Featured
-
-
Article |
 Organocatalytic aromatization-promoted umpolung reaction of imines
Organocatalytic aromatization-promoted umpolung reaction of imines
The umpolung functionalization of imines bears vast synthetic potential, but polarity inversion is less efficient compared with the carbonyl counterparts. Now, an alternative strategy exploiting chiral phosphoric acid catalytic aromatization has been developed, affording structures possessing a central chirality or a stereogenic C–N axis with high efficiency and enantiocontrol.
- Ye-Hui Chen
- , Meng Duan
- & Bin Tan
-
News & Views |
 An acid for an acid
An acid for an acid
Stereoselective decarboxylative protonation can produce diverse chiral molecules from widely available carboxylic acids. However, general and practical strategies are lacking. Now, a chiral spirocyclic phosphoric acid-catalysed decarboxylation of aminomalonic acids has enabled the modular synthesis of α-amino acids.
- Xufeng Lin
- & Alemayehu Gashaw Woldegiorgis
-
News & Views |
 Enyne difluorination
Enyne difluorination
Fluorination strategies are important in assisting the synthesis of pharmaceuticals. Iodine(I/III) catalysis has become particularly useful for installing gem-difluoro groups but is limited to styrenes. Now, the hypervalent iodane-catalysed difluorination of enynes has enabled access to diverse homopropargylic difluorides.
- Rachel C. Epplin
- & Tanja Gulder
-
Article
| Open Access Regioselective, catalytic 1,1-difluorination of enynes
Regioselective, catalytic 1,1-difluorination of enynes
Hypervalent iodine catalysis remains a powerful method to enable geminal difluoromethylenation of alkenes. However, the scope is mainly limited to styrene derivatives. Now, enynes have been validated as competent substrates where a formal 1,2-shift of the alkyne occurs, thereby enabling highly versatile homopropargylic difluorides to be generated.
- Zi-Xuan Wang
- , Keith Livingstone
- & Ryan Gilmour
-
Article |
 Reversible 2′-OH acylation enhances RNA stability
Reversible 2′-OH acylation enhances RNA stability
Stabilization of RNAs for storage, transport and biological application remains a profound challenge. Now, it has been shown that reversible 2′-OH acylation with easily accessible acylimidazoles unlocks efficient protection of RNA. RNA can be deprotected by non-basic nucleophiles or spontaneously in cells to restore RNA functions.
- Linglan Fang
- , Lu Xiao
- & Eric T. Kool
-
Article
| Open Access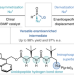 Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective approaches to access chiral organophosphorus compounds are rare. A two-stage catalytic strategy for the synthesis of diverse enantioenriched P(V) compounds has now been developed: a bifunctional iminophosphorane superbase enables enantioselective nucleophilic desymmetrization, followed by downstream enantiospecific diversification of the resulting intermediate by substitution with medicinally relevant O-, N- and S-based nucleophiles.
- Michele Formica
- , Tatiana Rogova
- & Darren J. Dixon
-
News & Views |
 Amino amide assembly
Amino amide assembly
Enantioenriched β-amino acid derivatives are attractive synthetic targets, considering the significance of these motifs in medicinal and material chemistry. Now, using ambiphilic ynamides as two-carbon synthons in a four-component reaction, three classes of β-amino amides with well-defined stereocentres can be accessed.
- Sunliang Cui
- & Linwei Zeng
-
Article |
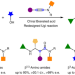 Modular enantioselective access to β-amino amides by Brønsted acid-catalysed multicomponent reactions
Modular enantioselective access to β-amino amides by Brønsted acid-catalysed multicomponent reactions
β-Amino acids are broadly found in biomimetic drugs and therapeutics, but the general synthesis of chiral β-amino amides remains limited. An organocatalytic four-component reaction has now been developed for their asymmetric synthesis with notable efficiency, chemoselectivity and stereoselectivity. This protocol shows broad product scope and provides a shortcut to other important structures.
- Jun Wei
- , Jian Zhang
- & Bin Tan
-
Article |
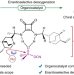 Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
The direct conversion of sulfones to chiral sulfinyl compounds is one of the major challenges in the fields of asymmetric synthesis and organosulfur chemistry. Now, through incorporation of a cyano group into the sulfone, an organocatalytic asymmetric deoxygenation strategy has been developed that enables the synthesis of chiral sulfinyl compounds.
