Key Points
- Eosinophilic esophagitis is a clinicopathologic disease characterized by upper intestinal symptoms and the finding of more than 15 or 20 eosinophils in the esophageal epithelium; these findings are unresponsive to proton pump inhibitor treatment.
- Eosinophilic esophagitis is a common disease that affects both children and adults.
- The prevalence around the world is unknown, but eosinophilic esophagitis appears to be increasing.
- Food impaction and dysphagia are the most common presenting symptoms in older children and adults.
- Eosinophilic esophagitis can be associated with a narrow esophagus and other esophageal strictures.
- Eosinophilic esophagitis has specific endoscopic findings that suggest, but are not pathognomonic for, the diagnosis.
- Several treatments are effective. Elemental and elimination diets, as well as corticosteroids in both oral and topical forms are successful.
- The natural history is unknown but includes the potential for fibrotic strictures.
Eosinophilic Esophagitis
Until recently esophageal eosinophilia was primarily attributed to acid reflux esophagitis, but in the last 5 years eosinophilic esophagitis (also known as allergic esophagitis, primary eosinophilic esophagitis, and idiopathic eosinophilic esophagitis) has emerged as an important independent clinicopathologic entity found to occur in children and in adults.1, 2
Definition
Eosinophilic esophagitis is a disease in which upper intestinal symptoms are associated with dense eosinophilic infiltration of the squamous esophageal epithelium or deeper esophageal tissue; neither symptoms nor eosinophilia respond to the administration of a proton pump inhibitor (PPI). The pathophysiologic mechanisms are likely related to allergic inflammation, not to an underlying motility defect as in gastroesophageal reflux disease (GERD).1, 3
History
Today, the diagnosis of eosinophilic esophagitis is made by the gastroenterologist following procurement of a mucosal biopsy. But the fascinating story of eosinophilic esophagitis has been shaped by a number of different subspecialists including pediatric and adult gastroenterologists, surgeons, radiologists, pathologists, and allergists. One of the first reports of eosinophilic esophagitis dates from 1977, when Dobbins et al.4 described a man with dysphagia who had dense esophageal eosinophilia. Then in 1978, Landres et al.5 identified a male patient with achalasia and esophageal eosinophilia. During the next 15 years, several other reports described the association of severe dysphagia, proximal esophageal strictures, esophageal eosinophilic infiltration, and food allergies.6
In 1993 Attwood et al.7 provided the first comparison between adults with eosinophilic esophagitis and those with peptic esophagitis. The next two reports of similarly affected patients were published in 1994 and 1996 by investigators from Switzerland and Mexico. Straumann et al.8 and Borda et al.9 described a group of males with dysphagia and peripheral eosinophilia with endoscopic features of whitish exudates and esophageal narrowing. In 1995 Vitellas et al.10 described a series of patients with eosinophilic esophagitis, allergic symptoms, and proximal strictures that responded to systemic corticosteroids.
During the next 10 years, eosinophilic esophagitis was more frequently recognized by pediatricians, with pediatric gastroenterologists, allergists, and pathologists recognizing and studying affected children most intensely.3 Kelly et al.11 reported the first pediatric patients in their series of 10 children with persistent GERD symptoms who were found to have 41 esophageal eosinophils/high power field (HPF). Six of the patients had previously underdone Nissen fundoplication for symptoms thought to be secondary to GERD. Following the diagnosis of eosinophilic esophagitis, all patients responded to exclusive use of an amino acid-based formula. The authors suggested that eosinophilic esophagitis is associated with an exaggerated immunologic response to specific food antigens.
In 1997, Gupta et al.12 began a series of observations regarding the endoscopic features associated with eosinophilic esophagitis. Vertical lines in the esophageal mucosa were identified as a feature of severe esophageal inflammation. Since then, a number of investigators have identified other features suggestive of eosinophilic esophagitis including small-caliber esophagus, white exudates, ringed esophagus, fragile esophageal mucosa, Schatzki's ring, and proximal esophageal strictures.3, 13 In 1999, Ruchelli et al.14 and Walsh et al.15 determined specific pathologic features of eosinophilic esophagitis. Orenstein et al.16 published the largest experience of children with eosinophilic esophagitis and emphasized nonspecific presentations including vomiting and abdominal pain and the associated symptoms including chronic respiratory disease and allergic history.
