Key Points
-
The activity of hypothalamic neurons is modified by inputs leading to heterogeneous activity; a small proportion of the total population can drive pituitary hormone pulsatility
-
Neurohormone output can vary following neuron excitation according to the physiological status, which might also lead to declining neuroendocrine output with age
-
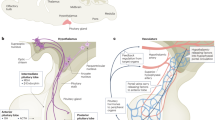
The release of hypothalamic factors into the blood is modified by alterations in the juxtaposition of nerve terminals with the vasculature and tanycytes in the median eminence
-
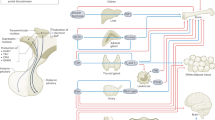
Cells in the pituitary gland form homotypic networks, and the organization and relationship of a network with the vasculature is distinct for each endocrine axis, which modifies responses to regulatory factors and patterns of output in response to demand
-
The reorganisation of the pituitary network can store long-term memories of increased output and enhance function on repeated challenge
-
Understanding the importance of coordinated hypothalamic–vasculature–pituitary function provides new understanding of a range of endocrine axes defects and targets for novel therapies
Abstract
The discoveries of novel functional adaptations of the hypothalamus and anterior pituitary gland for physiological regulation have transformed our understanding of their interaction. The activity of a small proportion of hypothalamic neurons can control complex hormonal signalling, which is disconnected from a simple stimulus and the subsequent hormone secretion relationship and is dependent on physiological status. The interrelationship of the terminals of hypothalamic neurons and pituitary cells with the vasculature has an important role in determining the pattern of neurohormone exposure. Cells in the pituitary gland form networks with distinct organizational motifs that are related to the duration and pattern of output, and modifications of these networks occur in different physiological states, can persist after cessation of demand and result in enhanced function. Consequently, the hypothalamus and pituitary can no longer be considered as having a simple stratified relationship: with the vasculature they form a tripartite system, which must function in concert for appropriate hypothalamic regulation of physiological processes, such as reproduction. An improved understanding of the mechanisms underlying these regulatory features has implications for current and future therapies that correct defects in hypothalamic–pituitary axes. In addition, recapitulating proper network organization will be an important challenge for regenerative stem cell treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sandow, A. Excitation-contraction coupling in skeletal muscle. Pharmacol. Rev. 17, 265–320 (1965).
Romano, N. et al. Plasticity of hypothalamic dopamine neurons during lactation results in dissociation of electrical activity and release. J. Neurosci. 33, 4424–4433 (2013).
Smith, J. T. et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152, 1001–1012 (2011).
Lim, N. F., Nowycky, M. C. & Bookman, R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature 344, 449–451 (1990).
Drouva, S. V., Epelbaum, J., Laplante, E. & Kordon, C. Calmodulin involvement on the Ca++-dependent release of LHRH and SRIF in vitro. Neuroendocrinology 38, 189–192 (1984).
Douglas, W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br. J. Pharmacol. 34, 451–474 (1968).
Tse, A., Tse, F. W., Almers, W. & Hille, B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science 260, 82–84 (1993).
Lafont, C. et al. Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc. Natl. Acad. Sci. USA 107, 4465–4470 (2010).
Thomas, P., Wong, J. G., Lee, A. K. & Almers, W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron 11, 93–104 (1993).
Tse, A. & Lee, A. K. Voltage-gated Ca2+ channels and intracellular Ca2+ release regulate exocytosis in identified rat corticotrophs. J. Physiol. 528, 79–90 (2000).
Raisman, G. An urge to explain the incomprehensible: Geoffrey Harris and the discovery of the neural control of the pituitary gland. Annu. Rev. Neurosci. 20, 533–566 (1997).
Stojilkovic, S. S., Tabak, J. & Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 31, 845–915 (2010).
Keenan, D. M. & Veldhuis, J. D. Pulsatility of hypothalamo-pituitary hormones: a challenge in quantification. Physiology (Bethesda) 31, 34–50 (2016).
