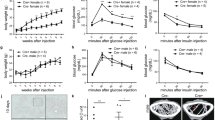
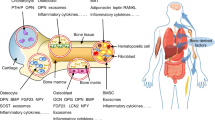
Abstract
Traditional textbook physiology has ascribed unitary functions to hormones from the anterior and posterior pituitary gland, mainly in the regulation of effector hormone secretion from endocrine organs. However, the evolutionary biology of pituitary hormones and their receptors provides evidence for a broad range of functions in vertebrate physiology. Over the past decade, we and others have discovered that thyroid-stimulating hormone, follicle-stimulating hormone, adrenocorticotropic hormone, prolactin, oxytocin and arginine vasopressin act directly on somatic organs, including bone, adipose tissue and liver. New evidence also indicates that pituitary hormone receptors are expressed in brain regions, nuclei and subnuclei. These studies have prompted us to attribute the pathophysiology of certain human diseases, including osteoporosis, obesity and neurodegeneration, at least in part, to changes in pituitary hormone levels. This new information has identified actionable therapeutic targets for drug discovery.
Key points
-
Contrary to textbook physiology, pituitary hormones have ubiquitous functions and are the basis of important regulatory circuits that continue to evolve.
-
Endocrine diseases, such as osteoporosis, that were traditionally attributed to a single effector hormone are far more complicated than originally thought.
-
Pituitary hormones, such as follicle-stimulating hormone, act directly on both peripheral and central targets to contribute to the development of several diseases, such as osteoporosis, obesity and Alzheimer disease.
-
The pituitary–multiorgan circuitry could be exploited therapeutically to prevent and treat diseases driven by pituitary hormones.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rosen, E. D. & Carter-Su, C. in Williams Textbook of Endocrinology (eds Melmed, S. et al.) Ch. 2, 13–41 (Elsevier, 2020).
Abe, E. et al. TSH is a negative regulator of skeletal remodeling. Cell 115, 151–162 (2003).
Sun, L. et al. FSH directly regulates bone mass. Cell 125, 247–260 (2006).
Liu, P. et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546, 107–112 (2017).
Xiong, J. et al. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 603, 470–476 (2022).
Gera, S. et al. FSH-blocking therapeutic for osteoporosis. eLife 11, e78022 (2022).
Gera, S. et al. First-in-class humanized FSH blocking antibody targets bone and fat. Proc. Natl Acad. Sci. USA 117, 28971–28979 (2020).
Yamashita, K. & Kitano, T. Molecular evolution of the oxytocin–oxytocin receptor system in eutherians. Mol. Phylogenet. Evol. 67, 520–528 (2013).
Knobloch, H. S. & Grinevich, V. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 8, 31 (2014).
Garrison, J. L. et al. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science 338, 540–543 (2012).
Gimpl, G. & Fahrenholz, F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 81, 629–683 (2001).
Kleinau, G. & Krause, G. Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr. Rev. 30, 133–151 (2009).
Davies, T., Marians, R. & Latif, R. The TSH receptor reveals itself. J. Clin. Invest. 110, 161–164 (2002).
Bogerd, J., Granneman, J. C., Schulz, R. W. & Vischer, H. F. Fish FSH receptors bind LH: how to make the human FSH receptor to be more fishy? Gen. Comp. Endocrinol. 142, 34–43 (2005).
Kobayashi, T. & Andersen, O. The gonadotropin receptors FSH-R and LH-R of Atlantic halibut (Hippoglossus hippoglossus), 1: isolation of multiple transcripts encoding full-length and truncated variants of FSH-R. Gen. Comp. Endocrinol. 156, 584–594 (2008).
Cooray, S. N. & Clark, A. J. Melanocortin receptors and their accessory proteins. Mol. Cell Endocrinol. 331, 215–221 (2011).
Wilson, M. G. et al. Proopiolipomelanocortin peptides in normal pituitary, pituitary tumor, and plasma of normal and Cushing’s horses. Endocrinology 110, 941–954 (1982).
Baudet, M. L., Sanders, E. J. & Harvey, S. Retinal growth hormone in the chick embryo. Endocrinology 144, 5459–5468 (2003).
Harvey, S., Kakebeeke, M. & Sanders, E. J. Growth hormone localization in the neural retina and retinal pigmented epithelium of embryonic chicks. J. Mol. Neurosci. 22, 139–145 (2004).
Martinez-Moreno, C. G. et al. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp. Eye Res. 166, 1–12 (2018).
Harvey, S. & Aramburo, C. Growth hormone: not just a pituitary endocrine. J. Endocr. Disord. 4, 1024 (2017).
Martin, B. T., List, E. O., Kopchick, J. J., Sauve, Y. & Harvey, S. Selective inner retinal dysfunction in growth hormone transgenic mice. Growth Horm. IGF Res. 21, 219–227 (2011).