The novel treatment modality of topical steroids was first published by Faubion et al.17 in 1998; this treatment reduced the need for systemic steroids. As more emphasis was placed on nutritional management of the disease, Spergel and colleagues18 demonstrated the utility of skin patch and prick testing in identifying food allergens. In recent years, there has been a return of interest in this disease among gastroenterologists who treat adults. Three independent studies documented clinicopathologic findings of 88 adults from Switzerland, North America, and Australia.1, 19, 20 These studies identified strictures, but not cancer, as a long-term complication.
Epidemiology
Eosinophilic esophagitis is a clinicopathologic disease that shows a worldwide distribution.21 It is distinctly more common in males, and it affects patients of all ages. Until recently there had been a preponderance of reports in the pediatric population, but it has emerged as a disease that also affects adults worldwide.1 Although it is possible that eosinophilic esophagitis occurs less often in adults, most likely it has been underdiagnosed, as recent clinical reports suggest. A recent study determined that eosinophilic esophagitis is the leading cause of food impaction in a suburban private practice.1
Clinical experience in both adult and pediatric centers suggests both increased awareness and increased incidence of eosinophilic esophagitis are the reasons for the recent explosion of cases. To date, several studies have examined the prevalence of the disease but only one study has carefully dissected this issue. In a population-based demographic study, Noel et al.21 reported that from 2000 to 2003, the annual incidence of pediatric eosinophilic esophagitis was approximately 1 in 10,000, with a prevalence of 4.296 cases per 10,000 children at the end of 2003. They also found a familial pattern, raising the possibility of either a genetic predisposition, or exposure to an unknown environmental factor.21 The incidence that those authors established may be higher than those for other well-recognized inflammatory gastrointestinal (GI) disorders in children (such as Crohn's disease).22 Another study of 214 children with esophageal eosinophilia evaluated in a tertiary care center between 1993 and 1995 found that 9.3% of these children were given a diagnosis of eosinophilic esophagitis.23 In our unpublished data 6.8% of patients with esophagitis demonstrated histologic features of eosinophilic esophagitis.3
Pathophysiology
Although the esophagus is typically considered a conduit for food to travel from the mouth to the stomach, an increasing body of evidence demonstrates its immunologic potential. For instance, the squamous epithelium is armed with a population of lymphocytes, mast cells, and dendritic cells poised to protect the underlying esophageal milieu from invasion of microbes, toxins, and other antigens. Although eosinophils typically reside in the lamina propria of the rest of the GI tract that is lined by a columnar epithelium, eosinophils do not populate the normal esophageal squamous epithelium.
In this light, the recent identification of eosinophilic esophagitis offers a fascinating opportunity to further explore the immunologic role of the esophagus and the inflammatory impact of eosinophils. Despite extensive descriptions of eosinophilic inflammation in mucosal tissues affected by a number of different allergic diseases, the exact role of this cell is still undetermined. These granulated leukocytes contain a number of biologically active mediators including cytokines, granule proteins, and leukotrienes. Importantly, both the eosinophil granule–derived protein, major basic protein, and cysteinyl-leukotriene C4 and D4 induce epithelial barrier dysfunction and airway smooth muscle contraction. Translational studies involving patients with eosinophilic esophagitis have identified the deposition of major basic protein and increased expression of interleukin-5 (IL-5), IL-13, and tumor necrosis factor-a within the affected esophageal epithelium, but their functional impact has yet to be identified.24, 25, 26, 27, 28