- Shengli Huang
- , Zhen Zeng
- & Hailong Yan
-
News & Views |
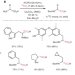 Enriched amino acids
Enriched amino acids
The direct carbon isotope exchange reaction on α-amino acids is highly desirable, as existing labelling methods require several synthetic steps and harsh conditions. Now, an aldehyde-catalysed carboxylate exchange with isotopically labelled *CO2 has enabled the direct formation of 11C, 13C and 14C-labelled α-amino acids.
- Karoline T. Neumann
- & Troels Skrydstrup
-
Article |
 Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2
Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2
Carbon-labelled α-amino acids are valuable compounds in drug development and nuclear medicine, but are difficult and time consuming to prepare. Now, an aldehyde-catalysed method has been developed for the direct C1-labelling of α-amino acids using *CO2 (* = 14, 13, 11), providing access to many proteinogenic and non-natural labelled α-amino acids.
- Odey Bsharat
- , Michael G. J. Doyle
- & Rylan J. Lundgren
-
Article |
 Tunable and recyclable polyesters from CO2 and butadiene
Tunable and recyclable polyesters from CO2 and butadiene
The direct copolymerization of carbon dioxide and commodity olefins has been a long-standing challenge in polymer science. Now, an indirect approach has been developed in which hydrogenated disubstituted valerolactones derived from telomerization of CO2 and butadiene can undergo ring-opening polymerization, yielding chemically recyclable and degradable aliphatic polyesters with high CO2 content.
- Rachel M. Rapagnani
- , Rachel J. Dunscomb
- & Ian A. Tonks
-
News & Views |
 Sustainable and degradable plastics
Sustainable and degradable plastics
Plastics that are developed from renewable resources and can be recycled are highly environmentally desirable alternatives to current petroleum-based non-degradable polymers. Now, an effective and robust industrially relevant strategy towards high-performance biomass-derived degradable poly(γ-thiobutyrolactone)s has been developed.
- Sophie M. Guillaume
-
Article |
 Organocatalyst-controlled site-selective arene C–H functionalization
Organocatalyst-controlled site-selective arene C–H functionalization
Although organocatalysis has emerged as a powerful catalysis platform, its application for the selective transformation of inactive arenes remains challenging. Now, an organocatalyst-controlled site-selective C–H functionalization of arenes has been developed, with wide substrate generality and applicability, offering a general strategy for arene transformation.
- Jian-Hui Mao
- , Yong-Bin Wang
- & Bin Tan
-
Article |
 Catalytic enantiocontrol over a non-classical carbocation
Catalytic enantiocontrol over a non-classical carbocation
In 1949, Winstein and Trifan proposed that the 2-norbornyl cation adopts a bridged, non-classical structure. Now, the generation of an asymmetric environment around the three-centre two-electron bond of such an ion has been reported, enabling highly enantioselective catalytic addition reactions to a simple, non-functionalized non-classical cation.
- Roberta Properzi
- , Philip S. J. Kaib
- & Benjamin List
-
News & Views |
 Mild map to quinone methides
Mild map to quinone methides
The widespread application of quinone methides — reactive intermediates in a variety of reactions — is limited by their tedious synthesis. Now, hypoiodite catalysis allows the efficient generation and use of these species in a plethora of tandem processes for the functionalization and synthesis of biologically active compounds.
- Boris J. Nachtsheim
-
Article |
 Chemoselective oxidative generation of ortho-quinone methides and tandem transformations
Chemoselective oxidative generation of ortho-quinone methides and tandem transformations
ortho-Quinone methides are highly reactive transient intermediates found in some organic syntheses and biological processes. The generation of these species often requires pre-functionalized substrates and/or metal oxidants, but now the chemoselective oxidative generation of ortho-quinone methides from ortho-alkylarenols has been achieved using hypoiodite catalysis under nearly neutral conditions.
