Key Points
-
Here, we discuss emerging evidence that causally links perinatal exposure to microbial and environmental factors with chronic inflammatory diseases of the intestinal and respiratory tracts.
-
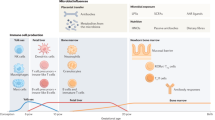
Structural and functional changes of the lung and gut mucosal immune systems occur during the pre- and postnatal periods, and are regulated by developmental and environmental factors. Concomitantly, the exposure to environmental stimuli increases and the resident microbiota at mucosal sites is established.
-
Adaptation to these changes is facilitated by complex molecular and cellular regulatory circuits that prevent inappropriate immune stimulation and promote the establishment of a stable microbial–host homeostasis.
-
A failure to acquire tolerance to food and environmental antigens, impaired epithelial barrier formation and an imbalanced mucosal cellular composition promote the development of inflammation. In addition, alterations to the composition of the resident microbiota influence nutrient metabolism, mucosal tissue maturation and immunoregulatory mechanisms.
-
Further insight is required to fully understand the links between early microbial and environmental exposures, perinatal mucosal tissue maturation, postnatal establishment of the mucosal microbiota and disease susceptibility; such information might be used to develop prophylactic and therapeutic strategies to overcome chronic inflammatory diseases.
Abstract
The mucosal surfaces of the gut and airways have important barrier functions and regulate the induction of immunological tolerance. The rapidly increasing incidence of chronic inflammatory disorders of these surfaces, such as inflammatory bowel disease and asthma, indicates that the immune functions of these mucosae are becoming disrupted in humans. Recent data indicate that events in prenatal and neonatal life orchestrate mucosal homeostasis. Several environmental factors promote the perinatal programming of the immune system, including colonization of the gut and airways by commensal microorganisms. These complex microbial–host interactions operate in a delicate temporal and spatial manner and have an important role in the induction of homeostatic mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Holt, P. G., Strickland, D. H., Wikstrom, M. E. & Jahnsen, F. L. Regulation of immunological homeostasis in the respiratory tract. Nature Rev. Immunol. 8, 142–152 (2008).
Guarner, F. et al. Mechanisms of disease: the hygiene hypothesis revisited. Nature Clin. Pract. Gastroenterol. Hepatol. 3, 275–284 (2006).
Tulic, M. K. et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J. Allergy Clin. Immunol. 127, 470–478 (2011).
Prescott, S. L. Role of dietary immunomodulatory factors in the development of immune tolerance. Nestle Nutr. Workshop Ser. Pediatr. Program. 64, 185–194 (2009).
Fasano, A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 91, 151–175 (2011).
Elinav, E., Strowig, T., Henao-Mejia, J. & Flavell, R. A. Regulation of the antimicrobial response by NLR proteins. Immunity 34, 665–679 (2011).
Garrett, W. S. et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 8, 292–300 (2010).
Hilty, M. et al. Disordered microbial communities in asthmatic airways. PLoS ONE 5, e8578 (2010).
Huang, Y. J. et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 127, 372–381 (2011).
McGovern, D. P. et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nature Genet. 42, 332–337 (2010).
Franke, A. et al. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL). Nature Genet. 42, 292–294 (2010).
Noguchi, E. et al. Genome-wide association study identifies HLA-DP as a susceptibility gene for pediatric asthma in asian populations. PLoS Genet. 7, e1002170 (2011).
Harper, J., Mould, A., Andrews, R. M., Bikoff, E. K. & Robertson, E. J. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc. Natl Acad. Sci. USA 13 Jun 2011 (doi:10.1073/pnas.1105852108).
de Santa, B. P., van den Brink, G. R. & Roberts, D. J. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. 60, 1322–1332 (2003).
Darmoul, D., Brown, D., Selsted, M. E. & Ouellette, A. J. Cryptdin gene expression in developing mouse small intestine. Am. J. Physiol. 272, G197–G206 (1997).
Bry, L. et al. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc. Natl Acad. Sci. USA 91, 10335–10339 (1994).
Menard, S. et al. Developmental switch of intestinal antimicrobial peptide expression. J. Exp. Med. 205, 183–193 (2008).
Putsep, K. et al. Germ-free and colonized mice generate the same products from enteric prodefensins. J. Biol. Chem. 275, 40478–40482 (2000).