Grimbly, C., Martin, B., Karpinski, E. & Harvey, S. Growth hormone production and action in N1E-115 neuroblastoma cells. J. Mol. Neurosci. 39, 117–124 (2009).
de Mello-Coelho, V. et al. Growth hormone and its receptor are expressed in human thymic cells. Endocrinology 139, 3837–3842 (1998).
Hull, K. L. & Harvey, S. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int. J. Endocrinol. 2014, 234014 (2014).
Schwarzler, P. et al. Selective growth hormone/placental lactogen gene transcription and hormone production in pre- and postmenopausal human ovaries. J. Clin. Endocrinol. Metab. 82, 3337–3341 (1997).
Hull, K. L. & Harvey, S. Growth hormone: roles in male reproduction. Endocrine 13, 243–250 (2000).
Baliram, R. et al. Thyroid and bone: macrophage-derived TSH-beta splice variant increases murine osteoblastogenesis. Endocrinology 154, 4919–4926 (2013).
Baliram, R., Latif, R., Morshed, S. A., Zaidi, M. & Davies, T. F. T3 regulates a human macrophage-derived TSH-beta splice variant: implications for human bone biology. Endocrinology 157, 3658–3667 (2016).
Vincent, B. H. et al. Bone marrow cells produce a novel TSHbeta splice variant that is upregulated in the thyroid following systemic virus infection. Genes Immun. 10, 18–26 (2009).
Smith, E. M., Phan, M., Kruger, T. E., Coppenhaver, D. H. & Blalock, J. E. Human lymphocyte production of immunoreactive thyrotropin. Proc. Natl Acad. Sci. USA 80, 6010–6013 (1983).
Harbour, D. V., Kruger, T. E., Coppenhaver, D., Smith, E. M. & Meyer, W. J. 3rd Differential expression and regulation of thyrotropin (TSH) in T cell lines. Mol. Cell. Endocrinol. 64, 229–241 (1989).
Klein, J. R. & Wang, H. C. Characterization of a novel set of resident intrathyroidal bone marrow-derived hematopoietic cells: potential for immune–endocrine interactions in thyroid homeostasis. J. Exp. Biol. 207, 55–65 (2004).
Colaianni, G. et al. Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochem. Biophys. Res. Commun. 411, 512–515 (2011).
Colaianni, G. et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J. Biol. Chem. 287, 29159–29167 (2012).
Yakar, S. et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 110, 771–781 (2002).
De Jesus, K., Wang, X. & Liu, J. L. A general IGF-I overexpression effectively rescued somatic growth and bone deficiency in mice caused by growth hormone receptor knockout. Growth Factors 27, 438–447 (2009).
Bachrach, L. K. et al. Bone mineral, histomorphometry, and body composition in adults with growth hormone receptor deficiency. J. Bone Min. Res. 13, 415–421 (1998).
Fritton, J. C. et al. Growth hormone protects against ovariectomy-induced bone loss in states of low circulating insulin-like growth factor (IGF-1). J. Bone Min. Res. 25, 235–246 (2010).
Sun, L. et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc. Natl Acad. Sci. USA 105, 4289–4294 (2008).
Baliram, R. et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J. Clin. Invest. 122, 3737–3741 (2012).
Kim, S. M. et al. Thyrotropin, hyperthyroidism, and bone mass. J. Clin. Endocrinol. Metab. 106, e4809–e4821 (2021).
Novack, D. V. TSH, the bone suppressing hormone. Cell 115, 129–130 (2003).
Hase, H. et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc. Natl Acad. Sci. USA 103, 12849–12854 (2006).
Ma, R., Morshed, S., Latif, R., Zaidi, M. & Davies, T. F. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid 21, 897–906 (2011).
Yamoah, K. et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol. Endocrinol. 22, 1141–1153 (2008).
Sun, L. et al. Genetic confirmation for a central role for TNFalpha in the direct action of thyroid stimulating hormone on the skeleton. Proc. Natl Acad. Sci. USA 110, 9891–9896 (2013).
Baliram, R. et al. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc. Natl Acad. Sci. USA 108, 16277–16282 (2011).
Sampath, T. K. et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J. Bone Min. Res. 22, 849–859 (2007).
Mazziotti, G. et al. Recombinant human TSH modulates in vivo C-telopeptides of type-1 collagen and bone alkaline phosphatase, but not osteoprotegerin production in postmenopausal women monitored for differentiated thyroid carcinoma. J. Bone Min. Res. 20, 480–486 (2005).
Karga, H. et al. The effects of recombinant human TSH on bone turnover in patients after thyroidectomy. J. Bone Min. Metab. 28, 35–41 (2010).
Martini, G. et al. The effects of recombinant TSH on bone turnover markers and serum osteoprotegerin and RANKL levels. Thyroid 18, 455–460 (2008).
Cho, S. W. et al. The presence of thyroid-stimulation blocking antibody prevents high bone turnover in untreated premenopausal patients with Graves’ disease. PLoS ONE 10, e0144599 (2015).
von Recklinghausen, F. in Festschrift für Rudolf Virchow (ed. Reimer, G.) (Druck und Verlag von Georg Reimer, 1891).
