Abstract
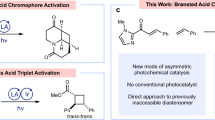
Visible-light-driven photoredox catalysis is known to be a powerful tool for organic synthesis. Its occurrence critically depends on the twice exothermic single-electron transfer processes of photosensitizers, which are governed by the redox properties of the species involved. Hence, the inherently narrow range of redox potentials of photosensitizers inevitably constrains their further availability. Sensitization-initiated electron transfer has recently been found to effectively overcome this substantial challenge. However, feasible and practical strategies for designing such complicated catalytic systems are rather scarce. Herein we report an elaborate dual-catalyst platform, with dicyanopyrazine as a visible light photosensitizer and a pyrenyl-incorporated chiral phosphoric acid as a co-sensitizer, and we demonstrate the applicability of this sensitization-initiated electron transfer strategy in an asymmetric formal de Mayo-type reaction. The catalysis platform enables otherwise thermodynamically unfavourable electron transfer processes to close the redox cycle and allows for precise access to valuable enantioenriched 1,5-diketones with a wide substrate range.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available in the paper and its Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2234002 (97), 2234003 (98), 2234004 (31), 2234005 (59), 2234006 (93), 2234007 (SX19168) and 2234008 (SX19171). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Bauer, A., Westkämper, F., Grimme, S. & Bach, T. Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 436, 1139–1140 (2005).
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322 (2013).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Yao, W., Bazan-Bergamino, E. A. & Ngai, M.-Y. Asymmetric photocatalysis enabled by chiral organocatalysts. ChemCatChem 14, e202101292 (2022).
Glaser, F., Kerzig, C. & Wenger, O. S. Multi-photon excitation in photoredox catalysis: concepts, applications, methods. Angew. Chem. Int. Ed. 59, 10266–10284 (2020).
Glaser, F., Kerzig, C. & Wenger, O. S. Sensitization-initiated electron transfer via upconversion: mechanism and photocatalytic applications. Chem. Sci. 12, 9922–9933 (2021).
Kerzig, C. & Goez, M. Combining energy and electron transfer in a supramolecular environment for the ‘green’ generation and utilization of hydrated electrons through photoredox catalysis. Chem. Sci. 7, 3862–3868 (2016).
Ghosh, I., Shaikh, R. S. & König, B. Sensitization-initiated electron transfer for photoredox catalysis. Angew. Chem. Int. Ed. 56, 8544–8549 (2017).
Marchini, M., Bergamini, G., Cozzi, P. G., Ceroni, P. & Balzani, V. Photoredox catalysis: the need to elucidate the photochemical mechanism. Angew. Chem. Int. Ed. 56, 12820–12821 (2017).
Ghosh, I., Bardagi, J. I. & König, B. Reply to ‘Photoredox catalysis: the need to elucidate the photochemical mechanism’. Angew. Chem. Int. Ed. 56, 12822–12824 (2017).
Pal, A. K., Li, C., Hanan, G. S. & Zysman-Colman, E. Blue-emissive cobalt(III) complexes and their use in the photocatalytic trifluoromethylation of polycyclic aromatic hydrocarbons. Angew. Chem. Int. Ed. 57, 8027–8031 (2018).
Coles, M. S., Quach, G., Beves, J. E. & Moore, E. G. A photophysical study of sensitization-initiated electron transfer: insights into the mechanism of photoredox activity. Angew. Chem. Int. Ed. 59, 9522–9526 (2020).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Zhou, Q.-Q., Zou, Y.-Q., Lu, L.-Q. & Xiao, W.-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew. Chem. Int. Ed. 58, 1586–1604 (2019).
De Mayo, P., Takeshita, H. & Sattar, A. B. M. A. The photochemical synthesis of 1,5-diketones and their cyclisation: a new annulation process. Proc. Chem. Soc. 1962, 119 (1962).
Begley, M. J., Mellor, M. & Pattenden, G. New synthetic approaches to fused-ring carbocycles based on intramolecular photocycloadditions of 1,3-dione enol esters. J. Chem. Soc. Perkin Trans. 1 1, 1905–1912 (1983).
Andrew, D., Hastings, D. J. & Weedon, A. C. The mechanism of the photochemical cycloaddition reaction between 2-cyclopentenone and polar alkenes: trapping of triplet 1,4-biradical intermediates with hydrogen selenide. J. Am. Chem. Soc. 116, 10870–10882 (1994).
Kandappa, S. K., Valloli, L. K., Jockusch, S. & Sivaguru, J. Uncovering new excited state photochemical reactivity by altering the course of the De Mayo reaction. J. Am. Chem. Soc. 143, 3677–3681 (2021).
Martinez-Haya, R., Marzo, L. & König, B. Reinventing the De Mayo reaction: synthesis of 1,5-diketones or 1,5-ketoesters via visible light [2+2] cycloaddition of β-diketones or β-ketoesters with styrenes. Chem. Commun. 54, 11602–11605 (2018).
Salaverri, N., Mas-Ballesté, R., Marzo, L. & Alemán, J. Visible light mediated photocatalytic [2 + 2] cycloaddition/ring-opening rearomatization cascade of electron-deficient azaarenes and vinylarenes. Commun. Chem. 3, 132 (2020).
Zhao, Q.-Q., Rehbein, J. & Reiser, O. Thermoneutral synthesis of spiro-1,4-cyclohexadienes by visible-light-driven dearomatization of benzylmalonates. Green Chem. 24, 2772–2776 (2022).
Paulisch, T. O. et al. Dynamic kinetic sensitization of β-dicarbonyl compounds‒access to medium-sized rings by De Mayo-type ring expansion. Angew. Chem. Int. Ed. 61, e202112695 (2022).