Sanos, S. L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nature Immunol. 10, 83–91 (2009).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Kai-Larsen, Y. et al. Antimicrobial components of the neonatal gut affected upon colonization. Pediatr. Res. 61, 530–536 (2007).
Salzman, N. H. et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nature Immunol. 11, 76–83 (2010). This is the first study to detail the functional consequences of decreased or increased antimicrobial peptide production on the enteric microbiota.
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010). An important study showing that the human neonatal intestinal microbiota is derived from the mother but depends on the mode of delivery.
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4578–4585 (2011).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Lotz, M. et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203, 973–984 (2006).
Gribar, S. C. et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 182, 636–646 (2009).
Claud, E. C. et al. Developmentally regulated IκB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc. Natl Acad. Sci. USA 101, 7404–7408 (2004).
Chassin, C. et al. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 8, 358–368 (2010). A detailed analysis of the molecular processes that control intestinal epithelial innate immune tolerance in neonatal mice.
Kajino-Sakamoto, R. et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J. Immunol. 181, 1143–1152 (2008).
Hooper, L. V. et al. Molecular analysis of commensal host–microbial relationships in the intestine. Science 291, 881–884 (2001).
Bates, J. M. et al. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 297, 374–386 (2006).
Broquet, A. H., Hirata, Y., McAllister, C. S. & Kagnoff, M. F. RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J. Immunol. 186, 1618–1626 (2011).
Pott, J. et al. IFN-λ determines the intestinal epithelial antiviral host defense. Proc. Natl Acad. Sci. USA 108, 7944–7949 (2011).
Weitkamp, J. H. et al. Ontogeny of FOXP3+ regulatory T cells in the postnatal human small intestinal and large intestinal lamina propria. Pediatr. Dev. Pathol. 12, 443–449 (2009).
Xiong, N. & Raulet, D. H. Development and selection of γδ T cells. Immunol. Rev. 215, 15–31 (2007).
van de Pavert, S. A. & Mebius, R. E. New insights into the development of lymphoid tissues. Nature Rev. Immunol. 10, 664–674 (2010).
Brandtzaeg, P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Invest. 39, 303–355 (2010).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Geuking, M. B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Brandtzaeg, P. Food allergy: separating the science from the mythology. Nature Rev. Gastroenterol. Hepatol. 7, 380–400 (2010).
Michaelsson, J., Mold, J. E., McCune, J. M. & Nixon, D. F. Regulation of T cell responses in the developing human fetus. J. Immunol. 176, 5741–5748 (2006). This study showed that CD4+CD25+ T Reg cells are present at high frequency in human fetal lymphoid tissue, including the mesenteric lymph nodes; these T Reg cells seem to be important in controlling T cell responses in utero and perhaps also neonatally.
Grindebacke, H. et al. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J. Immunol. 183, 4360–4370 (2009).
Hadis, U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246 (2011).
Haddeland, U. et al. Putative regulatory T cells are impaired in cord blood from neonates with hereditary allergy risk. Pediatr. Allergy Immunol. 16, 104–112 (2005). This study of cord blood cells presents data to indicate that neonates at high risk of atopy (familial allergic disease) might generate decreased numbers of CD4+CD25+ T Reg cells after stimulation.
Smith, M. et al. Children with egg allergy have evidence of reduced neonatal CD4+CD25+CD127lo/− regulatory T cell function. J. Allergy Clin. Immunol. 121, 1460–1466.e7 (2008).
O'Mahony, C. et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-κB activation. PLoS Pathog. 4, e1000112 (2008).
Wang, G. et al. “Default” generation of neonatal regulatory T cells. J. Immunol. 185, 71–78 (2010). Murine neonatal CD4+ T cells were shown to have an inborn ability to preferentially develop into FOXP3+ T Reg cells with suppressive function after stimulation, which was in marked contrast to results obtained with adult CD4+ T cells.
Lee, J. H., Ulrich, B., Cho, J., Park, J. & Kim, C. H. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into TH17 cells. J. Immunol. 187, 1778–1787 (2011).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009). References 54 and 55 demonstrate the striking effects of one particular gut commensal on mucosal immune development.
Jiang, H. Q., Bos, N. A. & Cebra, J. J. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect. Immun. 69, 3611–3617 (2001).
Brandtzaeg, P., Fjellanger, I. & Gjeruldsen, S. T. Adsorption of immunoglobulin A onto oral bacteria in vivo. J. Bacteriol. 96, 242–249 (1968).
van der Waaij, L. A., Limburg, P. C., Mesander, G. & van der Waaij, D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut 38, 348–354 (1996).