Blum, M. R. et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA 313, 2055–2065 (2015).
Flynn, R. W. et al. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J. Clin. Endocrinol. Metab. 95, 186–193 (2010).
Kim, M. K. et al. The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone 71, 101–105 (2015).
La Vignera, S. et al. l-thyroxin treatment and post-menopausal osteoporosis: relevance of the risk profile present in clinical history. Minerva Ginecol. 60, 475–484 (2008).
Svare, A. et al. Hyperthyroid levels of TSH correlate with low bone mineral density: the HUNT 2 study. Eur. J. Endocrinol. 161, 779–786 (2009).
Bauer, D. C., Ettinger, B., Nevitt, M. C. & Stone, K. L., Study of Osteoporotic Fractures Research Group. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann. Intern. Med. 134, 561–568 (2001).
Wang, L. Y. et al. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid 25, 300–307 (2015).
Karimifar, M. et al. Effects of levothyroxine and thyroid stimulating hormone on bone loss in patients with primary hypothyroidism. J. Res. Pharm. Pract. 3, 83–87 (2014).
Abrahamsen, B. et al. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. J. Bone Min. Res. 30, 898–905 (2015).
Abrahamsen, B. et al. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures — the OPENTHYRO register cohort. J. Bone Min. Res. 29, 2040–2050 (2014).
Grimnes, G., Emaus, N., Joakimsen, R. M., Figenschau, Y. & Jorde, R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromso study. Thyroid 18, 1147–1155 (2008).
Morris, M. S. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone 40, 1128–1134 (2007).
Lee, S. J. et al. Low normal TSH levels are associated with impaired BMD and hip geometry in the elderly. Aging Dis. 7, 734–743 (2016).
Ding, B. et al. Low thyroid stimulating hormone levels are associated with low bone mineral density in femoral neck in elderly women. Arch. Med. Res. 47, 310–314 (2016).
Waring, A. C. et al. A prospective study of thyroid function, bone loss, and fractures in older men: the MrOS study. J. Bone Min. Res. 28, 472–479 (2013).
Acar, B. et al. Evaluation of thyroid function status among postmenopausal women with and without osteoporosis. Int. J. Gynaecol. Obstet. 134, 53–57 (2016).
Noh, H. M., Park, Y. S., Lee, J. & Lee, W. A cross-sectional study to examine the correlation between serum TSH levels and the osteoporosis of the lumbar spine in healthy women with normal thyroid function. Osteoporos. Int. 26, 997–1003 (2015).
van der Deure, W. M. et al. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam study. Clin. Endocrinol. 68, 175–181 (2008).
Albagha, O. M. E., Natarajan, R., Reid, D. M. & Ralston, S. H. The D727E polymorphism of the human thyroid stimulating hormone receptor is associated with bone mineral density and bone loss in women from the UK. J. Bone Min. Res. 20, S341 (2005).
Liu, R. D. et al. The Glu727 allele of thyroid stimulating hormone receptor gene is associated with osteoporosis. N. Am. J. Med. Sci. 4, 300–304 (2012).
van Vliet, N. A. et al. Thyroid stimulating hormone and bone mineral density: evidence from a two-sample Mendelian randomization study and a candidate gene association study. J. Bone Min. Res. 33, 1318–1325 (2018).
Liu, S., Cheng, Y., Fan, M., Chen, D. & Bian, Z. FSH aggravates periodontitis-related bone loss in ovariectomized rats. J. Dent. Res. 89, 366–371 (2010).
Liu, S., Cheng, Y., Xu, W. & Bian, Z. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats. J. Endod. 36, 658–663 (2010).
Robinson, L. J. et al. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem. Biophys. Res. Commun. 394, 12–17 (2010).
Sun, L. et al. Further evidence for direct pro-resorptive actions of FSH. Biochem. Biophys. Res. Commun. 394, 6–11 (2010).
Wu, Y. et al. Bone microenvironment specific roles of ITAM adapter signaling during bone remodeling induced by acute estrogen-deficiency. PLoS ONE 2, e586 (2007).
Wang, J. et al. Follicle-stimulating hormone increases the risk of postmenopausal osteoporosis by stimulating osteoclast differentiation. PLoS ONE 10, e0134986 (2015).
Allan, C. M. et al. Follicle-stimulating hormone increases bone mass in female mice. Proc. Natl Acad. Sci. USA 107, 22629–22634 (2010).
Ritter, V. et al. Follicle-stimulating hormone does not impact male bone mass in vivo or human male osteoclasts in vitro. Calcif. Tissue Int. 82, 383–391 (2008).
Feng, Y. et al. Live imaging of follicle stimulating hormone receptors in gonads and bones using near infrared II fluorophore. Chem. Sci. 8, 3703–3711 (2017).
