Featured
-
-
-
Letter |
 Fructose 1,6-bisphosphate aldolase/phosphatase may be an ancestral gluconeogenic enzyme
Fructose 1,6-bisphosphate aldolase/phosphatase may be an ancestral gluconeogenic enzyme
Thermophilic bacteria and archaea use carbon dioxide or carbon monoxide as a starting material for making the organic substances used in cellular molecules. A central enzyme in this pathway has now been discovered, namely fructose 1,6-bisphosphate aldolase/phosphatase. This enzyme might represent the ancestral gluconeogenic enzyme.
- Rafael F. Say
- & Georg Fuchs
-
Letter |
 Phosphorylation of histone H3T6 by PKCβI controls demethylation at histone H3K4
Phosphorylation of histone H3T6 by PKCβI controls demethylation at histone H3K4
The amino-terminal tails of histone proteins are subject to a variety of post-translational modifications; addition or removal of these 'marks' facilitates gene activation or silencing. Here, a mechanism is defined that modulates the activity of the dual-specificity histone demethylase LSD1 during androgen-dependent transcription. Androgen-dependent signalling through protein kinase C beta I leads to phosphorylation of a histone amino acid, which prevents demethylation of an adjacent amino acid by LSD1.
- Eric Metzger
- , Axel Imhof
- & Roland Schüle
-
Correspondence |
The Rubisco enzyme and agricultural productivity
- John R. Porter
- & Bernd Wollenweber
-
Letter |
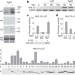 Differential innate immune signalling via Ca2+ sensor protein kinases
Differential innate immune signalling via Ca2+ sensor protein kinases
Plants and animals sense intruding pathogens by using proteins that recognize diverse microbe-associated molecular patterns (MAMPs) and initiate innate immune responses. Early signalling responses in the host include calcium influx, an oxidative burst and transcriptional reprogramming. Here, four calcium-dependent protein kinases are described that function as calcium sensors, act as convergence points for various MAMPs, and are crucial for transcriptional reprogramming and oxidative burst in plants.
- Marie Boudsocq
- , Matthew R. Willmann
- & Jen Sheen
-
Article |
 Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte
Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte
To survive and evade host responses, malaria parasites export several hundred proteins into the host cell on infection. A feature of these proteins is a conserved, pentameric motif that is cleaved by an unknown protease before export. This is one of two independent studies revealing the identity of the protease as plasmepsin V, an aspartic acid protease located in the endoplasmic reticulum. This enzyme is essential for parasite viability and is an attractive candidate for drug development.
- Ilaria Russo
- , Shalon Babbitt
- & Daniel E. Goldberg
-
Article |
 An aspartyl protease directs malaria effector proteins to the host cell
An aspartyl protease directs malaria effector proteins to the host cell
To survive and evade host responses, malaria parasites export several hundred proteins into the host cell on infection. A feature of these proteins is a conserved, pentameric motif that is cleaved by an unknown protease before export. This is one of two independent studies revealing the identity of the protease as plasmepsin V, an aspartic acid protease located in the endoplasmic reticulum. This enzyme is essential for parasite viability and is an attractive candidate for drug development.
- Justin A. Boddey
- , Anthony N. Hodder
- & Alan F. Cowman
-
Letter |
 Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency
Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency
The extent of epigenetic reprogramming in mammalian primordial germ cells (PGCs) and in early embryos, and its molecular mechanisms, are poorly understood. DNA methylation profiling in PGCs now reveals a genome–wide erasure of methylation, with female PGCs being less methylated than male ones. A deficiency of the cytidine deaminase AID interferes with the genome–wide erasure of DNA methylation, indicating that AID has a critical function in epigenetic reprogramming.
- Christian Popp
- , Wendy Dean
- & Wolf Reik
-
News & Views |
 Model offers intermediate insight
Model offers intermediate insight
Chemical models of enzymes' active sites aid our understanding of biological reactions. Such a model of a reaction intermediate promises to advance our knowledge of the biochemistry of iron-containing haem enzymes.
- Kenneth D. Karlin
-
News & Views |
Tackling unintelligent design
The key enzyme in photosynthesis, Rubisco, is a relic of a bygone age. The ability to assemble Rubisco in the test tube offers the prospect of genetically manipulating the enzyme to make it fit for the modern world.
- R. John Ellis

 G domain dimerization controls dynamin's assembly-stimulated GTPase activity
G domain dimerization controls dynamin's assembly-stimulated GTPase activity