Peterson, D. A., McNulty, N. P., Guruge, J. L. & Gordon, J. I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2, 328–339 (2007). In this study, Bacteroides thetaiotaomicron was introduced into germ-free and immunodeficient mice harbouring IgA-producing hybridoma cells with reactivity against a single immunomodulatory molecule; the antibody decreased intestinal pro-inflammatory signalling and bacterial epitope expression, thereby documenting the role of secretory IgA in a balanced mutualistic host–microbial relationship.
Mathias, A. & Corthesy, B. Recognition of gram-positive intestinal bacteria by hybridoma- and colostrum-derived secretory immunoglobulin A is mediated by carbohydrates. J. Biol. Chem. 286, 17239–17247 (2011).
Hanson, L. A. Session 1: Feeding and infant development breast-feeding and immune function. Proc. Nutr. Soc. 66, 384–396 (2007).
Pabst, R., Russell, M. W. & Brandtzaeg, P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol. 29, 206–208 (2008).
Mellander, L., Carlsson, B., Jalil, F., Soderstrom, T. & Hanson, L. A. Secretory IgA antibody response against Escherichia coli antigens in infants in relation to exposure. J. Pediatr. 107, 430–433 (1985).
Crabbe, P. A., Nash, D. R., Bazin, H., Eyssen, H. & Heremans, J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab. Invest. 22, 448–457 (1970). This is the first experimental study to show that the development of the intestinal population of IgA-producing plasma cells depends on colonization of the gut with a commensal microbiota.
Shroff, K. E., Meslin, K. & Cebra, J. J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 63, 3904–3913 (1995).
Hapfelmeier, S. et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705–1709 (2010).
Kaufman, D. R. et al. Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. J. Immunol. 187, 1877–1883 (2011).
Brandtzaeg, P. Update on mucosal immunoglobulin A in gastrointestinal disease. Curr. Opin. Gastroenterol. 26, 554–563 (2010).
Johansen, F. E. et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190, 915–922 (1999). This study describes for the first time the generation of knockout mice deficient for a functional polymeric immunoglobulin receptor (pIgR) in secretory epithelia and the liver. These mice could not therefore export IgA or IgM to the mucosal surfaces and had a 'leaky' gut mucosa, which was associated with an increased systemic immune responsiveness to commensal bacteria, but remarkably not to food antigens.
Sait, L. C. et al. Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int. Immunol. 19, 257–265 (2007).
Karlsson, M. R., Johansen, F. E., Kahu, H., Macpherson, A. & Brandtzaeg, P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy 65, 561–570 (2010). Mice deficient for pIgR were shown to be hyper-reactive and therefore predisposed to anaphylaxis after systemic antigen sensitization and challenge. However, they also had an increased capacity for the induction of oral tolerance, which after continuous feeding of cognate antigen protected the mice from IgG1-induced anaphylaxis and delayed type hypersensitivity. This study showed for the first time that a leaky gut mucosa not only results in hyper-reactivity but can also enhance mucosally induced tolerance to cognate antigen.
Boirivant, M. et al. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology 135, 1612–1623 (2008).
Karlsson, M. R., Rugtveit, J. & Brandtzaeg, P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J. Exp. Med. 199, 1679–1688 (2004). This study provides the first human data to indicate that oral tolerance to dietary antigens is associated with the development or proliferation of CD4+CD25+ T Reg cells.
Shreffler, W. G., Wanich, N., Moloney, M., Nowak-Wegrzyn, A. & Sampson, H. A. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J. Allergy Clin. Immunol. 123, 43–52 (2009).
Feng, T. & Elson, C. O. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 4, 15–21 (2011).
Menezes, J. S. et al. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int. Immunol. 15, 447–455 (2003).
Kang, W. & Kudsk, K. A. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J. Parenter. Enteral Nutr. 31, 246–258 (2007).
Barclay, A. R. et al. Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J. Pediatr. 155, 421–426 (2009).
Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L. & Gordon, J. I. Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 (2011).
Harju, K., Glumoff, V. & Hallman, M. Ontogeny of Toll-like receptors TLR2 and TLR4 in mice. Pediatr. Res. 49, 81–83 (2001).