Moenter, S. M. Leap of faith: does serum luteinizing hormone always accurately reflect central reproductive neuroendocrine activity? Neuroendocrinology 102, 256–266 (2015).
Petersenn, S. & Schulte, H. M. Structure and function of the growth-hormone-releasing hormone receptor. Vitam. Horm. 59, 35–69 (2000).
Bonnefont, X. et al. Revealing the large-scale network organization of growth hormone-secreting cells. Proc. Natl. Acad. Sci. USA 102, 16880–16885 (2005).
Han, S. K., Todman, M. G. & Herbison, A. E. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145, 495–499 (2004).
Han, S. K., Lee, K., Bhattarai, J. P. & Herbison, A. E. Gonadotrophin-releasing hormone (GnRH) exerts stimulatory effects on GnRH neurons in intact adult male and female mice. J. Neuroendocrinol. 22, 188–195 (2010).
Dierschke, D. J., Bhattacharya, A. N., Atkinson, L. E. & Knobil, E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology 87, 850–853 (1970).
Carmel, P. W., Araki, S. & Ferin, M. Pituitary stalk portal blood collection in rhesus monkeys: evidence for pulsatile release of gonadotropin-releasing hormone (GnRH). Endocrinology 99, 243–248 (1976).
Knobil, E. The neuroendocrine control of the menstrual cycle. Recent Prog. Horm. Res. 36, 53–88 (1980).
Clarke, I. J. & Cummins, J. T. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111, 1737–1739 (1982).
Levine, J. E., Pau, K. Y., Ramirez, V. D. & Jackson, G. L. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111, 1449–1455 (1982).
Caraty, A., Locatelli, A. & Martin, G. B. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J. Endocrinol. 123, 375–382 (1989).
Moenter, S. M., Brand, R. C. & Karsch, F. J. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130, 2978–2984 (1992).
Levine, J. E. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol. Reprod. 56, 293–302 (1997).
Karsch, F. J., Bowen, J. M., Caraty, A., Evans, N. P. & Moenter, S. M. Gonadotropin-releasing hormone requirements for ovulation. Biol. Reprod. 56, 303–309 (1997).
Steyn, F. J. et al. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 154, 4939–4945 (2013).
Campos, P. & Herbison, A. E. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc. Natl. Acad. Sci. USA 111, 18387–18392 (2014).
Kokoris, G. J., Lam, N. Y., Ferin, M., Silverman, A. J. & Gibson, M. J. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology 48, 45–52 (1988).
Herbison, A. E., Porteous, R., Pape, J. R., Mora, J. M. & Hurst, P. R. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149, 597–604 (2008).
Jasoni, C. L., Romano, N., Constantin, S., Lee, K. & Herbison, A. E. Calcium dynamics in gonadotropin-releasing hormone neurons. Front. Neuroendocrinol. 31, 259–269 (2010).
Lehman, M. N., Coolen, L. M. & Goodman, R. L. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151, 3479–3489 (2010).
Navarro, V. M. et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J. Neurosci. 32, 2388–2397 (2012).
Campbell, R. E., Gaidamaka, G., Han, S. K. & Herbison, A. E. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc. Natl. Acad. Sci. USA 106, 10835–10840 (2009).
Herde, M. K., Iremonger, K. J., Constantin, S. & Herbison, A. E. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci. 33, 12689–12697 (2013).
Prevot, V. et al. Evidence that members of the TGFβ superfamily play a role in regulation of the GnRH neuroendocrine axis: expression of a type I serine-threonine kinase receptor for TGRβ and activin in GnRH neurones and hypothalamic areas of the female rat. J. Neuroendocrinol. 12, 665–670 (2000).
Knauf, C. et al. Evidence for a spontaneous nitric oxide release from the rat median eminence: influence on gonadotropin-releasing hormone release. Endocrinology 142, 2343–2350 (2001).
De Seranno, S. et al. Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J. Neurosci. 24, 10353–10363 (2004).
Hanchate, N. K. et al. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J. Neurosci. 32, 932–945 (2012).
