Featured
-
-
News & Views |
 Copper catalysed asymmetric amination
Copper catalysed asymmetric amination
Chiral amines possessing a stereogenic carbon atom bearing three carbon substituents and one nitrogen substituent are challenging structural motifs to prepare enantioselectively. Now, such motifs have been accessed in high enantiopurities by asymmetric Cu-catalysed propargylic amination using sterically confined ligands.
- Joshua D. Sieber
-
Article |
 Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioenriched α-disubstituted α-ethynylamines are valuable synthons to chiral α-tertiary amines and azacycles, but their facile access remains challenging. Now, sterically confined pyridinebisoxazoline ligands have been developed to facilitate highly enantioselective Cu(I)-catalysed propargylic amination of both aliphatic and aryl ketone-derived propargylic carbonates to give α-tertiary ethynylamines. Related tandem sequences are reported to synthesize quaternary azacycles.
- Zheng Zhang
- , Ying Sun
- & Jian Zhou
-
In Your Element |
 A blueprint for catalysis
A blueprint for catalysis
Ciro Romano, Jack I. Mansell, and David J. Procter have explored the versatility and selectivity of samarium diiodide, and its use as a radical relay catalyst.
- Ciro Romano
- , Jack I. Mansell
- & David J. Procter
-
Article
| Open Access Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Achieving selectivity control in allylic arylations is a long-standing challenge in catalysis. Now a rhodium-catalysed system demonstrates chemo-, regio- and enantioselectivity, enabling Suzuki–Miyaura-type arylation with racemic, non-symmetrical, acyclic allylic systems; chelation is speculated to facilitate oxidative addition and enable both enantiomers of the starting material to converge onto a single product.
- Violeta Stojalnikova
- , Stephen J. Webster
- & Stephen P. Fletcher
-
News & Views |
 Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Tetracoordinate boron molecules are the key intermediates in many organoboron-related chemical transformations. Now, using alkynyl tetracoordinate boron species, a nickel-catalysed asymmetric 1,3-metallate shift towards axial chirality has been developed, giving access to various axially chiral alkenes in high efficiency.
- Li-Qing Ren
- & Chuan He
-
Article |
 Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Robust protocols for the synthesis of chiral α-tertiary amino acids remain scarce due to the challenge of constructing congested tetrasubstituted stereocentres. Now a cobalt-catalysed enantioselective aza-Barbier reaction of ketimines with various unactivated alkyl halides has been developed, forming diverse chiral α-tertiary amino esters with high enantioselectivity and excellent functional group tolerance.
- Xianqing Wu
- , Hanyu Xia
- & Yifeng Chen
-
Article |
 A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution
A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution
Methods for transition-metal-catalysed enantioselective C(sp3)–S bond construction are underdeveloped. Now, by taking advantage of the biomimetic radical homolytic substitution manifold, the copper-catalysed enantioconvergent C(sp3)–S cross-coupling of racemic secondary and tertiary alkyl halides with highly transformable sulfur nucleophiles has been realized. This reaction provides access to an array of α-chiral alkyl organosulfur compounds.
- Yu Tian
- , Xi-Tao Li
- & Xin-Yuan Liu
-
Article |
 Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer
Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer
The inherent rigidity of the azaarene ring structure has made it challenging to achieve remote stereocontrol through asymmetric catalysis on these substrates. Now, through a photoenzymatic process, an ene-reductase system facilitates the production of diverse azaarenes with distant γ-stereocentres, highlighting the potential of biocatalysts for stereoselectivity at remote sites.
- Maolin Li
- , Wesley Harrison
- & Huimin Zhao
-
Article |
 Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Asymmetric decarboxylation can transform abundant carboxylic acids into valuable chiral molecules but faces major limitations due to the challenging enantiocontrol of proton transfer. Now the use of Brønsted acid catalysis in conjunction with an anchoring group strategy has enabled the decarboxylative protonation of aminomalonic acids to access diverse amino acids.
- Wei-Feng Zheng
- , Jingdan Chen
- & Zhongxing Huang
-
Article |
 Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
The synthesis of optically enriched atropisomers has so far been limited to molecules containing aryl groups. Now a variant of non-aryl atropisomerism has been identified in vinyl sulfoxonium ylides, and an organocatalytic method has been developed to produce these molecules. This type of axial chirality is characterized by restricted rotation of the central C(sp2)–C(sp2) bond.