Petrikin, J. E., Gaedigk, R., Leeder, J. S. & Truog, W. E. Selective Toll-like receptor expression in human fetal lung. Pediatr. Res. 68, 335–338 (2010).
Zhang, X., Shan, P., Jiang, G., Cohn, L. & Lee, P. J. Toll-like receptor 4 deficiency causes pulmonary emphysema. J. Clin. Invest. 116, 3050–3059 (2006).
Saenz, S. A., Taylor, B. C. & Artis, D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226, 172–190 (2008).
Barrett, N. A. & Austen, K. F. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity 31, 425–437 (2009).
Hiemstra, P. S. The role of epithelial β-defensins and cathelicidins in host defense of the lung. Exp. Lung Res. 33, 537–542 (2007).
Bienenstock, J., Johnston, N. & Perey, D. Y. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab. Invest. 28, 686–692 (1973).
Racz, P., Tenner-Racz, K., Myrvik, Q. N. & Fainter, L. K. Functional architecture of bronchial associated lymphoid tissue and lymphoepithelium in pulmonary cell-mediated reactions in the rabbit. J. Reticuloendothel. Soc. 22, 59–83 (1977).
Finke, D., Baribaud, F., Diggelmann, H. & Acha-Orbea, H. Extrafollicular plasmablast B cells play a key role in carrying retroviral infection to peripheral organs. J. Immunol. 166, 6266–6275 (2001).
Delventhal, S., Hensel, A., Petzoldt, K. & Pabst, R. Effects of microbial stimulation on the number, size and activity of bronchus-associated lymphoid tissue (BALT) structures in the pig. Int. J. Exp. Pathol. 73, 351–357 (1992).
Tschernig, T. & Pabst, R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 68, 1–8 (2000).
Chvatchko, Y., Kosco-Vilbois, M. H., Herren, S., Lefort, J. & Bonnefoy, J. Y. Germinal center formation and local immunoglobulin E (IgE) production in the lung after an airway antigenic challenge. J. Exp. Med. 184, 2353–2360 (1996).
Corbett, N. P. et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS ONE 5, e15041 (2010).
Yerkovich, S. T. et al. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr. Res. 62, 547–552 (2007).
Holt, P. G., Batty, J. E. & Turner, K. J. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology 42, 409–417 (1981).
Uthoff, H. et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J. Immunol. 171, 3485–3492 (2003).
Polte, T., Hennig, C. & Hansen, G. Allergy prevention starts before conception: maternofetal transfer of tolerance protects against the development of asthma. J. Allergy Clin. Immunol. 122, 1022–1030 (2008).
Blumer, N., Pfefferle, P. I. & Renz, H. Development of mucosal immune function in the intrauterine and early postnatal environment. Curr. Opin. Gastroenterol. 23, 655–660 (2007).
Holloway, J. A. et al. Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet 356, 1900–1902 (2000).
Vance, G. H. et al. Exposure of the fetus and infant to hens' egg ovalbumin via the placenta and breast milk in relation to maternal intake of dietary egg. Clin. Exp. Allergy 35, 1318–1326 (2005).
Verhasselt, V. et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nature Med. 14, 170–175 (2008). Using an asthma model in mice, these authors showed that allergen-exposed dams could transfer small amounts of allergen from their airways to their breast-fed pups; this resulted in the induction of oral tolerance in the pups, which mediated protection against asthma in the same model.
Gereke, M., Jung, S., Buer, J. & Bruder, D. Alveolar type II epithelial cells present antigen to CD4+ T cells and induce Foxp3+ regulatory T cells. Am. J. Respir. Crit. Care Med. 179, 344–355 (2009).
Vestermark, V., Hogdall, C. K., Birch, M., Plenov, G. & Toftager-Larsen, K. Influence of the mode of delivery on initiation of breast-feeding. Eur. J. Obstet. Gynecol. Reprod. Biol. 38, 33–38 (1991).
Ege, M. J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364, 701–709 (2011).
Decker, E. et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 125, e1433–e1440 (2010).
Koplin, J. et al. Is caesarean delivery associated with sensitization to food allergens and IgE-mediated food allergy: a systematic review. Pediatr. Allergy Immunol. 19, 682–687 (2008).