- Muhammet Uyanik
- , Kohei Nishioka
- & Kazuaki Ishihara
-
News & Views |
 Mitsunobu gets a makeover
Mitsunobu gets a makeover
Nucleophiles directly substitute alcohols in the Mitsunobu reaction, but the process uses hazardous materials and generates an excessive amount of toxic waste. More than 50 years on from its initial discovery, chemists report a new catalytic version of the reaction, with water as the only by-product.
- Andy McNally
-
Article |
 Unravelling mechanistic features of organocatalysis with in situ modifications at the secondary sphere
Unravelling mechanistic features of organocatalysis with in situ modifications at the secondary sphere
Secondary-sphere interactions serve a fundamental role in controlling the reactivity and selectivity of organometallic and enzyme catalysts, but their study in organocatalytic systems is scarce. Now, it has been shown that the in situ secondary-sphere modification of organocatalysts combined with machine-learning techniques can uncover reaction mechanisms and streamline catalyst optimization.
- Vasudevan Dhayalan
- , Santosh C. Gadekar
- & Anat Milo
-
Meeting Report |
 Safe, sustainable and scalable
Safe, sustainable and scalable
Two recent back-to-back meetings conveyed a common set of ongoing challenges for the fields of organocatalysis, photoredox catalysis and photochemistry.
- Craig P. Johnston
-
Article |
 Approaching sub-ppm-level asymmetric organocatalysis of a highly challenging and scalable carbon–carbon bond forming reaction
Approaching sub-ppm-level asymmetric organocatalysis of a highly challenging and scalable carbon–carbon bond forming reaction
Chiral tertiary aldols are encountered in a variety of biologically relevant molecules. Making these valuable compounds directly from unbiased ketones has proven to be extremely challenging. Now it has been shown that sub-ppm levels of in situ generated silylium-based organic Lewis acid catalysts can give quantitative product formation in very high enantiopurity through a Mukaiyama aldol reaction.
- Han Yong Bae
- , Denis Höfler
- & Benjamin List
-
Article |
 Direct, enantioselective α-alkylation of aldehydes using simple olefins
Direct, enantioselective α-alkylation of aldehydes using simple olefins
The catalytic asymmetric α-alkylation of aldehydes has historically been a significant challenge within organic synthesis. Now, this elusive transformation has been achieved through the merger of organocatalysis, photoredox catalysis and hydrogen-atom transfer catalysis to enable the coupling of simple olefins and aldehydes.
- Andrew G. Capacci
- , Justin T. Malinowski
- & David W. C. MacMillan
-
Article |
 Visible-light excitation of iminium ions enables the enantioselective catalytic β-alkylation of enals
Visible-light excitation of iminium ions enables the enantioselective catalytic β-alkylation of enals
Chiral iminium ions generated from an amine catalyst and enals are key organocatalytic intermediates in thermal asymmetric processes. Now, visible-light excitation of these iminium ions can turn these compounds into strong oxidants to enable enantioselective photochemical β-alkylations of enals with silanes, which are unachievable via conventional ground state pathways.
- Mattia Silvi
- , Charlie Verrier
- & Paolo Melchiorre
-
Article |
 Unique physicochemical and catalytic properties dictated by the B3NO2 ring system
Unique physicochemical and catalytic properties dictated by the B3NO2 ring system
Amidation is one of the most widely utilized organic reactions for the synthesis of pharmaceuticals and functional materials. DATB, characterized by the B3NO2 heterocycle, proved to act as a superb catalyst for the direct amidation with a distinct mechanistic pathway, displaying broadened applicability to a wide range of substrates.
- Hidetoshi Noda
- , Makoto Furutachi
- & Naoya Kumagai
-
Article |
 Catalytic enantioselective synthesis of atropisomeric biaryls by a cation-directed O-alkylation
Catalytic enantioselective synthesis of atropisomeric biaryls by a cation-directed O-alkylation
A chiral ammonium salt mediates a dynamic kinetic resolution of racemic α-aryl ketones by atropselective O-alkylation. Oxidation with DDQ gives access to C2-symmetric and non-symmetric BINOL derivatives in high yields and with high enantioselectivity.