Murine studies have begun to shed light on mechanisms that eosinophils use to home to the esophagus during allergic inflammation. Interleukin-5 and eotaxin are two cytokines with critical roles in eosinophil's growth development and chemotactic responses. To determine the role of these cytokines in eosinophilic esophagitis, Mishra et al.24, 25 induced esophageal eosinophilia with an ubiquitous aeroallergen, Aspergillus fumigatus, in IL-5 knockout mice and eotaxin knockout mice. Eotaxin null mice showed diminished esophageal eosinophilia compared to wild-type challenged mice, whereas IL-5 null mice were completely protected from eosinophilic infiltration. These results suggest critical roles for IL-5 in esophageal eosinophilia and point to the potential therapeutic role of IL-5 antagonists.25, 29
The exact etiology of eosinophilic esophagitis is not known. Most investigators agree that eosinophilic esophagitis is driven by an aberrant immune-mediated response.30 The known association between eosinophils with food allergies suggested that food antigens may cause eosinophilic esophagitis, and this assumption has proven true in many patients. The most common food allergens that have been identified include milk, soy, egg, and wheat.31 Environmental allergens have also been implicated as possible contributors.32 A 21-year-old woman who had pollen-induced asthma and allergic rhinoconjunctivitis was found to have acute exacerbations of her eosinophilic esophagitis when she was exposed to pollens.32 Similar findings have been described in a murine model.29
The pathogenesis of the mucosal rings associated with eosinophilic esophagitis is unknown. Speculation suggests that the release of histamine, eosinophilic chemotactic factor, or platelet-activating factor by the mast cells in the esophageal wall in response to allergens could lead to ring formation. Those substances activate eosinophils to release toxic cationic proteins. Activation of acetyl choline by histamine or leukotrienes may cause contraction of the muscle fibers in the muscularis mucosae, resulting in the formation of esophageal rings.33
Pathology
The normal esophageal mucosa is composed of a stratified squamous epithelium that contains a scattering of lymphocytes, dendritic cells, and mast cells but no leukocytes including eosinophils. Deeper to the epithelium is an intricate quilt composed of circular and longitudinal muscle layers woven together with fibroblasts and neurons.
Eosinophilic inflammation of the esophagus can be found in a number of diseases including GERD, eosinophilic gastroenteritis (EOG), hypereosinophilic syndrome, food allergies, inflammatory bowel disease, parasitic infection, and collagen vascular diseases.15, 21, 30 To date, no studies have clearly defined the upper limit of normal eosinophils seen in the epithelium of adults and children with GERD. Typically, this number is thought to be <5 per HPF, with the eosinophilia being limited to the distal esophagus. But recent unpublished data in children suggest that the number of esophageal eosinophils in GERD might be significantly greater than this and that esophageal eosinophils extend to the proximal esophageal mucosa. In that light, it is imperative that GERD be excluded as a cause of esophageal eosinophilia before making a diagnosis of eosinophilic esophagitis.
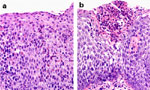
When clinical features suggest a diagnosis of eosinophilic esophagitis, several histologic features can be seen15, 21, 30 (Figure 1). First, the epithelium contains a dense infiltration of more than 15 or 20 eosinophils/HPF. Second, eosinophils often layer along the superficial surface of the epithelium. Third, eosinophilic microabscess (>4 eosinophils clustering adjacent to each other) can be seen in 40% to 50% of affected tissue sections. Abscesses form the histologic correlate of the whitish exudates or pinpoint papules often seen at endoscopy. Fourth, evidence of eosinophilic degranulation can be so profound that intact eosinophils might appear to be absent in some sections. Last, these described patterns of eosinophilia are present on a background of intense basal zone hyperplasia. All these patterns persist despite proton pump inhibition.
Figure 1: Histologic features of eosinophilic esophagitis.
a: Photomicrograph showing large numbers of eosinophils with squamous epithelium from a child with eosinophilic esophagitis (H&E original magnification  400). b: Photomicrograph showing eosinophilic microabscess burgeoning through the squamous epithelium (H&E original magnification
400). b: Photomicrograph showing eosinophilic microabscess burgeoning through the squamous epithelium (H&E original magnification  400). (Source: Photomicrographs courtesy of Kamran Badizadegan, M.D., Boston. Figure adapted from Fox et al.,3 with permission from the American Society for Gastrointestinal Endoscopy.)