Kuitunen, M. et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 123, 335–341 (2009). In this study, mothers with infants at high risk of IgE-mediated allergy received a probiotic mixture during the last month of pregnancy and their infants received the probiotic mixture combined with prebiotics until the age of 6 months. No allergy-preventive effect of the probiotic mixture was observed at 5 years of age except in children who had been delivered by Caesarean section.
Furuhjelm, C. et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. 98, 1461–1467 (2009).
Newson, R. B. et al. Umbilical cord and maternal blood red cell fatty acids and early childhood wheezing and eczema. J. Allergy Clin. Immunol. 114, 531–537 (2004).
Dirix, C. E. et al. Prenatal arachidonic acid exposure and selected immune-related variables in childhood. Br. J. Nutr. 102, 387–397 (2009).
Maslowski, K. M. & Mackay, C. R. Diet, gut microbiota and immune responses. Nature Immunol. 12, 5–9 (2011). A timely and interesting review focusing on the relationship between the fields of immunology, microbiology, nutrition and metabolism. It is also pointed out how the diet in various parts of the world influences the composition of the gut microbiome.
Sandin, A., Braback, L., Norin, E. & Bjorksten, B. Faecal short chain fatty acid pattern and allergy in early childhood. Acta Paediatr. 98, 823–827 (2009).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009).
Malmborg, P., Bahmanyar, S., Grahnquist, L., Hildebrand, H. & Montgomery, S. Cesarean section and the risk of pediatric Crohn's disease. Inflamm. Bowel Dis. 27 Apr 2011 (doi:10.1002/ibd.21741).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Garrett, W. S., Gordon, J. I. & Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell 140, 859–870 (2010). References 114 and 115 demonstrate the transmissibility of an altered 'colitogenic' microbiota that can induce disease in genetically wild-type animals.
Cadwell, K. et al. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145 (2010). Exciting work describing the requirement for viral infection to promote cellular dysfunction and disease in a genetically predisposed animal model of Crohn's disease.
Stecher, B. et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5, 2177–2189 (2007).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Sodhi, C. P. et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 (2010).
Alfaleh, K. & Bassler, D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. CD005496 (2008).
Bottcher, M. F., Nordin, E. K., Sandin, A., Midtvedt, T. & Bjorksten, B. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin. Exp. Allergy 30, 1590–1596 (2000).
Penders, J. et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 56, 661–667 (2007).
Murray, C. S. et al. Fecal microbiota in sensitized wheezy and non-sensitized non-wheezy children: a nested case–control study. Clin. Exp. Allergy 35, 741–745 (2005).
Adlerberth, I. et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J. Allergy Clin. Immunol. 120, 343–350 (2007).
Wang, M. et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 121, 129–134 (2008).
Arnold, I. C. et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J. Clin. Invest. 121, 3088–3093 (2011).
Noverr, M. C., Falkowski, N. R., McDonald, R. A., McKenzie, A. N. & Huffnagle, G. B. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73, 30–38 (2005).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Thavagnanam, S., Fleming, J., Bromley, A., Shields, M. D. & Cardwell, C. R. A meta-analysis of the association between Caesarean section and childhood asthma. Clin. Exp. Allergy 38, 629–633 (2008).
Bager, P., Wohlfahrt, J. & Westergaard, T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin. Exp. Allergy 38, 634–642 (2008).
Metsala, J. et al. Perinatal factors and the risk of asthma in childhood — a population-based register study in Finland. Am. J. Epidemiol. 168, 170–178 (2008).
von Mutius, E. & Vercelli, D. Farm living: effects on childhood asthma and allergy. Nature Rev. Immunol. 10, 861–868 (2010).
Ege, M. J. et al. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J. Allergy Clin. Immunol. 122, 407–412.e4 (2008).
Heinrich, J. et al. Environmental surveys in the areas of Bitterfeld, Hettstedt and a comparative area in 1992–2000. Gesundheitswesen 64, 675–682 (2002) (in German).
Pfefferle, P. I. et al. Cord blood allergen-specific IgE is associated with reduced IFN-γ production by cord blood cells: the Protection against Allergy — Study in Rural Environments (PASTURE) Study. J. Allergy Clin. Immunol. 122, 711–716 (2008).
Braun-Fahrlander, C. et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 347, 869–877 (2002).
Ege, M. J. et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J. Allergy Clin. Immunol. 117, 817–823 (2006).
Patel, M. et al. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J. Immunol. 174, 7558–7563 (2005).
Velasco, G. et al. Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am. J. Respir. Cell Mol. Biol. 32, 218–224 (2005).