Bellefontaine, N. et al. Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction. Neuroendocrinology 93, 74–89 (2011).
Page, R. B. Pituitary blood flow. Am. J. Physiol. 243, E427–E442 (1982).
Belchetz, P. E., Plant, T. M., Nakai, Y., Keogh, E. J. & Knobil, E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202, 631–633 (1978).
Wildt, L. et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109, 376–385 (1981).
Pohl, C. R., Richardson, D. W., Hutchison, J. S., Germak, J. A. & Knobil, E. Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology 112, 2076–2080 (1983).
McArdle, C. A. & Roberson, M. S. Knobil and Neill's Physiology of Reproduction 4th edn 335–397 (Academic Press, 2015).
Tsutsumi, R. & Webster, N. J. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr. J. 56, 729–737 (2009).
Cooke, B., Hegstrom, C. D., Villeneuve, L. S. & Breedlove, S. M. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 19, 323–362 (1998).
Simerly, R. B. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav. Brain Res. 92, 195–203 (1998).
Levine, J. E. & Ramirez, V. D. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111, 1439–1448 (1982).
Sarkar, D. K., Chiappa, S. A., Fink, G. & Sherwood, N. M. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264, 461–463 (1976).
Park, O. K. & Ramirez, V. D. Spontaneous changes in LHRH release during the rat estrous cycle, as measured with repetitive push-pull perfusions of the pituitary gland in the same female rats. Neuroendocrinology 50, 66–72 (1989).
Herbison, A. E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 12, 452–466 (2016).
Plant, T. M. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front. Neuroendocrinol. 33, 160–168 (2012).
Moenter, S. M., Caraty, A., Locatelli, A. & Karsch, F. J. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129, 1175–1182 (1991).
Clarke, I. J. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology 133, 1624–1632 (1993).
Caraty, A. et al. Nature and bioactivity of gonadotropin-releasing hormone (GnRH) secreted during the GnRH surge. Endocrinology 136, 3452–3460 (1995).
Kozlowski, G. P. & Coates, P. W. Ependymoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res. 242, 301–311 (1985).
King, J. C. & Rubin, B. S. Dynamic alterations in luteinizing hormone-releasing hormone (LHRH) neuronal cell bodies and terminals of adult rats. Cell. Mol. Neurobiol. 15, 89–106 (1995).
Parkash, J. et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat. Commun. 6, 6385 (2015).
Giacobini, P. et al. Brain endothelial cells control fertility through ovarian-steroid-dependent release of semaphorin 3A. PLoS Biol. 12, e1001808 (2014).
King, J. C. & Letourneau, R. J. Luteinizing hormone-releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology 134, 1340–1351 (1994).
Prevot, V. et al. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience 94, 809–819 (1999).
Budry, L. et al. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc. Natl. Acad. Sci. USA 108, 12515–12520 (2011).
Alim, Z. et al. Gonadotrope plasticity at cellular and population levels. Endocrinology 153, 4729–4739 (2012).
Thomas, S. G., Takahashi, M., Neill, J. D. & Clarke, I. J. Components of the neuronal exocytotic machinery in the anterior pituitary of the ovariectomised ewe and the effects of oestrogen in gonadotropes as studied with confocal microscopy. Neuroendocrinology 67, 244–259 (1998).
Barkan, A. L., Regiani, S. R., Duncan, J. A. & Marshall, J. C. Pituitary gonadotropin-releasing hormone receptors during gonadotropin surges in ovariectomized-estradiol-treated rats. Endocrinology 112, 1042–1048 (1983).
Qiao, S. et al. Molecular plasticity of male and female murine gonadotropes revealed by mRNA sequencing. Endocrinology 157, 1082–1093 (2016).
Navratil, A. M., Knoll, J. G., Whitesell, J. D., Tobet, S. A. & Clay, C. M. Neuroendocrine plasticity in the anterior pituitary: gonadotropin-releasing hormone-mediated movement in vitro and in vivo. Endocrinology 148, 1736–1744 (2007).