400). (Source: Photomicrographs courtesy of Kamran Badizadegan, M.D., Boston. Figure adapted from Fox et al.,3 with permission from the American Society for Gastrointestinal Endoscopy.)
These histologic features can extend between the proximal and distal esophagus. The gastric and duodenal tissues are normal; this feature separates eosinophilic esophagitis from one of the other eosinophilic GI diseases, eosinophilic gastroenteritis. This distinction is important to make because treatments for these two diseases can be different; topical corticosteroids that relieve clinicopathologic features of eosinophilic esophagitis are unlikely to benefit the gastric or duodenal mucosa. In addition, basic studies suggest that the pathogenesis of eosinophilic esophagitis and EOG is different with eosinophilic esophagitis being IL-5 mediated and EOG being mediated by eotaxin.21
Whether this inflammation extends to the deeper levels of the esophageal tissues is not certain because mucosal biopsies are limited in their depth. Case reports in which full-thickness biopsies were obtained and endoscopic ultrasonographic evidence suggest extension of the eosinophilic inflammation to the submucosal and muscular layer; this finding lends support to the speculation that eosinophils and their mediators induce esophageal dysmotility.34, 35
Clinical Features
Eosinophilic esophagitis is distinctly more common in males for unknown reasons. Interestingly, the presenting symptoms if eosinophilic esophagitis may vary with age. Parents often bring in young children for evaluation of failure to thrive and food refusal, whereas older children complain of vomiting, regurgitation, and epigastric and chest pain. Adolescents and adults usually present with heartburn and dysphagia. In a recent report of 103 children, 12.5% presented with feeding difficulties (mean age 3.5 years), 25% with vomiting (mean age 7.9 years), 25% with abdominal pain (mean age 12 years), 26% with dysphagia (mean age 13 years), and 6.8% with food impaction (mean age 16 years).21
The most characteristic symptom in adults is intermittent dysphagia, often accompanied by food impaction.1, 3, 19, 20 In a series of 29 adults with eosinophilic esophagitis, food impaction was present in 33%.1 A recent series found that 17 of 31 adults from a suburban private practice who presented with food impaction were found to have clinicopathologic features consistent with eosinophilic esophagitis.36 There is often a long delay between the onset of symptoms and the diagnosis. A study of 30 adult patients found that patients developed dysphagia at an average age of 28 years, but the diagnosis was not made until an average age of 33 years. The attacks of dysphagia occurred for >1 week in 66%.13 Although the symptom of dysphagia is usually longstanding, it is often intermittent, not interfering with daily life.16
Heartburn is often present, particularly in older children and adults, making the differentiation with GERD difficult. In a series of 29 adults, 28% had a history of heartburn.1 There may be partial improvement in symptoms after acid blockade, but eosinophilia persists.30 In fact most patients with eosinophilic esophagitis have been previously treated unsuccessfully for GERD with some patients receiving antireflux surgery.3 For instance, six of 10 children in the series of Kelly et al.11 and two reported by Liacouras37 had already undergone fundoplication.