Gerhold, K., Bluemchen, K., Franke, A., Stock, P. & Hamelmann, E. Exposure to endotoxin and allergen in early life and its effect on allergen sensitization in mice. J. Allergy Clin. Immunol. 112, 389–396 (2003).
Blumer, N., Herz, U., Wegmann, M. & Renz, H. Prenatal lipopolysaccharide exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin. Exp. Allergy 35, 397–402 (2005).
Sel, S. et al. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL-12 and IL-10. J. Immunol. 178, 7805–7813 (2007).
Kim, Y. S., Kwon, K. S., Kim, D. K., Choi, I. W. & Lee, H. K. Inhibition of murine allergic airway disease by Bordetella pertussis. Immunology 112, 624–630 (2004).
Debarry, J. et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J. Allergy Clin. Immunol. 119, 1514–1521 (2007).
Tulic, M. K. et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J. Allergy Clin. Immunol. 127, 470–478 (2011).
Conrad, M. L. et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 206, 2869–2877 (2009).
Pattenden, S. et al. Parental smoking and children's respiratory health: independent effects of prenatal and postnatal exposure. Tob. Control 15, 294–301 (2006).
Alati, R., Al Mamun, A., O'Callaghan, M., Najman, J. M. & Williams, G. M. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology 17, 138–144 (2006).
Gilliland, F. D. et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax 55, 271–276 (2000).
Blacquiere, M. J. et al. Maternal smoking during pregnancy induces airway remodelling in mice offspring. Eur. Respir. J. 33, 1133–1140 (2009).
Nakamura, Y. et al. Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. Am. J. Respir. Crit. Care Med. 151, 1497–1503 (1995).
Blacquiere, M. J. et al. Maternal smoking during pregnancy decreases Wnt signalling in neonatal mice. Thorax 65, 553–554 (2010).
Noakes, P. S. et al. Maternal smoking is associated with impaired neonatal Toll-like-receptor-mediated immune responses. Eur. Respir. J. 28, 721–729 (2006).
Noakes, P. S., Holt, P. G. & Prescott, S. L. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy 58, 1053–1058 (2003).
Gentile, D. et al. Association between environmental tobacco smoke and diminished dendritic cell interleukin-10 production during infancy. Ann. Allergy Asthma Immunol. 92, 433–437 (2004).
Singh, S. P. et al. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J. Immunol. 183, 2115–2121 (2009).
Seymour, B. W. et al. Gender differences in the allergic response of mice neonatally exposed to environmental tobacco smoke. Dev. Immunol. 9, 47–54 (2002).
Romagnani, S. Regulation of the development of type 2 T-helper cells in allergy. Curr. Opin. Immunol. 6, 838–846 (1994).
Singh, S. P. et al. Early postnatal exposure to cigarette smoke impairs the antigen-specific T-cell responses in the spleen. Toxicol. Lett. 167, 231–237 (2006).
Gilliland, F. D. et al. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am. J. Respir. Crit. Care Med. 166, 457–463 (2002).
Salam, M. T., Gauderman, W. J., McConnell, R., Lin, P. C. & Gilliland, F. D. Transforming growth factor-1 C-509T polymorphism, oxidant stress, and early-onset childhood asthma. Am. J. Respir. Crit. Care Med. 176, 1192–1199 (2007).
Ramadas, R. A. et al. Interleukin-1R antagonist gene and pre-natal smoke exposure are associated with childhood asthma. Eur. Respir. J. 29, 502–508 (2007).
Harnett, W. & Harnett, M. M. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nature Rev. Immunol. 10, 278–284 (2010).
Kukkonen, K. et al. High intestinal IgA associates with reduced risk of IgE-associated allergic diseases. Pediatr. Allergy Immunol. 21, 67–73 (2010). In this study, prenatal and postnatal probiotic intervention tended to increase IgA levels in the faeces of children at the age of 6 months; this increase of faecal IgA seemed to be associated with a mild inflammatory response in the gut mucosa and a decreased risk of IgE-mediated allergic diseases.
Salminen, S., Collado, M. C., Isolauri, E. & Gueimonde, M. Microbial–host interactions: selecting the right probiotics and prebiotics for infants. Nestle Nutr. Workshop Ser. Pediatr. Program 64, 201–213 (2009).