- Fengjin Wu
- , Yichi Zhang
- & Yong Huang
-
Research Briefing |
 Iron(iii) porphyrin complexes as metalloradical catalysts
Iron(iii) porphyrin complexes as metalloradical catalysts
Experimental and computational studies establish the operation of Fe(iii)-based metalloradical catalysis for the asymmetric cyclopropanation of alkenes with different classes of diazo compounds. The reaction proceeds through a stepwise radical mechanism involving α-Fe(iv)-alkyl and γ-Fe(iv)-alkyl radical intermediates. This work provides a future direction for the development of metalloradical catalysis.
-
Article |
 Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Cobalt(II) complexes of porphyrins have dominated the development of metalloradical catalysts. Now it has been shown that five-coordinate iron(III) complexes of porphyrins with an axial ligand are also potent metalloradical catalysts for olefin cyclopropanation. They are shown to react with different classes of diazo compounds via a stepwise radical mechanism.
- Wan-Chen Cindy Lee
- , Duo-Sheng Wang
- & X. Peter Zhang
-
Article
| Open Access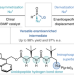 Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective approaches to access chiral organophosphorus compounds are rare. A two-stage catalytic strategy for the synthesis of diverse enantioenriched P(V) compounds has now been developed: a bifunctional iminophosphorane superbase enables enantioselective nucleophilic desymmetrization, followed by downstream enantiospecific diversification of the resulting intermediate by substitution with medicinally relevant O-, N- and S-based nucleophiles.
- Michele Formica
- , Tatiana Rogova
- & Darren J. Dixon
-
Article |
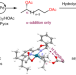 Regio- and enantioselective remote dioxygenation of internal alkenes
Regio- and enantioselective remote dioxygenation of internal alkenes
Functionalization of unsymmetrical internal alkenes usually takes place with low regioselectivity, giving a mixture of isomers. Now, a highly regio- and enantioselective remote 1,n-dioxygenation of internal alkenes using a palladium catalyst has been developed for the synthesis of chiral 1,n-diols. Regioselectivity tuning was demonstrated by altering the rate-determining step, enabled by the phenyl-substituted Pyox ligand.
- Xiaonan Li
- , Tilong Yang
- & Guosheng Liu
-
Article |
 Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Methods for the direct construction of 1,3-disubstituted planar chiral ferrocenes are elusive. Now, a modular platform enables the construction of planar chirality in 1,3-disubstituted ferrocenes/ruthenocenes via enantioselective relay remote C–H arylation. The strategy involves an initial enantiodetermining ortho-C‒H activation enabled by a Pd(II)/chiral amino-acid ligand, followed by relay to the remote meta-position by a bridgehead-substituted norbornene mediator.
- Lan Zhou
- , Hong-Gang Cheng
- & Qianghui Zhou
-
Article
| Open Access A C–H activation-based enantioselective synthesis of lower carbo[n]helicenes
A C–H activation-based enantioselective synthesis of lower carbo[n]helicenes
A simple and general enantioselective method for the synthesis of non-fused lower carbo[n]helicenes (n = 4–6) is reported. The helicene scaffold is constructed with high enantioselectivity by Pd0-catalysed C–H arylation with aryl bromides. A bifunctional ligand provides a precise chiral environment that allows fine control of the enantioselectivity.
- Shu-Min Guo
- , Soohee Huh
- & Olivier Baudoin
-
News & Views |
 Chiral sulfinyls from sulfones
Chiral sulfinyls from sulfones
The selective removal of one oxygen atom from sulfones, without over-reduction to sulfide, is a challenging task. Now, through organocatalysis and incorporation of a cyano group into the sulfone, an asymmetric deoxygenation strategy has been developed, providing an efficient method for the synthesis of chiral sulfinyl compounds.