Zhang, W. & Luo, S. Visible-light promoted De Mayo reaction by zirconium catalysis. Chem. Commun. 58, 12979–12982 (2022).
Gentry, E. C. & Knowles, R. R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 49, 1546–1556 (2016).
Lv, X., Xu, H., Yin, Y., Zhao, X. & Jiang, Z. Visible light-driven cooperative DPZ and chiral hydrogen-bonding catalysis. Chin. J. Chem. 38, 1480–1488 (2020).
Proctor, R. S. J. & Phipps, R. J. Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed. 58, 13666–13699 (2019).
Yin, Y., Zhao, X., Qiao, B. & Jiang, Z. Cooperative photoredox and chiral hydrogen-bonding catalysis. Org. Chem. Front. 7, 1283–1296 (2020).
Yin, Y. et al. Conjugate addition–enantioselective protonation of N-aryl glycines to α-branched 2-vinylazaarenes via cooperative photoredox and asymmetric catalysis. J. Am. Chem. Soc. 140, 6083–6087 (2018).
Proctor, R. S. J., Davis, H. J. & Phipps, R. J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science 360, 419–422 (2018).
Fu, M.-C., Shang, R., Zhao, B., Wang, B. & Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 363, 1429–1434 (2019).
Li, Y. et al. Catalytic asymmetric reductive azaarylation of olefins via enantioselective radical coupling. J. Am. Chem. Soc. 144, 7805–7814 (2022).
Hloušková, Z. et al. Structure–catalytic activity in a series of push–pull dicyanopyrazine/dicyanoimidazole photoredox catalysts. ChemistrySelect 3, 4262–4270 (2018).
Müller, C., Bauer, A. & Bach, T. Light-driven enantioselective organocatalysis. Angew. Chem. Int. Ed. 48, 6640–6642 (2009).
Müller, C. et al. Enantioselective intramolecular [2 + 2]-photocycloaddition reactions of 4-substituted quinolones catalysed by a chiral sensitizer with a hydrogen-bonding motif. J. Am. Chem. Soc. 133, 16689–16697 (2011).
Alonso, R. & Bach, T. A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light. Angew. Chem. Int. Ed. 53, 4369–4371 (2014).
Ding, W. et al. Bifunctional photocatalysts for enantioselective aerobic oxidation of β-ketoeaters. J. Am. Chem. Soc. 139, 63–66 (2017).
Plaza, M., Großkopf, J., Breitenlechner, S., Bannwarth, C. & Bach, T. Photochemical deracemization of primary allene amides by triplet energy transfer: a combined synthetic and theoretical study. J. Am. Chem. Soc. 143, 11209–11217 (2021).
Ryder, A. S. H. et al. Photocatalytic α-tertiary amine synthesis via C–H alkylation of unmasked primary amines. Angew. Chem. Int. Ed. 59, 14986–14991 (2020).
Majumdar, K. C. & Chattopadhyay, S. K. Heterocycles in Natural Product Synthesis 267−341 (Wiley-VCH Verlag, 2011).
Li, J. J. Heterocyclic Chemistry in Drug Discovery 397−611 (John Wiley & Sons, 2013).
Chelucci, G. Metal-complexes of optically active amino- and imino-based pyridine ligands in asymmetric catalysis. Coord. Chem. Rev. 257, 1887–1932 (2013).
Guan, A.-Y., Liu, C.-L., Sun, X.-F., Xie, Y. & Wang, M.-A. Discovery of pyridine-based agrochemicals by using intermediate derivatization methods. Bioorg. Med. Chem. 24, 342–353 (2016).
Chatterjee, A. & König, B. Birch-type photoreduction of arenes and heteroarenes by sensitized electron transfer. Angew. Chem. Int. Ed. 58, 14289–14294 (2019).
Acknowledgements
This work was financially supported by the National Science Foundation of China (21925103 and 22171072), Henan University and Henan Normal University.
Author information
Authors and Affiliations
Contributions
Z.J. and X.S. conceived and designed the experiments. X.S. and Y.L. performed the experiments. X.S., Y.Y., X.Z. and X.B. analysed and interpreted the results. X.S., X.B. and Z.J. prepared the Supplementary Information. Z.J. and X.B. wrote the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Christoph Kerzig and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
General information, general experimental procedures, synthetic applications, mechanistic studies, determination of the absolute configurations, characterization of adducts, copies of NMR spectra and references.
Supplementary Data 1
Crystallographic data for compound 97; CCDC reference 2234002.
Supplementary Data 2
Crystallographic data for compound 98; CCDC reference 2234003.
Supplementary Data 3
Crystallographic data for compound 31; CCDC reference 2234004.
Supplementary Data 4
Crystallographic data for compound 59; CCDC reference 2234005.
Supplementary Data 5
Crystallographic data for compound 93; CCDC reference 2234006.
Supplementary Data 6
Crystallographic data for compound SX19168 derived from product 73; CCDC reference 2234007.
Supplementary Data 7
Crystallographic data for compound SX19171 derived from product 89; CCDC reference 2234008.
Supplementary Data 8
Structure factors for compound 97; CCDC reference 2234002.
Supplementary Data 9
Structure factors for compound 98; CCDC reference 2234003.
Supplementary Data 10
Structure factors for compound 31; CCDC reference 2234004.
Supplementary Data 11
Structure factors for compound 59; CCDC reference 2234005.
Supplementary Data 12
Structure factors for compound 93; CCDC reference 2234006.
Supplementary Data 13
Structure factors for compound SX19168 derived from product 73; CCDC reference 2234007.
Supplementary Data 14
Structure factors for compound SX19171 derived from product 89; CCDC reference 2234008.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Liu, Y., Yin, Y. et al. Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01502-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01502-3