Hormannsperger, G. & Haller, D. Molecular crosstalk of probiotic bacteria with the intestinal immune system: clinical relevance in the context of inflammatory bowel disease. Int. J. Med. Microbiol. 300, 63–73 (2010).
Rachmilewitz, D. et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126, 520–528 (2004).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010). This study showed that a single microbial immunomodulatory molecule — polysaccharide A from the commensal bacterium Bacteroides fragilis — can induce T Reg cells in the murine gut, which express Foxp3 and secrete anti-inflammatory cytokines such as IL-10.
Solt, L. A. et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 (2011). This study used a high-affinity synthetic ligand to block specifically the retinoic acid receptor-related orphan receptors (RORs) that are indispensable for the development of pro-inflammatory T H 17 cells; the results indicate that such compounds have potential for the future treatment of chronic inflammatory diseases.
Uibo, R., Tian, Z. & Gershwin, M. E. Celiac disease: a model disease for gene-environment interaction. Cell. Mol. Immunol. 8, 93–95 (2011).
Zhernakova, A. et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 7, e1002004 (2011).
Eder, W. & von Mutius, E. Genetics in asthma: the solution to a lasting conundrum? Allergy 60, 1482–1484 (2005).
Hong, X., Tsai, H. J. & Wang, X. Genetics of food allergy. Curr. Opin. Pediatr. 21, 770–776 (2009).
Chabra, S. et al. Vitamin A status in preterm neonates with and without chronic lung disease. J. Paediatr. Child Health 30, 432–435 (1994).
Schuster, G. U., Kenyon, N. J. & Stephensen, C. B. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J. Immunol. 180, 1834–1842 (2008).
Marin, L., Dufour, M. E., Nguyen, T. M., Tordet, C. & Garabedian, M. Maturational changes induced by 1α, 25-dihydroxyvitamin D3 in type II cells from fetal rat lung explants. Am. J. Physiol. 265, L45–L52 (1993).
Nguyen, M., Guillozo, H., Garabedian, M. & Balsan, S. Lung as a possible additional target organ for vitamin D during fetal life in the rat. Biol. Neonate 52, 232–240 (1987).
Litonjua, A. A. Childhood asthma may be a consequence of vitamin D deficiency. Curr. Opin. Allergy Clin. Immunol. 9, 202–207 (2009).
Adorini, L. & Penna, G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 70, 345–352 (2009).
Manavalan, J. S. et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl. Immunol. 11, 245–258 (2003).
Rochat, M. K. et al. Maternal vitamin D intake during pregnancy increases gene expression of ILT3 and ILT4 in cord blood. Clin. Exp. Allergy 40, 786–794 (2010).
Greenough, A., Shaheen, S. O., Shennan, A., Seed, P. T. & Poston, L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax 65, 998–1003 (2010).
Turner, S. W. et al. Associations between fetal size, maternal α-tocopherol and childhood asthma. Thorax 65, 391–397 (2010).
Dunstan, J. A. et al. The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy 19 Sep 2011 (doi:10.1111/j.1398-9995.2011.02714.x).
Waterland, R. A. & Michels, K. B. Epigenetic epidemiology of the developmental origins hypothesis. Annu. Rev. Nutr. 27, 363–388 (2007).
Hollingsworth, J. W. et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J. Clin. Invest. 118, 3462–3469 (2008).
Whitrow, M. J., Moore, V. M., Rumbold, A. R. & Davies, M. J. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am. J. Epidemiol. 170, 1486–1493 (2009).
Yang, Q. H. et al. Race-ethnicity differences in folic acid intake in women of childbearing age in the United States after folic acid fortification: findings from the National Health and Nutrition Examination Survey, 2001–2002. Am. J. Clin. Nutr. 85, 1409–1416 (2007).
Acknowledgements
H.R. is supported by the SFB/TR22 DFG-funded network 'Allergic Immune Responses of the Lung' and by the Universities of Giessen and Marburg Lung Centre (UGMLC), a LOEWE excellence initiative of the Hessian Government, Germany. P.B. is supported by the Research Council of Norway, The University of Oslo and Oslo University Hospital, through the joint financing of the Center of Excellence named 'Centre for Immune Regulation'. M.H. is supported by the German Research Foundation (Ho2236/5-3), the Federal Ministry of Education and Research (DLR 01GU0825 and DLR 01KI1003D) and the Collaborative Research Center grants SFB621 and SFB900.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Oral tolerance
-
This form of tolerance is established through the intestinal mucosa to avoid local and systemic hypersensitivity to innocuous antigens that have breached the epithelial barrier. It is mediated mainly through the induction of regulatory T cells but probably also through other mechanisms, such as T cell anergy and clonal deletion.