- Elżbieta Wojaczyńska
- & Jacek Wojaczyński
-
Article |
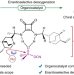 Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
The direct conversion of sulfones to chiral sulfinyl compounds is one of the major challenges in the fields of asymmetric synthesis and organosulfur chemistry. Now, through incorporation of a cyano group into the sulfone, an organocatalytic asymmetric deoxygenation strategy has been developed that enables the synthesis of chiral sulfinyl compounds.
- Shengli Huang
- , Zhen Zeng
- & Hailong Yan
-
Article |
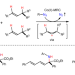 Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Controlling various selectivities in radical reactions presents both formidable challenges and great opportunities. Now, Co(II)-based metalloradical catalysis has enabled the concurrent control of multiple convergences and selectivities in intermolecular radical allylic C−H amination. The reaction provides access to valuable chiral α-tertiary amines directly from an isomeric mixture of alkenes.
- Pan Xu
- , Jingjing Xie
- & X. Peter Zhang
-
Article
| Open Access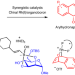 A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
Asymmetric systems for catalytic carbohydrate functionalization are mostly limited to chiral copper complexes and organocatalysts. Now, a synergistic chiral Rh(I)- and organoboron-catalysed site-selective functionalization of carbohydrate polyols has been developed, giving stereocontrolled access to biologically relevant arylhydronaphthalene glycosides. Enantio-, diastereo-, regio- and anomeric control and dynamic kinetic resolution were found to be concomitantly operative.
- V. U. Bhaskara Rao
- , Caiming Wang
- & Charles C. J. Loh
-
Article |
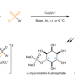 Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Heteroatom–heteroatom cross-couplings have so far remained elusive. Now, a copper-catalysed enantioselective S–O cross-coupling of diverse diols or triols with sulfonyl chlorides has been realized via a single-electron reductive elimination manifold. The reaction provides access to value-added chiral C3 building blocks and inositol phosphates through enantioselective desymmetrization of biomass-derived alcohols.
- Yong-Feng Cheng
- , Zhang-Long Yu
- & Xin-Yuan Liu
-
Article |
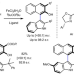 Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Direct oxidative methods for the enantioselective synthesis of heterobiaryl compounds that exhibit axial chirality remain elusive. Now, the use of an iron catalyst in the presence of a chiral PyBOX ligand and an oxidant enables the direct coupling of naphthols and indoles with high levels of enantio- and cross-selectivity.
- Richard R. Surgenor
- , Xiangqian Liu
- & Martin D. Smith
-
Article |
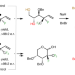 Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Methods to access organofluorine compounds with a trifluoromethyl- and fluoro-substituted carbon stereogenic centre are severely limited. Now, a flexible and stereodivergent approach has been developed for the efficient preparation of homoallylic alcohols containing this moiety. Conversion to polyfluoro furanosides and pyranosides has been demonstrated, which is relevant to antiviral drug development.
- Shibo Xu
- , Juan del Pozo
- & Amir H. Hoveyda
-
Article |
 Nickel-catalysed asymmetric hydrogenation of oximes
Nickel-catalysed asymmetric hydrogenation of oximes
The asymmetric hydrogenation of oximes to chiral hydroxylamines is a long-standing challenge because of the labile N–O bond and inert C=N bond. Now, it has been shown that this reaction can be catalysed with a chiral nickel complex, and the weak interactions between catalyst and substrate are found to play a crucial role.
- Bowen Li
- , Jianzhong Chen
- & Wanbin Zhang
-
Article |
 Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
A general copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of diverse racemic tertiary alkyl halides with terminal alkynes has been developed, forging all-carbon quaternary stereocentres. Key to the success is the rational design of chiral anionic N,N,N-ligands tailor-made for the computationally predicted outer-sphere radical group transfer pathway.
- Fu-Li Wang
- , Chang-Jiang Yang
- & Xin-Yuan Liu
-
Article |
 Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
A straightforward method for synthesizing optically active α-amino acids from abundant carboxylic acids has been developed. Based on a nitrene-mediated stereocontrolled 1,3-nitrogen shift, this approach provides access to a large variety of unnatural α-amino acids with aryl, allyl, propargyl and alkyl side chains and enables late-stage amination of carboxylic-acid-containing drugs.