Ji, Y. et al. Epitope-specific monoclonal antibodies to FSHbeta increase bone mass. Proc. Natl Acad. Sci. USA 115, 2192–2197 (2018).
Meher, B. R., Dixit, A., Bousfield, G. R. & Lushington, G. H. Glycosylation effects on FSH–FSHR interaction dynamics: a case study of different FSH glycoforms by molecular dynamics simulations. PLoS ONE 10, e0137897 (2015).
Cannon, J. G., Kraj, B. & Sloan, G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine 53, 141–144 (2011).
Iqbal, J., Sun, L., Kumar, T. R., Blair, H. C. & Zaidi, M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc. Natl Acad. Sci. USA 103, 14925–14930 (2006).
Cannon, J. G. et al. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R790–R798 (2010).
Gertz, E. R. et al. Contribution of serum inflammatory markers to changes in bone mineral content and density in postmenopausal women: a 1-year investigation. J. Clin. Densitom. 13, 277–282 (2010).
Zhu, L. L. et al. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc. Natl Acad. Sci. USA 109, 14574–14579 (2012).
Gao, J. et al. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology 148, 2613–2621 (2007).
Danilovich, N. et al. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 141, 4295–4308 (2000).
Abel, M. H., Huhtaniemi, I., Pakarinen, P., Kumar, T. R. & Charlton, H. M. Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHbeta subunit knockout mice. Reproduction 125, 165–173 (2003).
Oz, O. K. et al. Bone has a sexually dimorphic response to aromatase deficiency. J. Bone Min. Res. 15, 507–514 (2000).
Couse, J. F., Yates, M. M., Walker, V. R. & Korach, K. S. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol. Endocrinol. 17, 1039–1053 (2003).
Sims, N. A. et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30, 18–25 (2002).
Khalid, A. B. & Krum, S. A. Estrogen receptors alpha and beta in bone. Bone 87, 130–135 (2016).
Devleta, B., Adem, B. & Senada, S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J. Bone Min. Metab. 22, 360–364 (2004).
Kawai, H., Furuhashi, M. & Suganuma, N. Serum follicle-stimulating hormone level is a predictor of bone mineral density in patients with hormone replacement therapy. Arch. Gynecol. Obstet. 269, 192–195 (2004).
Podfigurna-Stopa, A. et al. Skeletal status and body composition in young women with functional hypothalamic amenorrhea. Gynecol. Endocrinol. 28, 299–304 (2012).
Drake, M. T., McCready, L. K., Hoey, K. A., Atkinson, E. J. & Khosla, S. Effects of suppression of follicle-stimulating hormone secretion on bone resorption markers in postmenopausal women. J. Clin. Endocrinol. Metab. 95, 5063–5068 (2010).
Rendina, D. et al. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur. J. Endocrinol. 163, 165–172 (2010).
Mendoza, N. et al. Estrogen-related genes and postmenopausal osteoporosis risk. Climacteric 15, 587–593 (2012).
Randolph, J. F. Jr. et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J. Clin. Endocrinol. Metab. 88, 1516–1522 (2003).
Sowers, M. R. et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos. Int. 14, 191–197 (2003).
Sowers, M. R. et al. Hormone predictors of bone mineral density changes during the menopausal transition. J. Clin. Endocrinol. Metab. 91, 1261–1267 (2006).
Greendale, G. A. et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J. Bone Min. Res. 27, 111–118 (2012).
Sowers, M. et al. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am. J. Epidemiol. 163, 950–958 (2006).
Greendale, G. A. et al. Changes in body composition and weight during the menopause transition. JCI Insight 4, e124865 (2019).
Greendale, G. A. et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72, 1850–1857 (2009).
Lukefahr, A. L. et al. Decreased bone mineral density in rats rendered follicle-deplete by an ovotoxic chemical correlates with changes in follicle-stimulating hormone and inhibin A. Calcif. Tissue Int. 90, 239–249 (2012).
Xu, Z. R. et al. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clin. Chim. Acta 400, 8–13 (2009).
Wu, X. Y. et al. Age-related changes in biochemical markers of bone turnover and gonadotropin levels and their relationship among Chinese adult women. Osteoporos. Int. 21, 275–285 (2010).
Cheung, E. et al. Bone loss during menopausal transition among southern Chinese women. Maturitas 69, 50–56 (2011).
Wang, B. et al. Correlation analysis for follicle-stimulating hormone and C-terminal cross-linked telopetides of type I collagen in menopausal transition women with osteoporosis. Int. J. Clin. Exp. Med. 8, 2417–2422 (2015).
Gallagher, C. M., Moonga, B. S. & Kovach, J. S. Cadmium, follicle-stimulating hormone, and effects on bone in women age 42–60 years, NHANES III. Environ. Res. 110, 105–111 (2010).
Adami, S. et al. Determinants of bone turnover markers in healthy premenopausal women. Calcif. Tissue Int. 82, 341–347 (2008).