- Crypt–villus architecture
-
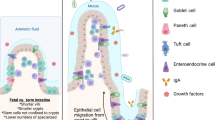
Crypts are glandular invaginations of the intestinal epithelium. Paneth cells localize to the base of crypts in the small intestine and secrete antimicrobial peptides. Intestinal stem cells in crypts divide continuously and provide rapid epithelial cell renewal. In the small intestine, villi are finger-like protrusions into the gut lumen, which increase the absorptive surface of the gut epithelium. Villi mainly consist of mature, absorptive enterocytes, but also contain mucus-secreting goblet cells.
- Cathelicidin
-
Cathelicidins are a large family of antimicrobial peptides in ruminants. In mice and humans, only one cathelicidin — CRAMP or LL37, respectively — is expressed. The genes encoding CRAMP and LL37 are expressed by most epithelial cells, as well as granulocytes, and their expression is regulated at the level of transcription. They encode a highly conserved 'cathelin' sequence of 12 kDa at the amino terminus, followed by the mature antimicrobial peptide, which requires enzymatic cleavage to become active.
- Defensins
-
A class of antimicrobial peptides with broad antibacterial, antifungal and, in part, antiviral activity. α-defensins are produced constitutively by small intestinal Paneth cells and, in humans, also by neutrophils, whereas many β-defensins are transcriptionally regulated and expressed by most epithelial cells.
- RORγt+NKp46+ lymphocytes
-
A population of innate lymphoid cells in the intestinal lamina propria that expresses the transcription factor retinoic acid receptor-related orphan receptor-γt (RORγt) and the natural killer cell marker NKp46. RORγt+NKp46+ lymphocytes support the expression of the antimicrobial peptide REG3γ by epithelial cells through secretion of IL-22.
- Cryptopatches
-
Clusters of KIT+IL-7R+THY1+ T cell progenitors found in the murine intestinal lamina propria. Cryptopatches are absent in germ-free mice.
- Isolated lymphoid follicles
-
Small lymphoid aggregates located in the lamina propria of the small and large intestines that contain B cells, dendritic cells, stromal cells and some T cells and that might form germinal centres. In mice, isolated lymphoid follicles were shown to develop from cryptopatches and they are absent in germ-free animals.
- Anaphylaxis
-
A severe and rapid allergic reaction triggered by the activation of high-affinity Fc receptors for IgE in sensitized individuals. Anaphylactic shock is the most severe type of anaphylaxis and can lead to death in minutes if left untreated. In various mouse models, it has been shown that IgG1 rather than IgE antibodies trigger the anaphylactic reaction.
- Tight junctions
-
These are specialized intercellular junctions that seal the apical epithelium, in which two plasma membranes form a sealing gasket around a cell (also known as the zonula occludens). They are formed by several proteins, including occludin and claudin. Tight junctions prevent fluid moving through the intercellular gaps and prevent the lateral movement of membrane proteins between the apical and basolateral cellular domains.
- Goblet cells
-
Mucus-producing cells that are found in the epithelial cell lining of the intestines and lungs.
- Clara cells
-
Dome-shaped cells with short microvilli that are found in the small airways (bronchioles) of the lungs. These cells can secrete glycosaminoglycans to protect the lining of the bronchioles and are also known as bronchiolar exocrine cells.
Rights and permissions
About this article
Cite this article
Renz, H., Brandtzaeg, P. & Hornef, M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol 12, 9–23 (2012). https://doi.org/10.1038/nri3112
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3112
This article is cited by
-
The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases
Science China Life Sciences (2024)
-
Perinatal foodborne titanium dioxide exposure-mediated dysbiosis predisposes mice to develop colitis through life
Particle and Fibre Toxicology (2023)
-
Comparative analysis of intestinal microbiota composition and transcriptome in diploid and triploid Carassius auratus
BMC Microbiology (2023)
-
Developmentally programmed early-age skin localization of iNKT cells supports local tissue development and homeostasis
Nature Immunology (2023)
-
Mining chicken ileal microbiota for immunomodulatory microorganisms
The ISME Journal (2023)