- John D. Jolliffe
- , Roly J. Armstrong
- & Martin D. Smith
-
Article |
 Organocatalytic stereoselective [8+2] and [6+4] cycloadditions
Organocatalytic stereoselective [8+2] and [6+4] cycloadditions
Higher-order cycloadditions — cycloadditions involving more than six π electrons — provide easy access to complex molecules containing heterocyclic or carbocyclic scaffolds. Now, it has been shown that aminocatalytic activation of 2-cycloalkenones affords mixtures of dienamines that can undergo [6 + 4] cycloadditions with heptafulvenes through a cross dienamine, or [8 + 2] cycloadditions through a linear dienamine.
- Rasmus Mose
- , Gert Preegel
- & Karl Anker Jørgensen
-
Article |
 Fast and selective ring-opening polymerizations by alkoxides and thioureas
Fast and selective ring-opening polymerizations by alkoxides and thioureas
By simply deprotonating a neutral hydrogen-bond donor thiourea it is possible to generate a class of highly efficient and tunable thioimidates that can simultaneously activate a pro-nucleophile and an electrophile. These bifunctional thioimidates exhibit fast kinetics and high selectivity for ring-opening polymerizations of cyclic lactones and carbonates.
- Xiangyi Zhang
- , Gavin O. Jones
- & Robert M. Waymouth
-
News & Views |
 More than just a game
More than just a game
Catalytic methods are among the most valuable tools for sustainable synthesis. Domino catalysis enables multiple reactions to be combined so that synthetic efficiency may begin to approach that of nature, but significant challenges remain before this promising approach can fulfil the needs of pharmaceutical and materials chemistry.
- Thomas Broja
- , Patrick J. W. Fuchs
- & Kirsten Zeitler
-
Article |
 Organocatalytic removal of formaldehyde adducts from RNA and DNA bases
Organocatalytic removal of formaldehyde adducts from RNA and DNA bases
Formaldehyde is universally employed in the fixation of tissue specimens, where it forms adducts with biomolecules, but this hinders the analysis of nucleic acids in the specimen. Bifunctional organocatalysts that speed the reversal of formaldehyde adducts of RNA and DNA are now reported, and show promise for general use in clinical specimens.
- Saswata Karmakar
- , Emily M. Harcourt
- & Eric T. Kool
-
-
-
News & Views |
 An internal affair
An internal affair
Preparing powerful reactive intermediates such as enolates and homoenolates for C–C bond formation used to require strong bases and stoichiometric reagents. They can now be catalytically generated from α-functionalized aldehydes or even from saturated esters under mild conditions using N-heterocyclic carbene catalysts.
- Jeffrey W. Bode
-
-
-
Article |
 Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst
Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst
Spirooxindoles are important structural motifs found in an array of bioactive compounds. Here a Michael–aldol domino reaction for the construction of bispirooxindoles is presented, creating four stereocentres, including three quaternary carbon centres. Novel multifunctional organocatalysts have been developed for this transformation.
- Bin Tan
- , Nuno R. Candeias
- & Carlos F. Barbas III
-
Article |
 Synergistic organocatalysis in the kinetic resolution of secondary thiols with concomitant desymmetrization of an anhydride
Synergistic organocatalysis in the kinetic resolution of secondary thiols with concomitant desymmetrization of an anhydride
Chiral thiols and organosulfur compounds are important in many areas of chemistry but there are relatively few methods available for their efficient enantioselective synthesis. Here, a kinetic resolution of chiral thiols is reported along with a demonstration that a concomitant desymmetrization of the acylating agent is beneficial for the selectivity of both processes.
- Aldo Peschiulli
- , Barbara Procuranti
- & Stephen J. Connon
-
Review Article |
 Organocatalytic cascade reactions as a new tool in total synthesis
Organocatalytic cascade reactions as a new tool in total synthesis
The field of organocatalysis has grown rapidly in the past decade to become, along with metal catalysis and biocatalysis, a third pillar of asymmetric catalysis. Here, progress in the use of organocatalytic cascade reactions for total synthesis is reviewed. The elegance and efficiency of such cascades mean that they have emerged as a powerful tool in synthetic organic chemistry.
- Christoph Grondal
- , Matthieu Jeanty
- & Dieter Enders

 Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer
Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer Addition to alkynoates
Addition to alkynoates Higher or lower?
Higher or lower?