Veldhuis-Vlug, A. G. et al. Serum FSH is associated with BMD, bone marrow adiposity, and body composition in the AGES-Reykjavik Study of older adults. J. Clin. Endocrinol. Metab. 106, e1156–e1169 (2021).
Crandall, C. J. et al. Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. J. Clin. Endocrinol. Metab. 98, E654–E663 (2013).
Hofbauer, L. C. & Rauner, M. Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol. Endocrinol. 23, 1525–1531 (2009).
Compston, J. Glucocorticoid-induced osteoporosis: an update. Endocrine 61, 7–16 (2018).
Minetto, M. et al. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing’s syndrome. Osteoporos. Int. 15, 855–861 (2004).
Zhong, Q. et al. Multiple melanocortin receptors are expressed in bone cells. Bone 36, 820–831 (2005).
Isales, C. M., Zaidi, M. & Blair, H. C. ACTH is a novel regulator of bone mass. Ann. N. Y. Acad. Sci. 1192, 110–116 (2010).
Sato, T. et al. Bone phenotype in melanocortin 2 receptor-deficient mice. Bone Rep. 13, 100713 (2020).
Zaidi, M. et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc. Natl Acad. Sci. USA 107, 8782–8787 (2010).
Tourkova, I. L. et al. Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D(3) enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab. Invest. 97, 1072–1083 (2017).
Sadeghi, F., Vahednia, E., Naderi Meshkin, H. & Kerachian, M. A. The effect of adrenocorticotropic hormone on alpha-2-macroglobulin in osteoblasts derived from human mesenchymal stem cells. J. Cell. Mol. Med. 24, 4784–4790 (2020).
Elabd, C. et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cell 26, 2399–2407 (2008).
Tamma, R. et al. Oxytocin is an anabolic bone hormone. Proc. Natl Acad. Sci. USA 106, 7149–7154 (2009).
Sun, L. et al. Functions of vasopressin and oxytocin in bone mass regulation. Proc. Natl Acad. Sci. USA 113, 164–169 (2016).
Tamma, R. et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc. Natl Acad. Sci. USA 110, 18644–18649 (2013).
Athonvarangkul, D. & Wysolmerski, J. J. Crosstalk within a brain–breast–bone axis regulates mineral and skeletal metabolism during lactation. Front. Physiol. 14, 1121579 (2023).
Di Benedetto, A. et al. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc. Natl Acad. Sci. USA 111, 16502–16507 (2014).
Sun, L. et al. Oxytocin regulates body composition. Proc. Natl Acad. Sci. USA 116, 26808–26815 (2019).
Liu, X. et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochem. Biophys. Res. Commun. 388, 161–166 (2009).
Yu, W. J. et al. Association between serum oxytocin, bone mineral density and body composition in Chinese adult females. Medicina 58, 1625 (2022).
Breuil, V. et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: analysis of the OPUS cohort. J. Clin. Endocrinol. Metab. 99, E634–E641 (2014).
Breuil, V. et al. Oxytocin and bone status in men: analysis of the MINOS cohort. Osteoporos. Int. 26, 2877–2882 (2015).
Sejling, A. S., Pedersen-Bjergaard, U. & Eiken, P. Syndrome of inappropriate ADH secretion and severe osteoporosis. J. Clin. Endocrinol. Metab. 97, 4306–4310 (2012).
Sejling, A. S., Thorsteinsson, A. L., Pedersen-Bjergaard, U. & Eiken, P. Recovery from SIADH-associated osteoporosis: a case report. J. Clin. Endocrinol. Metab. 99, 3527–3530 (2014).
Murthy, K. et al. The effects of hyponatremia on bone density and fractures: a systematic review and meta-analysis. Endocr. Pract. 25, 366–378 (2019).
Upala, S. & Sanguankeo, A. Association between hyponatremia, osteoporosis, and fracture: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 101, 1880–1886 (2016).
Kinsella, S., Moran, S., Sullivan, M. O., Molloy, M. G. & Eustace, J. A. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin. J. Am. Soc. Nephrol. 5, 275–280 (2010).
Coss, D. et al. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am. J. Physiol. Endocrinol. Metab. 279, E1216–E1225 (2000).
Seriwatanachai, D. et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone 42, 535–546 (2008).
Clement-Lacroix, P. et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology 140, 96–105 (1999).
Sowers, M. et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J. Clin. Endocrinol. Metab. 92, 895–901 (2007).
Liu, X. M. et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell 14, 409–420 (2015).
Han, X. et al. FSH promotes fat accumulation by activating PPARgamma signaling in surgically castrated, but not immunocastrated, male pigs. Theriogenology 160, 10–17 (2021).
Han, X. et al. A novel follicle-stimulating hormone vaccine for controlling fat accumulation. Theriogenology 148, 103–111 (2020).
Abildgaard, J. et al. Changes in abdominal subcutaneous adipose tissue phenotype following menopause is associated with increased visceral fat mass. Sci. Rep. 11, 14750 (2021).