Schaeffer, M., Hodson, D. J., Lafont, C. & Mollard, P. Endocrine cells and blood vessels work in tandem to generate hormone pulses. J. Mol. Endocrinol. 47, R59–R66 (2011).
Padmanabhan, V. Polycystic ovary syndrome — “a riddle wrapped in a mystery inside an enigma”. J. Clin. Endocrinol. Metab. 94, 1883–1885 (2009).
McCartney, C. R., Eagleson, C. A. & Marshall, J. C. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin. Reprod. Med. 20, 317–326 (2002).
Francou, M. et al. Characterization of pituitary cell populations in rats with induced polycystic ovaries. Cells Tissues Organs 188, 310–319 (2008).
Roland, A. V. & Moenter, S. M. Reproductive neuroendocrine dysfunction in polycystic ovary syndrome: insight from animal models. Front. Neuroendocrinol. 35, 494–511 (2014).
Cardoso, R. C., Puttabyatappa, M. & Padmanabhan, V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology 102, 226–237 (2015).
Boehm, U. et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 11, 547–564 (2015).
Pitteloud, N., Durrani, S., Raivio, T. & Sykiotis, G. P. Complex genetics in idiopathic hypogonadotropic hypogonadism. Front. Horm. Res. 39, 142–153 (2010).
Christin-Maitre, S. et al. Grossesses obtenues par administration pulsatile de GnRH: résultats d'une large étude rétrospective multicentrique. J. Gynecol. Obstet. Biol. Reprod. (Paris) 36, 8–12 (in French) (2007).
Sidhoum, V. F. et al. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J. Clin. Endocrinol. Metab. 99, 861–870 (2014).
Grattan, D. R. & Kokay, I. C. Prolactin: a pleiotropic neuroendocrine hormone. J. Neuroendocrinol. 20, 752–763 (2008).
Arbogast, L. A. & Voogt, J. L. Hyperprolactinemia increases and hypoprolactinemia decreases tyrosine hydroxylase messenger ribonucleic acid levels in the arcuate nuclei, but not the substantia nigra or zona incerta. Endocrinology 128, 997–1005 (1991).
Stagkourakis, S., Kim, H., Lyons, David, J. & Broberger, C. Dopamine autoreceptor regulation of a hypothalamic dopaminergic network. Cell Rep. 15, 735–747 (2016).
Lyons, D. J., Horjales-Araujo, E. & Broberger, C. Synchronized network oscillations in rat tuberoinfundibular dopamine neurons: switch to tonic discharge by thyrotropin-releasing hormone. Neuron 65, 217–229 (2010).
Freeman, M. E., Reichert, L. E. Jr & Neill, J. D. Regulation of the proestrus surge of prolactin secretion by gonadotropin and estrogens in the rat. Endocrinology 90, 232–238 (1972).
Butcher, R. L., Fugo, N. W. & Collins, W. E. Semicircadian rhythm in plasma levels of prolactin during early gestation in the rat. Endocrinology 90, 1125–1127 (1972).
Larsen, C. M. & Grattan, D. R. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151, 3805–3814 (2010).
Mai, L. M., Shieh, K. R. & Pan, J. T. Circadian changes of serum prolactin levels and tuberoinfundibular dopaminergic neuron activities in ovariectomized rats treated with or without estrogen: the role of the suprachiasmatic nuclei. Neuroendocrinology 60, 520–526 (1994).
Egli, M., Bertram, R., Sellix, M. T. & Freeman, M. E. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology 145, 3386–3394 (2004).
Hodson, D. J. & Mollard, P. Navigating pituitary structure and function - defining a roadmap for hormone secretion. J. Neuroendocrinol. 25, 674–675 (2013).
Hodson, D. J. et al. Coordination of calcium signals by pituitary endocrine cells in situ. Cell Calcium 51, 222–230 (2012).