About half the patients present with other allergic symptoms, including eczema, allergic rhinitis, and bronchospasm.1, 30 Orenstein et al.16 identified chronic respiratory symptoms including wheezing, pneumonia, sinusitis, and congestion in 62% of 21 patients. A family history of allergy, such as asthma, rhinitis, and eczema, was found in 30% to 50% of patients.30 In the largest epidemiologic study of 103 children, 57% had a history of rhinoconjunctivitis, 37% of wheezing, and 46% of food allergy. In 73% a family history of atopy was present, 9% had a family history of eosinophilic esophagitis, and 6.8% had a family history of esophageal dilatation.21 In a series of 30 adults, a history of allergic disease was present in 53%.13
Laboratory, Radiologic, and Endoscopic Findings
Laboratory Findings
The presence of eosinophilia is common, but not universal, ranging from 30% to 100%.38 In a recent adult series, mild eosinophilia occurred in 50%.13 In another study, 30 children were found to have a median absolute eosinophil count of 582, with a minority having an eosinophil count >800.16
Allergy Evaluation
The underlying pathogenesis of eosinophilic esophagitis is likely related to an allergic response. In this light, a number of studies have focused on trying to identify specific allergens in an attempt to remove them from the diet. Early evidence suggested a role for an immunoglobulin E (IgE)-mediated process; in a series of 30 children, 60% of those tested showed either a positive radioallergosorbent test (RAST) or skin prick test.30 The most common food allergens identified in a study of 26 children by using skin prick included milk, eggs, peanuts, shellfish, peas, beef, fish, rye, tomato, and wheat.18 Patients had and average of 2.7  3.3 foods. Recent studies suggest that eosinophilic esophagitis may represent a delayed response, and thus allergy evaluations have turned to skin patch testing. This form of testing has been traditionally used to assess the etiologic agent in contact hypersensitivity responses and suggests benefit in determining foods associated with eosinophilic esophagitis. When compared to skin prick and RAST testing, patch testing was able to identify food allergens in three of 16 patients who were prick and RAST negative.16 Patch testing identified sensitivity to the allergens wheat, corn, beef, milk, soy, rye, egg, chicken, oats, and potato.18 Patients were positive to an average of 2.7
3.3 foods. Recent studies suggest that eosinophilic esophagitis may represent a delayed response, and thus allergy evaluations have turned to skin patch testing. This form of testing has been traditionally used to assess the etiologic agent in contact hypersensitivity responses and suggests benefit in determining foods associated with eosinophilic esophagitis. When compared to skin prick and RAST testing, patch testing was able to identify food allergens in three of 16 patients who were prick and RAST negative.16 Patch testing identified sensitivity to the allergens wheat, corn, beef, milk, soy, rye, egg, chicken, oats, and potato.18 Patients were positive to an average of 2.7  1.8 foods. Definitive evidence that a food is causing eosinophilic esophagitis depends on showing resolution of symptoms, and on biopsy normalization with an elimination diet, as well as return of esophageal eosinophils after the reintroduction of the foods.38 Despite its profound impact on some patients, patch testing is not well standardized, and is not available in many centers, so additional studies are needed before its routine use can be recommended.
1.8 foods. Definitive evidence that a food is causing eosinophilic esophagitis depends on showing resolution of symptoms, and on biopsy normalization with an elimination diet, as well as return of esophageal eosinophils after the reintroduction of the foods.38 Despite its profound impact on some patients, patch testing is not well standardized, and is not available in many centers, so additional studies are needed before its routine use can be recommended.
Barium Esophagram
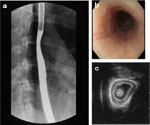
Eosinophilic esophagitis is a disorder with symptoms similar to those described in patients with Schatzki's ring (SR). We recently described a series of 18 patient with SR, and of those 8 (44%) fulfilled criteria for eosinophilic esophagitis including lack of response to acid blockade, normal pH probe monitoring of the distal esophagus, esophageal exudates and furrows, and severe eosinophilic inflammation of the esophageal mucosa39 (Figure 2). Although the majority of children with SR had peptic inflammation and evidence of a ring upon endoscopic investigation, these eight children with eosinophilic esophagitis did not have any endoscopic evidence of a SR.
These findings suggest that another mechanism may be responsible for the radiographic appearance of the SR. We speculate that the SR is formed by a plication of esophageal mucosa as suggested previously,40 but in the case of eosinophilic esophagitis that SR represents edema or thickening of redundant, inflamed esophageal mucosa. Our previous work supports this speculation, as our endoscopic ultrasonographic evidence suggests that eosinophilic inflammation associated with eosinophilic esophagitis extends beyond the squamous epithelium and into the esophageal submucosa and muscularis propria.35 The infolding of redundant esophageal mucosa may occur because it is loosely attached to underlying tissues except at the squamocolumnar junction, where it is firmly adherent.40, 41 The impetus for mucosal redundancy and ring formation is esophageal shortening, produced by the contraction of the longitudinal muscle.40 The ring appears and disappears as the longitudinal muscle of the esophagus shortens (contracts) and lengthens (relaxes).40
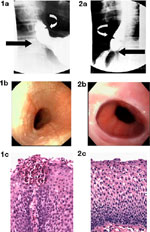
A variety of other radiologic findings characterize eosinophilic esophagitis but none is pathognomonic. Featureless small-caliber esophagus, isolated esophageal narrowing (Figure 3), and ringed esophagus are suggestive findings. When compared to GERD, proximal, not distal, stenosis is common. Esophageal narrowing may be subtle, extending the length of the esophagus, and thus may go unappreciated. In a pediatric series, 13 of 18 children with eosinophilic esophagitis had a normal study, whereas in three a stricture was seen.16 In one adult series in which barium studies were specifically done to assess the radiologic features in patients with eosinophilic esophagitis, esophageal narrowing was missed in four patients in whom it was identified endoscopically.