Araujo, A. B. & Wittert, G. A. Endocrinology of the aging male. Best. Pract. Res. Clin. Endocrinol. Metab. 25, 303–319 (2011).
Ostergren, P. B. et al. Metabolic consequences of gonadotropin-releasing hormone agonists vs orchiectomy: a randomized clinical study. BJU Int. 123, 602–611 (2019).
Lundback, V., Kulyte, A., Dahlman, I. & Marcus, C. Adipose-specific inactivation of thyroid stimulating hormone receptors in mice modifies body weight, temperature and gene expression in adipocytes. Physiol. Rep. 8, e14538 (2020).
Draman, M. S. et al. The role of thyrotropin receptor activation in adipogenesis and modulation of fat phenotype. Front. Endocrinol. 8, 83 (2017).
Lu, M. & Lin, R. Y. TSH stimulates adipogenesis in mouse embryonic stem cells. J. Endocrinol. 196, 159–169 (2008).
Endo, T. & Kobayashi, T. Expression of functional TSH receptor in white adipose tissues of hyt/hyt mice induces lipolysis in vivo. Am. J. Physiol. Endocrinol. Metab. 302, E1569–E1575 (2012).
Kumar, S., Coenen, M. J., Scherer, P. E. & Bahn, R. S. Evidence for enhanced adipogenesis in the orbits of patients with Graves’ ophthalmopathy. J. Clin. Endocrinol. Metab. 89, 930–935 (2004).
Haraguchi, K., Shimura, H., Lin, L., Endo, T. & Onaya, T. Differentiation of rat preadipocytes is accompanied by expression of thyrotropin receptors. Endocrinology 137, 3200–3205 (1996).
Lu, S. et al. Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 11, 17 (2012).
Haraguchi, K. et al. Effects of thyrotropin on the proliferation and differentiation of cultured rat preadipocytes. Thyroid 9, 613–619 (1999).
Haluzik, M. et al. Effects of hypo- and hyperthyroidism on noradrenergic activity and glycerol concentrations in human subcutaneous abdominal adipose tissue assessed with microdialysis. J. Clin. Endocrinol. Metab. 88, 5605–5608 (2003).
Fox, C. S. et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch. Intern. Med. 168, 587–592 (2008).
Ittermann, T. et al. Low serum TSH levels are associated with low values of fat-free mass and body cell mass in the elderly. Sci. Rep. 11, 10547 (2021).
Dvorakova, M. et al. Relationship between pituitary–thyroid axis hormones and anthropometric parameters in Czech adult population. Physiol. Res. 57, S127–S134 (2008).
Nyrnes, A., Jorde, R. & Sundsfjord, J. Serum TSH is positively associated with BMI. Int. J. Obes. 30, 100–105 (2006).
Ruhla, S. et al. A high normal TSH is associated with the metabolic syndrome. Clin. Endocrinol. 72, 696–701 (2010).
Sakurai, M. et al. Association between a serum thyroid-stimulating hormone concentration within the normal range and indices of obesity in Japanese men and women. Intern. Med. 53, 669–674 (2014).
Zhang, J. et al. TSH promotes adiposity by inhibiting the browning of white fat. Adipocyte 9, 264–278 (2020).
Jiang, D. et al. Thyroid-stimulating hormone inhibits adipose triglyceride lipase in 3T3-L1 adipocytes through the PKA pathway. PLoS ONE 10, e0116439 (2015).
Janson, A. et al. Effects of stimulatory and inhibitory thyrotropin receptor antibodies on lipolysis in infant adipocytes. J. Clin. Endocrinol. Metab. 80, 1712–1716 (1995).
Endo, T. & Kobayashi, T. Thyroid-stimulating hormone receptor in brown adipose tissue is involved in the regulation of thermogenesis. Am. J. Physiol. Endocrinol. Metab. 295, E514–E518 (2008).
Zhang, L. et al. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest. Ophthalmol. Vis. Sci. 47, 5197–5203 (2006).
Comas, F. et al. Adipose tissue TSH as a new modulator of human adipocyte mitochondrial function. Int. J. Obes. 43, 1611–1619 (2019).
Elgadi, A., Zemack, H., Marcus, C. & Norgren, S. Tissue-specific knockout of TSHr in white adipose tissue increases adipocyte size and decreases TSH-induced lipolysis. Biochem. Biophys. Res. Commun. 393, 526–530 (2010).
Moreno-Navarrete, J. M. et al. TSHB mRNA is linked to cholesterol metabolism in adipose tissue. FASEB J. 31, 4482–4491 (2017).
Blevins, J. E., Schwartz, M. W. & Baskin, D. G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R87–R96 (2004).
Son, S. et al. Whole-brain wiring diagram of oxytocin system in adult mice. J. Neurosci. 42, 5021–5033 (2022).
Maejima, Y. et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 588, 4404–4412 (2014).
Olson, B. R. et al. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12, 113–118 (1991).
Arletti, R., Benelli, A. & Bertolini, A. Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 48, 825–830 (1990).