Featherstone, K. et al. Spatially coordinated dynamic gene transcription in living pituitary tissue. eLife 5, e08494 (2016).
Harper, C. V. et al. Dynamic organisation of prolactin gene expression in living pituitary tissue. J. Cell Sci. 123, 424–430 (2010).
Long, T. et al. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol. 7, e68 (2009).
Weber, W. et al. Streptomyces-derived quorum-sensing systems engineered for adjustable transgene expression in mammalian cells and mice. Nucleic Acids Res. 31, e71 (2003).
Andrews, Z. B., Kokay, I. C. & Grattan, D. R. Dissociation of prolactin secretion from tuberoinfundibular dopamine activity in late pregnant rats. Endocrinology 142, 2719–2724 (2001).
Ciofi, P. et al. Plasticity in expression of immunoreactivity for neuropeptide Y, enkephalins and neurotensin in the hypothalamic tubero-infundibular dopaminergic system during lactation in mice. J. Neuroendocrinol. 5, 599–602 (1993).
Merchenthaler, I. Induction of enkephalin in tuberoinfundibular dopaminergic neurons during lactation. Endocrinology 133, 2645–2651 (1993).
Le Tissier, P. R., Hodson, D. J., Martin, A. O., Romano, N. & Mollard, P. Plasticity of the prolactin (PRL) axis: mechanisms underlying regulation of output in female mice. Adv. Exp. Med. Biol. 846, 139–162 (2015).
Castrique, E., Fernandez-Fuente, M., Le Tissier, P., Herman, A. & Levy, A. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J. Endocrinol. 205, 49–60 (2010).
Hodson, D. J. et al. Existence of long-lasting experience-dependent plasticity in endocrine cell networks. Nat. Commun. 3, 605 (2012).
Guillou, A. et al. Assessment of lactotroph axis functionality in mice: longitudinal monitoring of PRL secretion by ultrasensitive-ELISA. Endocrinology 156, 1924–1930 (2015).
Byrnes, E. M. & Bridges, R. S. Lactation reduces prolactin levels in reproductively experienced female rats. Horm. Behav. 48, 278–282 (2005).
Musey, V. C., Collins, D. C., Musey, P. I., Martino-Saltzman, D. & Preedy, J. R. Long-term effect of a first pregnancy on the secretion of prolactin. N. Engl. J. Med. 316, 229–234 (1987).
Byrnes, E. M. & Bridges, R. S. Reproductive experience and expression of dopamine D2 receptor mRNA: a possible mechanism for reduced prolactin secretion in primiparous rats. J. Neuroendocrinol. 19, 773–778 (2007).
Wong, A., Eloy, J. A., Couldwell, W. T. & Liu, J. K. Update on prolactinomas. Part 1: clinical manifestations and diagnostic challenges. J. Clin. Neurosci. 22, 1562–1567 (2015).
Holt, R. I. & Peveler, R. C. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin. Endocrinol. (Oxf.) 74, 141–147 (2011).
Melmed, S. et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 273–288 (2011).
Wong, A., Eloy, J. A., Couldwell, W. T. & Liu, J. K. Update on prolactinomas. Part 2: treatment and management strategies. J. Clin. Neurosci. 22, 1568–1574 (2015).
Brown, R. S., Herbison, A. E. & Grattan, D. R. Prolactin regulation of kisspeptin neurones in the mouse brain and its role in the lactation-induced suppression of kisspeptin expression. J. Neuroendocrinol. 26, 898–908 (2014).
Sonigo, C. et al. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J. Clin. Invest. 122, 3791–3795 (2012).
Li, Q., Rao, A., Pereira, A., Clarke, I. J. & Smith, J. T. Kisspeptin cells in the ovine arcuate nucleus express prolactin receptor but not melatonin receptor. J. Neuroendocrinol. 23, 871–882 (2011).
Adcock, C. J. et al. The use of an automated microsampling system for the characterization of growth hormone pulsatility in newborn babies. Pediatr. Res. 42, 66–71 (1997).