Figure 3: Radiographic and endoscopic studies from an 18-year-old woman with a longstanding history of dysphagia that began in early childhood.
a: Barium esophagram showing a small caliber esophagus. b: Endoscopic view of pale mucosa with absent vascular pattern and linear furrows in proximal esophagus. c: Endosonographic image (20 MHz catheter probe) showing thick-walled esophagus with prominent mucosa/submucosa layer and narrow lumen. (Source: Endoscopic ultrasound courtesy of Victor Fox, M.D., Boston. Figure adapted from Fox et al.,3 with permission from the American Society for Gastrointestinal Endoscopy.)
Endoscopy
Endoscopic findings seen on the esophageal mucosa can range from the subtle to dramatic. The mucosa can appear normal or have nonspecific features of inflammation such as erythema, edema, and friability. On the basis of these findings, one can appreciate the difficulty in distinguishing eosinophilic esophagitis from peptic esophagitis; this emphasizes the necessity of obtaining esophageal biopsies in all suspected patients.
A number of investigators have independently identified other endoscopic features such as granularity, absent vascular margins, linear fissuring, vertical furrowing, longitudinal tears, felinization, corrugation, fixed or transient concentric rings, and proximal strictures1 (Figures 2, 3, and 4) that are suggestive of eosinophilic esophagitis. Although none of the features are pathognomonic, taken together they likely represent evidence of acute eosinophilic inflammation (granularity and exudates) and longstanding chronic eosinophilic inflammation (strictures and longitudinal tears). In a series of 30 adult patients, different endoscopic features were described including (1) signs of exudation: 53.3% had white exudates, 43.3% nodules, 40% plaques; (2) signs of obstruction: short segment stricture in 3.3%, long segment stricture in 3.3%, solitary ring in 36.7%, corrugated rings in 16.7%; and (3) signs of other lesions: erosions in 6.7%, crepe paper appearance in 13.3%, loss of vascular pattern in 93.3%, circular folds in 20.0%, serpiginous erythema in 36.7%.13
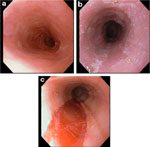
Figure 4: Endoscopic features of eosinophilic esophagitis.
a: Endoscopic view of proximal esophagus showing normal vascular pattern and distal narrowing in a 14-year-old boy with a history of obstructive dysphagia and allergic disease. b: Midesophageal view of whitish exudate coating mucosa. c: Linear shearing of a long segment stricture in distal esophagus after balloon dilation. (Source: Adapted from Fox et al.,3 with permission from the American Society for Gastrointestinal Endoscopy.)