- Chen-Xi Ye
- , Xiang Shen
- & Eric Meggers
-
Article |
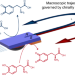 Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers
Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers
Self-propelled artificial chemical swimmers have previously been developed for chemical sensing. Now, hybrid bioelectrochemical swimmers, capable of translating chiral molecular information into macroscopic motion, have been developed. Diastereomeric interactions between enantiopure oligomers immobilized on the swimmer and a chiral molecule present in solution control the trajectory of the device.
- Serena Arnaboldi
- , Gerardo Salinas
- & Alexander Kuhn
-
Article |
 Asymmetric dearomatization catalysed by chiral Brønsted acids via activation of ynamides
Asymmetric dearomatization catalysed by chiral Brønsted acids via activation of ynamides
Chiral Brønsted acid catalysis is mostly limited to the activation of imine and carbonyl moieties. Now, by direct activation of alkynes, chiral Brønsted acids have been used to enable the catalytic asymmetric dearomatization of naphthol-, phenol- and pyrrole-ynamides for the construction of various spirocyclic enones and 2H-pyrroles bearing a chiral quaternary carbon stereocentre.
- Ying-Qi Zhang
- , Yang-Bo Chen
- & Long-Wu Ye
-
Article |
 A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes
A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes
Complex substituted cyclobutanes are common motifs in bioactive molecules but are difficult to make enantioselectively. Now, it has been shown that cross-coupling reactions between cyclobutenes and arylboronic acids—initiated by Rh-catalysed asymmetric carbometallation—can provide access to a diverse range of chiral cyclobutanes.
- F. Wieland Goetzke
- , Alexander M. L. Hell
- & Stephen P. Fletcher
-
News & Views |
 The gains from breaking symmetry
The gains from breaking symmetry
All-carbon quaternary centres are challenging targets in organic synthesis. Now, the development of a zinc-catalysed desymmetrization method enables the synthesis of chiral alcohols with all-carbon quaternary centres by the selective reduction of symmetrical α,α-disubstituted malonates.
- Jadwiga Gajewy
- & Marcin Kwit
-
Article |
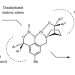 Catalytic reductive desymmetrization of malonic esters
Catalytic reductive desymmetrization of malonic esters
The desymmetrization of easily accessible malonic esters represents an attractive approach towards the formation of chiral quaternary stereocentres, but is largely limited to enzymatic hydrolysis. Now, a zinc-catalysed asymmetric hydrosilylation reaction—that works with a broad scope of substrates—has been shown to reduce one of the esters to a primary alcohol with excellent enantiocontrol.
- Pengwei Xu
- & Zhongxing Huang
-
Article |
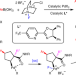 C–C bond activation enabled by dyotropic rearrangement of Pd(iv) species
C–C bond activation enabled by dyotropic rearrangement of Pd(iv) species
Many C–C bond activation methods involve strain-releasing cleavage of small rings to compensate for unfavourable kinetics and thermodynamics. Now, the 1,2-positional interchange of vicinal C–C and C–Pd bonds has been reported, giving access to quaternary carbon–palladium bonds. This dyotropic rearrangement has been used for the enantioselective synthesis of functionalized fluorinated cyclopentanes.
- Jian Cao
- , Hua Wu
- & Jieping Zhu
-
Article |
 Asymmetric, visible light-mediated radical sulfinyl-Smiles rearrangement to access all-carbon quaternary stereocentres
Asymmetric, visible light-mediated radical sulfinyl-Smiles rearrangement to access all-carbon quaternary stereocentres
The assembly of a single configuration of an all-carbon quaternary centre within acyclic systems remains a challenge for synthetic chemists. Now, it has been shown that α-all-carbon quaternary centres can be installed in acyclic amides, with excellent levels of absolute stereocontrol, through a radical sulfinyl Truce–Smiles rearrangement.
- Cédric Hervieu
- , Mariia S. Kirillova
- & Cristina Nevado
-
News & Views |
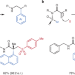 An asymmetric smile
An asymmetric smile
The formation of all-carbon quaternary centres is a challenging problem in organic chemistry, with far-reaching implications for functional molecule discovery. Now an inventive solution has been developed, using sulfinamides as traceless linkers for an asymmetric radical Truce–Smiles rearrangement.