Smith, A. S., Korgan, A. C. & Young, W. S. Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacol. Res. 146, 104324 (2019).
Maejima, Y. et al. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 3, 1169–1177 (2011).
Klockars, A., Brunton, C., Li, L., Levine, A. S. & Olszewski, P. K. Intravenous administration of oxytocin in rats acutely decreases deprivation-induced chow intake, but it fails to affect consumption of palatable solutions. Peptides 93, 13–19 (2017).
Wronski, M. L. et al. A randomized, double-blind, placebo-controlled clinical trial of 8-week intranasal oxytocin administration in adults with obesity: rationale, study design, and methods. Contemp. Clin. Trials 122, 106909 (2022).
Wu, Z. et al. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE 7, e45167 (2012).
Deblon, N. et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE 6, e25565 (2011).
Yi, K. J. et al. The regulation of oxytocin receptor gene expression during adipogenesis. J. Neuroendocrinol. 27, 335–342 (2015).
Blevins, J. E. et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R431–R438 (2015).
Yuan, J., Zhang, R., Wu, R., Gu, Y. & Lu, Y. The effects of oxytocin to rectify metabolic dysfunction in obese mice are associated with increased thermogenesis. Mol. Cell Endocrinol. 514, 110903 (2020).
Noble, E. E., Billington, C. J., Kotz, C. M. & Wang, C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R737–R745 (2014).
Kasahara, Y. et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 154, 4305–4315 (2013).
Xi, D. et al. Ablation of oxytocin neurons causes a deficit in cold stress response. J. Endocr. Soc. 1, 1041–1055 (2017).
Qian, W. et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J. Clin. Endocrinol. Metab. 99, 4683–4689 (2014).
Froemke, R. C. & Young, L. J. Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci. 44, 359–381 (2021).
Stevens, F. L., Weisman, O., Feldman, R., Hurley, R. A. & Taber, K. H. Oxytocin and behavior: evidence for effects in the brain. J. Neuropsychiatry Clin. Neurosci. 25, 96–102 (2013).
Gainer, H. Cell-type specific expression of oxytocin and vasopressin genes: an experimental odyssey. J. Neuroendocrinol. 24, 528–538 (2012).
Eliava, M. et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89, 1291–1304 (2016).
Ishunina, T. A. & Swaab, D. F. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J. Clin. Endocrinol. Metab. 84, 4637–4644 (1999).
Yoshikawa, T. et al. Spatiotemporal profiles of arginine vasopressin transcription in cultured suprachiasmatic nucleus. Eur. J. Neurosci. 42, 2678–2689 (2015).
Jenkins, J. S., Ang, V. T., Hawthorn, J., Rossor, M. N. & Iversen, L. L. Vasopressin, oxytocin and neurophysins in the human brain and spinal cord. Brain Res. 291, 111–117 (1984).
Mens, W. B., Witter, A. & Van Wimersma Greidanus, T. B. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 262, 143–149 (1983).
Pow, D. V. & Morris, J. F. Dendrites of hypothalamic magnocellular neurons release neurohypophyseal peptides by exocytosis. Neuroscience 32, 435–439 (1989).
Hirasawa, M. et al. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J. Physiol. 559, 611–624 (2004).
Brussaard, A. B., Kits, K. S. & de Vlieger, T. A. Postsynaptic mechanism of depression of GABAergic synapses by oxytocin in the supraoptic nucleus of immature rat. J. Physiol. 497, 495–507 (1996).
Carter, C. S. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 65, 17–39 (2014).
Carter, C. S. et al. Is oxytocin ‘Nature’s Medicine’? Pharmacol. Rev. 72, 829–861 (2020).
Cochran, D. M., Fallon, D., Hill, M. & Frazier, J. A. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv. Rev. Psychiatry 21, 219–247 (2013).
Ryu, V. et al. Brain atlas for glycoprotein hormone receptors at single-transcript level. eLife 11, e79612 (2022).
Fonseca, T. L. et al. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J. Clin. Invest. 123, 1492–1500 (2013).
Sáenz de Miera, C., Sage-Ciocca, D., Simonneaux, V., Pévet, P. & Monecke, S. Melatonin-independent photoperiodic entrainment of the circannual TSH rhythm in the pars tuberalis of the European Hamster. J. Biol. Rhythm. 33, 302–317 (2018).
Hanon, E. A. et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 18, 1147–1152 (2008).
Barrett, P. & Bolborea, M. Molecular pathways involved in seasonal body weight and reproductive responses governed by melatonin. J. Pineal Res. 52, 376–388 (2012).
Ikegami, K. et al. Tissue-specific posttranslational modification allows functional targeting of thyrotropin. Cell Rep. 9, 801–810 (2014).
Prevot, V. et al. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 39, 333–368 (2018).
Yang, R. et al. Association of subclinical hypothyroidism with anxiety symptom in young first-episode and drug-naive patients with major depressive disorder. Front. Psychiatry 13, 920723 (2022).