Coxam, V., Davicco, M. J., Robelin, J. & Barlet, J. P. Growth hormone secretory pattern and somatomedin C plasma concentrations in newborn calves. J. Dev. Physiol. 9, 113–121 (1987).
Davicco, M. J. et al. Growth hormone (GH) secretory pattern and GH response to GH-releasing factor (GRF) or thyrotropin-releasing hormone (TRH) in newborn foals. J. Dev. Physiol. 19, 143–147 (1993).
Robinson, I. C. A. F. & Hindmarsh, P. C. Comprehensive Physiology (John Wiley & Sons, 2010).
Tannenbaum, G. S. Genesis of episodic growth hormone secretion. J. Pediatr. Endocrinol. 6, 273–282 (1993).
Steyn, F. J., Tolle, V., Chen, C. & Epelbaum, J. Neuroendocrine regulation of growth hormone secretion. Compr. Physiol. 6, 687–735 (2016).
Balthasar, N. et al. Growth hormone-releasing hormone (GHRH) neurons in GHRH-enhanced green fluorescent protein transgenic mice: a ventral hypothalamic network. Endocrinology 144, 2728–2740 (2003).
Gouty-Colomer, L. A. et al. Specific involvement of gonadal hormones in the functional maturation of growth hormone releasing hormone (GHRH) neurons. Endocrinology 151, 5762–5774 (2010).
Romero, M. I. & Phelps, C. J. Identification of growth hormone-releasing hormone and somatostatin neurons projecting to the median eminence in normal and growth hormone-deficient Ames dwarf mice. Neuroendocrinology 65, 107–116 (1997).
Chowen, J. A., Frago, L. M. & Argente, J. The regulation of GH secretion by sex steroids. Eur. J. Endocrinol. 151 (Suppl. 3), 95–100 (2004).
Baccam, N. et al. Dual-level afferent control of growth hormone-releasing hormone (GHRH) neurons in GHRH-green fluorescent protein transgenic mice. J. Neurosci. 27, 1631–1641 (2007).
Osterstock, G. et al. Somatostatin triggers rhythmic electrical firing in hypothalamic GHRH neurons. Sci. Rep. 6, 24394 (2016).
Sanchez-Cardenas, C. et al. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc. Natl. Acad. Sci. USA 107, 21878–21883 (2010).
Schaeffer, M. et al. Influence of estrogens on GH-cell network dynamics in females: a live in situ imaging approach. Endocrinology 152, 4789–4799 (2011).
Alatzoglou, K. S., Webb, E. A., Le Tissier, P. & Dattani, M. T. Isolated growth hormone deficiency (GHD) in childhood and adolescence: recent advances. Endocr. Rev. 35, 376–432 (2014).
Pekic, S. & Popovic, V. Alternative causes of hypopituitarism: traumatic brain injury, cranial irradiation, and infections. Handb. Clin. Neurol. 124, 271–290 (2014).
Hindmarsh, P. C. & Dattani, M. T. Use of growth hormone in children. Nat. Clin. Pract. Endocrinol. Metab. 2, 260–268 (2006).
Andoniadou, C. L. et al. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13, 433–445 (2013).
Olarescu, N. C. & Bollerslev, J. The impact of adipose tissue on insulin resistance in acromegaly. Trends Endocrinol. Metab. 27, 226–237 (2016).
Dimaraki, E. V., Jaffe, C. A., DeMott-Friberg, R., Chandler, W. F. & Barkan, A. L. Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J. Clin. Endocrinol. Metab. 87, 3537–3542 (2002).
Holly, J. M. et al. Inter-relations between growth hormone, insulin, insulin-like growth factor-I (IGF-I), IGF-binding protein-1 (IGFBP-1) and sex hormone-binding globulin in acromegaly. Clin. Endocrinol. (Oxf.) 34, 275–280 (1991).
Narayanaswamy, S. et al. Subcutaneous infusion of kisspeptin-54 stimulates gonadotrophin release in women and the response correlates with basal oestradiol levels. Clin. Endocrinol. (Oxf.) 84, 939–945 (2016).