- David M. Whalley
- & Michael F. Greaney
-
News & Views |
 Hydride surprise
Hydride surprise
Harnessing the unique catalytic properties of enzymes for abiotic reactions is a prized goal that has inspired a variety of approaches in enzyme design and engineering. Now, the transfer hydrogenation of ketones with silanes has been reported, catalysed by a native carbonic anhydrase.
- Hans Martin Senn
-
Article |
 Quaternary stereocentres via catalytic enantioconvergent nucleophilic substitution reactions of tertiary alkyl halides
Quaternary stereocentres via catalytic enantioconvergent nucleophilic substitution reactions of tertiary alkyl halides
A wide variety of bioactive molecules contain stereogenic quaternary carbons, and developing methods for the construction of these stereocentres continues to be an active area of research. Now, it has been shown that a nickel-catalysed enantioconvergent coupling of tertiary alkyl electrophiles with alkenylmetal nucleophiles—which probably proceeds via a radical pathway—can form and set quaternary stereocentres efficiently under mild conditions.
- Zhaobin Wang
- , Ze-Peng Yang
- & Gregory C. Fu
-
Article |
 Enantioselective C(sp3)–C(sp3) cross-coupling of non-activated alkyl electrophiles via nickel hydride catalysis
Enantioselective C(sp3)–C(sp3) cross-coupling of non-activated alkyl electrophiles via nickel hydride catalysis
Methods for producing organic molecules rich in sp3-hybridized carbon centres can be particularly useful for drug development. Now, it has been shown that the enantioselective cross-coupling of non-activated alkyl halides with alkenyl boronates enables the synthesis of chiral alkyl boronates. The reaction proceeds via nickel hydride insertion into an internal alkene followed by nickel-catalysed alkyl–alkyl cross-coupling.
- Srikrishna Bera
- , Runze Mao
- & Xile Hu
-
Review Article |
 Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates
Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates
The past decade has seen unprecedented growth in the development of chemical methods that proceed by mechanisms involving radical intermediates, but controlling absolute stereochemistry has been a longstanding challenge in this area. This Review Article examines how attractive non-covalent interactions between a chiral catalyst and the substrate can exert enantiocontrol in radical reactions.
- Rupert S. J. Proctor
- , Avene C. Colgan
- & Robert J. Phipps
-
Article |
 Catalytic enantiocontrol over a non-classical carbocation
Catalytic enantiocontrol over a non-classical carbocation
In 1949, Winstein and Trifan proposed that the 2-norbornyl cation adopts a bridged, non-classical structure. Now, the generation of an asymmetric environment around the three-centre two-electron bond of such an ion has been reported, enabling highly enantioselective catalytic addition reactions to a simple, non-functionalized non-classical cation.
- Roberta Properzi
- , Philip S. J. Kaib
- & Benjamin List
-
Article |
 Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions
Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions
Using readily accessible tropones and γ-methylidene-δ-valerolactones, the divergent synthesis of three classes of challenging [5.5.0] or [4.4.1] bicyclic systems has been achieved—with high efficiency and stereoselectivity—through Pd-catalysed higher-order cycloaddition. Mechanistic studies and density functional theory calculations indicate that the divergent reactions arise from the different reactivity of two diastereomeric intermediates.
- Li-Cheng Yang
- , Ya-Nong Wang
- & Yu Zhao
-
Article |
 Time-dependent enantiodivergent synthesis via sequential kinetic resolution
Time-dependent enantiodivergent synthesis via sequential kinetic resolution
Access to both enantiomers of a chiral target compound typically relies on reversing the absolute configuration of the chiral component in the reaction system that is used to make them. A time-dependent enantiodivergent synthesis is reported in which the same enantiomer of a chiral catalyst can give both enantiomers of the product, depending on the reaction time.
- Hang-Fei Tu
- , Pusu Yang
- & Shu-Li You
-
Article |
 Dual electrocatalysis enables enantioselective hydrocyanation of conjugated alkenes
Dual electrocatalysis enables enantioselective hydrocyanation of conjugated alkenes
A general method for the enantioselective hydrocyanation of alkenes has been a long-standing synthetic challenge. Now, using a dual electrocatalytic approach that combines two synergistic redox catalytic cycles, a wide variety of chiral nitriles can be synthesized from conjugated alkenes in high enantioselectivity.