Dayan, C. M. & Panicker, V. Hypothyroidism and depression. Eur. Thyroid J. 2, 168–179 (2013).
Luan, S. et al. Thyrotropin receptor signaling deficiency impairs spatial learning and memory in mice. J. Endocrinol. 246, 41–55 (2020).
Burgos, J. R., Iresjo, B. M., Warnaker, S. & Smedh, U. Presence of TSH receptors in discrete areas of the hypothalamus and caudal brainstem with relevance for feeding controls — support for functional significance. Brain Res. 1642, 278–286 (2016).
Bi, W. K. et al. FSH signaling is involved in affective disorders. Biochem. Biophys. Res. Commun. 525, 915–920 (2020).
Blair, J. A., Bhatta, S. & Casadesus, G. CNS luteinizing hormone receptor activation rescues ovariectomy-related loss of spatial memory and neuronal plasticity. Neurobiol. Aging 78, 111–120 (2019).
Gale, S. D., Baxter, L. & Thompson, J. Greater memory impairment in dementing females than males relative to sex-matched healthy controls. J. Clin. Exp. Neuropsychol. 38, 527–533 (2016).
Chêne, G. et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 11, 310–320 (2015).
Lin, K. A. et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. 1, 103–110 (2015).
Shumaker, S. A. et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291, 2947–2958 (2004).
Espeland, M. A. et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291, 2959–2968 (2004).
Zandi, P. P. et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288, 2123–2129 (2002).
Greendale, G. A. et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am. J. Epidemiol. 171, 1214–1224 (2010).
Bowen, R. L., Isley, J. P. & Atkinson, R. L. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J. Neuroendocrinol. 12, 351–354 (2000).
Short, R. A., Bowen, R. L., O’Brien, P. C. & Graff-Radford, N. R. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin. Proc. 76, 906–909 (2001).
Corbo, R. M., Gambina, G., Broggio, E. & Scacchi, R. Influence of variation in the follicle-stimulating hormone receptor gene (FSHR) and age at menopause on the development of Alzheimer’s disease in women. Dement. Geriatr. Cogn. Disord. 32, 63–69 (2011).
Espinoza, S. E. et al. Intranasal oxytocin improves lean muscle mass and lowers LDL cholesterol in older adults with sarcopenic obesity: a pilot randomized controlled trial. J. Am. Med. Dir. Assoc. 22, 1877–1882 (2021).
Zhu, L. L. et al. Blocking FSH action attenuates osteoclastogenesis. Biochem. Biophys. Res. Commun. 422, 54–58 (2012).
Rojekar, S. et al. Development and biophysical characterization of a humanized FSH-blocking monoclonal antibody therapeutic formulated at an ultra-high concentration. eLife 12, e88898 (2023).
Sant, D., Rokekar, S. & Gera, S. Optimizing therapeutic humanized FSH-blocking antibody formulation using protein thermal shift assay. Ann. N. Y. Acad. Sci. 1521, 67–78 (2023).
Geng, W. et al. Immunization with FSHbeta fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochem. Biophys. Res. Commun. 434, 280–286 (2013).
Acknowledgements
Work at Icahn School of Medicine at Mount Sinai carried out at the Center for Translational Medicine and Pharmacology was supported by R01 AG071870 to M.Z., T.Y. and S.-M.K.; R01 AG074092 and U01 AG073148 to T.Y. and M.Z.; and U19 AG060917 and R01 DK113627 to M.Z. M.Z. also thanks the Harrington Discovery Institute for the Innovator-Scholar Award towards the development of anti-FSH antibody. The authors are grateful to V. Ryu (Center for Translational Medicine and Pharmacology, Icahn School of Medicine at Mount Sinai, New York, NY, USA) for his insights on the central actions of pituitary hormones.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.Z. is an inventor on issued patents on inhibiting FSH for the prevention and treatment of osteoporosis and obesity (US Patents 8,435,948 and 11,034,761). M.Z. is also an inventor on a patent application on the composition and use of humanized monoclonal anti-FSH antibodies and is a co-inventor of a pending patent on the use of FSH as a target for preventing Alzheimer disease. M.Z. and T.Y. are co-inventors on a pending patent application relating to the effect of luteinizing hormone on body composition and another patent relating to the ultra-high formulation of an FSH-blocking antibody. These patents are owned by Icahn School of Medicine at Mount Sinai (ISMMS), and the inventors and co-inventors would be recipients of royalties, per institutional policy. M.Z. also consults for Rani Pharmaceuticals, and several financial platforms, including Gerson Lehman Group and Guidepoint, on drugs for osteoporosis and genetic bone diseases. S.-M.K. declares no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaidi, M., Yuen, T. & Kim, SM. Pituitary crosstalk with bone, adipose tissue and brain. Nat Rev Endocrinol 19, 708–721 (2023). https://doi.org/10.1038/s41574-023-00894-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-023-00894-5