Alonso, G. et al. Selective alteration at the growth-hormone-releasing-hormone nerve terminals during aging in GHRH-green fluorescent protein mice. Aging Cell 6, 197–207 (2007).
Castinetti, F., Davis, S. W., Brue, T. & Camper, S. A. Pituitary stem cell update and potential implications for treating hypopituitarism. Endocr. Rev. 32, 453–471 (2011).
Andoniadou, C. L. et al. Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol. 124, 259–271 (2012).
Eckstrum, K. S., Weis, K. E., Baur, N. G., Yoshihara, Y. & Raetzman, L. T. Icam5 expression exhibits sex differences in the neonatal pituitary and is regulated by estradiol and bisphenol A. Endocrinology 157, 1408–1420 (2016).
Le Tissier, P. R. & Mollard, P. Bisphenol A effects on gonadotroph function: disruption of pituitary cell-cell communication? Endocrinology 157, 1324–1325 (2016).
Le Tissier, P. R. et al. Anterior pituitary cell networks. Front. Neuroendocrinol. 33, 252–266 (2012).
Mollard, P., Hodson, D. J., Lafont, C., Rizzoti, K. & Drouin, J. A tridimensional view of pituitary development and function. Trends Endocrinol. Metabolism 23, 261–269 (2012).
Schaeffer, M., Hodson, D. J., Lafont, C. & Mollard, P. Functional importance of blood flow dynamics and partial oxygen pressure in the anterior pituitary. Eur. J. Neurosci. 32, 2087–2095 (2010).
Ward, R. D., Stone, B. M., Raetzman, L. T. & Camper, S. A. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol. Endocrinol. 20, 1378–1390 (2006).
Waite, E. et al. Different degrees of somatotroph ablation compromise pituitary growth hormone cell network structure and other pituitary endocrine cell types. Endocrinology 151, 234–243 (2010).
Denef, C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J. Neuroendocrinol. 20, 1–70 (2008).
Acknowledgements
The authors thank F. Castinetti (Aix-Marseille Universite, France) for helpful comments and suggestions. P.L.T. was supported by a grant from the Biotechnology and Biological Sciences Research Council, UK, (BB/N007026/1). N.R. was supported by a Medical Research Council, UK, project grant (MR/J008893/1). D.J.H. was supported by an R.D. Lawrence Fellowship, Diabetes UK (12/0004431); European Foundation for the Study of Diabetes/Novo Nordisk Rising Star and Birmingham Fellowships; a Medical Research Council, UK project grant (MR/N00275X/1); Imperial Confidence in Concept, UK, and Wellcome Trust Institutional Support Awards, UK; and an European Research Council (ERC) Starting Grant (OptoBETA; 715884). P.M. was supported by funding from the Agence Nationale de la Recherche (ANR 12 BSV1 0032–01 and ANR-15-CE14-0012-01); INSERM; Centre National de la Recherche Scientifique; Université de Montpellier; Fondation pour la Recherche Médicale (DEQ20150331732); and IPAM-Biocampus of Montpellier and France-Bioimaging, all in France.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Le Tissier, P., Campos, P., Lafont, C. et al. An updated view of hypothalamic–vascular–pituitary unit function and plasticity. Nat Rev Endocrinol 13, 257–267 (2017). https://doi.org/10.1038/nrendo.2016.193
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.193
This article is cited by
-
Spatial transcriptomics in neuroscience
Experimental & Molecular Medicine (2023)
-
Intra-pituitary follicle-stimulating hormone signaling regulates hepatic lipid metabolism in mice
Nature Communications (2023)
-
Evidence that the pituitary gland connects type 2 diabetes mellitus and schizophrenia based on large-scale trans-ethnic genetic analyses
Journal of Translational Medicine (2022)
-
Hypothyroidism
Nature Reviews Disease Primers (2022)
-
A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region
Scientific Data (2020)