- Lu Song
- , Niankai Fu
- & Song Lin
-
Article |
 Enantioselective radical C–H amination for the synthesis of β-amino alcohols
Enantioselective radical C–H amination for the synthesis of β-amino alcohols
The synthesis of chiral amino alcohols from simple alcohol starting materials can be achieved through a catalytic, asymmetric radical relay. Multiple catalysts work in concert to harness a H-atom transfer mechanism to enable enantio- and regioselective C–H amination by transient N-centred radicals.
- Kohki M. Nakafuku
- , Zuxiao Zhang
- & David A. Nagib
-
Article |
 Ruthenium-catalysed multicomponent synthesis of the 1,3-dienyl-6-oxy polyketide motif
Ruthenium-catalysed multicomponent synthesis of the 1,3-dienyl-6-oxy polyketide motif
A ruthenium-catalysed multicomponent reaction provides rapid and tunable access to 1,3-dienyl-6-oxy polyketide motifs. An initial alkene–alkyne coupling produces unsymmetrical 3-boryl-1,4-dienes. Allylation of aldehydes and ketones with these products is highly diastereoselective and results in the formation of two carbon–carbon bonds, two stereodefined olefins and up to three contiguous sp3 stereocentres.
- Barry M. Trost
- , James J. Cregg
- & Jacob S. Tracy
-
Article |
 A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling
A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling
Asymmetric Sonogashira C(sp3)–C(sp) couplings provide complementary approaches to established C(sp3)–C(sp2/sp3) couplings for chiral C–C bond formation; however, relatively few reactions have been developed. Now, a versatile, enantioconvergent Sonogashira coupling via a radical intermediate has been developed. The approach uses a copper catalyst featuring a multidentate electron-rich cinchona alkaloid-derived ligand.
- Xiao-Yang Dong
- , Yu-Feng Zhang
- & Xin-Yuan Liu
-
Article |
 An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds
An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds
Stereoselective functionalization of C(sp3)–H bonds could greatly simplify the construction of complex molecular scaffolds. Now, a series of enzyme catalysts derived from a cytochrome P450 have been developed using directed evolution. The catalysts enable the enantioselective amination of primary, secondary and tertiary C(sp3)–H bonds.
- Yang Yang
- , Inha Cho
- & Frances H. Arnold
-
Article |
 Energy threshold for chiral symmetry breaking in molecular self-replication
Energy threshold for chiral symmetry breaking in molecular self-replication
Asymmetric autocatalysis—such as that observed experimentally in the Soai reaction—may have been responsible for the origin of biological homochirality. The magnitude of the energy imbalance required to induce directed symmetry breaking and asymmetric amplification in the Soai reaction has now been identified and compared to the parity violation energy difference.
- Neil A. Hawbaker
- & Donna G. Blackmond
-
Article |
 Unravelling mechanistic features of organocatalysis with in situ modifications at the secondary sphere
Unravelling mechanistic features of organocatalysis with in situ modifications at the secondary sphere
Secondary-sphere interactions serve a fundamental role in controlling the reactivity and selectivity of organometallic and enzyme catalysts, but their study in organocatalytic systems is scarce. Now, it has been shown that the in situ secondary-sphere modification of organocatalysts combined with machine-learning techniques can uncover reaction mechanisms and streamline catalyst optimization.
- Vasudevan Dhayalan
- , Santosh C. Gadekar
- & Anat Milo
-
Article |
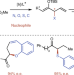 Enantioselective α-functionalizations of ketones via allylic substitution of silyl enol ethers
Enantioselective α-functionalizations of ketones via allylic substitution of silyl enol ethers
Typical methods for the enantioselective α-functionalizations of ketones join ketone enolate nucleophiles with carbon or heteroatom electrophiles. We report an umpolung strategy to achieve this transformation with masked ketone electrophiles and a wide range of conventional heteroatom and carbon nucleophiles catalysed by a metallacyclic iridium catalyst in high yield and enantioselectivity.
- Zhi-Tao He
- & John F. Hartwig

 Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer
Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer