. Agric. Sci. 31, 289–295 (2018).
Pieters, A. J. & El Souki, S. Effects of drought during grain filling on PSII activity in rice. J. Plant Physiol. 162, 903–911 (2005).
Singh, A., Sengar, K. & Sengar, R. S. Gene regulation and biotechnology of drought tolerance in rice. Int. J. Biotechnol. Bioenergy Res. 4, 547–552 (2013).
Yang, P. M. et al. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 52, 193–202 (2014).
Apel, K. & Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 (2004).
Basu, S. et al. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 60, 51 (2010).
Liu, L. et al. Osmotic regulation substance contents and activities of protective enzymes in leaves of different hybrid rice combinations as affected by shading. Chin. J. Rice Sci. 26, 569–575 (2012).
Hu, X. et al. Cross-talks between Ca2+/CaM and H2O2 in abscisic acid-induced antioxidant defense in leaves of maize plants exposed to water stress. Plant Growth Regul. 55, 183 (2008).
Volkov, R. A. et al. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 61, 733–746 (2006).
Ahammed, G. J. et al. Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2-mediated stomatal closure. Environ. Exp. Bot. https://doi.org/10.1016/j.envexpbot.2019.103960 (2020).
Ganie, S. A. et al. Vascular plant one zinc-finger (VOZ) transcription factors: novel regulators of abiotic stress tolerance in rice (Oryza sativa L.). Genet. Resour. Crop Evol. 67, 799–807 (2020).
Ahammed, G. J. et al. SlWRKY81 reduces drought tolerance by attenuating proline biosynthesis in tomato. Sci. Hortic. 270, 109444 (2020).
Gao, Z. F. et al. Exogenous γ-aminobutyric acid (GABA) application at different growth stages regulates 2-acetyl-1-pyrroline, yield, quality and antioxidant attributes in fragrant rice. J. Plant Interact. 15, 139–152 (2020).
Liu, X. W. et al. Selenium–silicon (Se–Si) induced modulations in physio-biochemical responses, grain yield, quality, aroma formation and lodging in fragrant rice. Ecotoxicol. Environ. Saf. 196, 110525 (2020).
Xie, W. J. et al. Enhancement of 2-acetyl-1-pyrroline (2AP) concentration, total yield, and quality in fragrant rice through exogenous γ-aminobutyric acid (GABA) application. J. Cereal Sci. 91, 102900 (2020).
Xie, W. J. et al. Application of γ-aminobutyric acid (GABA) and nitrogen regulates aroma biochemistry in fragrant rice. Food Sci. Nutr. 7, 3784–3796 (2019).
Huang, Z. L. et al. Response of rice genotypes with differential nitrate reductase-dependent NO synthesis to melatonin under ZnO nanoparticles’ (NPs) stress. Chemosphere 250, 126337 (2020).
Wu, Y. et al. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 265, 109205 (2020).
Yu, L. et al. Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol. 162, 1378–1391 (2013).
Yang, J. et al. Involvement of polyamines in the drought resistance of rice. J. Exp. Bot. 58, 1545–1555 (2007).
Mo, Z. W. et al. Silicon fertilization modulates 2-acetyl-1-pyrroline content, yield formation and grain quality of aromatic rice. J. Cereal Sci. 75, 17–24 (2017).
Li, S. Y. et al. Responses of plant growth, physiological, gas exchange parameters of super and non-super rice to rhizosphere temperature at the tillering stage. Sci. Rep. 9, 1–17 (2019).
Huang, Z. et al. Effects of increasing aroma cultivation on aroma and grain yield of aromatic rice and their mechanism. Sci. Agric. Sin. 45, 1054–1065 (2012).
Wu, M. L. et al. Characterization and the possible formation mechanism of 2-acetyl-1-pyrroline in aromatic vegetable soybean (Glycine max L.). J. Food Sci. 74, S192–S197 (2009).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Zhao, D. et al. Determination of γ-aminobutyric acid in barley. J. Triticeae Crops 29, 69–72 (2009).
Yao, S. et al. The variation of γ-aminobutyric acid content in germinated brown rice among different cultivars. Sci. Agric. Sin. 41, 3974–3982 (2008).
Ncube, B., Finnie, J. F. & Van Staden, J. Dissecting the stress metabolic alterations in in vitro Cyrtanthus regenerants. Plant Physiol. Biochem. 65, 102–110 (2013).
Zhang, C. S., Lu, Q. & Verma, D. P. S. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J. Biol. Chem. 270, 20491–20496 (1995).
Su, G., An, Z., Zhang, W. & Liu, Y. Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls. J. Plant Physiol. 162, 1297–1303 (2005).
Du, P. et al. Different tillage induces regulation in 2-acetyl-1-pyrroline biosynthesis in direct-seeded fragrant rice. BMC Plant Biol. 19, 308 (2019).
Acknowledgements
The authors acknowledge the funding provided by the National Natural Science Foundation of China (31601244, 31271646) and the Key-Area Research and Development Program of Guangdong Province (No. 2019B020221003).
Author information
Authors and Affiliations
Contributions
Z.M. designed the experiments; Y.L., L.L., X.F., and Z.G. investigated the traits; Y.L. and L.L. analyzed the data and wrote the manuscript; Z.M., H.L., J.T., M.P.P., X.T., S.P., H.T., M.D. revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Liang, L., Fu, X. et al. Light and water treatment during the early grain filling stage regulates yield and aroma formation in aromatic rice. Sci Rep 10, 14830 (2020). https://doi.org/10.1038/s41598-020-71944-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71944-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.