White pinpoint or granular exudate, at times reminiscent of candidiasis or swallowed topical anesthetic spray, can be found.13, 42 When the exudate is biopsied it is found to consist of eosinophilic microabscesses. Some evidence suggests that the white pinpoint exudate is more common in children than in adults.1 In a retrospective study of 153 children with eosinophilic esophagitis who underwent endoscopy, 31 had white specks. The sensitivity of the white specks for eosinophilic esophagitis was 30%, but the specificity was 95%.42
Endoscopy is the best method to detect esophageal narrowing.1 When a proximal stricture is severe, insertion of the endoscope may be impossible. The narrowing may extend the length of the esophagus, and the esophageal mucosa can be quite friable. If this is not recognized, splitting of the epithelium may occur after passage of the endoscope, and this may not be apparent until the endoscope is being withdrawn.1 The term crepe paper esophageal mucosa has been used to describe this friability.13 Esophageal rings have also been reported; this finding can correspond to true congenital or fibrous rings, or can at times signify intermittent contractions of the circular muscle of the esophagus. It is likely that many of the early associations of GERD with a ringed esophagus, in both children43 and adults,44 in fact represented patients with eosinophilic esophagitis.3 In the pediatric series, three of six patients with esophageal rings had a history of dysphagia and food impaction unresponsive to acid blockade, only slightly abnormal pH monitoring (reflux index 5–7%), and increased intraepithelial eosinophils.43 In the adult series eight of 19 adults (17 men) with esophageal rings and a longstanding history of food impaction had severe esophageal eosinophilia.44
Mucosal Biopsy
Biopsies of the esophageal epithelium after at least 4 weeks of high-dose proton pump inhibition are required to make the diagnosis. Characteristically there are more than 15 or 20 eosinophils per HPF (Figure 1). Given the propensity of eosinophilic esophagitis to affect the proximal esophagus, biopsies should be performed from the whole length of the esophagus. It is interesting than mucosal biopsies of the stomach, antrum, and duodenum tend to be normal.
Our experience suggests that the specific histologic findings associated with eosinophilic esophagitis also occur in children with GERD (unpublished data). This findings stress the recommendation that the diagnosis of eosinophilic esophagitis should be made only after aggressive acid blockade.
pH Monitoring
Multiple studies have shown that patients with eosinophilic esophagitis usually have normal pH monitoring of the distal esophagus.3, 15, 16, 23, 26, 34 Therefore, the presence of severe intractable esophagitis that is found after aggressive acid blockade in the setting of a normal pH monitoring is virtually diagnostic of the diagnosis of eosinophilic esophagitis. Most importantly a normal pH monitoring nearly eliminates GERD as an etiology of the intractable esophagitis. In recent years, however, it has become evident that there are patients with eosinophilic esophagitis and some underlying gastroesophageal reflux. This finding may explain why some patients may have a partial response to acid blockade.3
Esophageal Manometry
The pathophysiology of dysphagia associated with eosinophilic esophagitis is not well understood. Many studies utilizing stationary manometry have usually showed normal peristalsis. Some series described tertiary contractions, aperistalsis, simultaneous contractions, diffuse spasm, and nutcracker esophagus.7 In 1997 Dobbins et al.4 described one patient with eosinophilic esophagitis who had esophageal spasm. In a report from 1978 Landres et al.5 described a patient with vigorous achalasia who had underlying severe eosinophilic infiltration of the esophageal mucosa. In that case the symptoms were probably related to the achalasia, but it suggested the possibility that eosinophilic esophagitis may predispose to a motor esophageal disorder. In the initial report by Attwood et al.,7 10 of 12 patients had abnormal esophageal peristalsis by stationary manometry; two had diffuse esophageal spasm, and two nutcracker esophagus. Three others had a mean amplitude of contractions that was less than the 2.5 percentile of normal, and four had contractions of short duration. All 12 patients had normal lower esophageal sphincter (LES) pressure and function.
To gain further insights into the dynamic nature of this disease, we performed stationary and 24-hour ambulatory pH/manometry measurements on 16 children with eosinophilic esophagitis, all of whom were found to have normal stationary manometry. When these patients underwent 24-hour ambulatory pH motility, they had a significantly higher percent of ineffective peristalsis, high-amplitude contractions (>180 mmHg), and isolated contractions compared to controls.45 Importantly, abnormal esophageal peristaltic events correlated with dysphagia during the study. These changes were absent in children with documented peptic esophagitis.46 It is therefore possible that the intermittent nature of the dysphagia may be associated with motor abnormalities.
Endoscopic Ultrasound
Because the symptoms of this disease suggest motor dysfunction and because mucosal biopsies allow only sampling of the superficial mucosa, endoscopic ultrasound (EUS) has been used to establish if the esophageal inflammation extends beyond the mucosa.35 It demonstrated hypoechogenicity and thickening of esophageal wall layers35 (Figures 3 and 5). Those findings can be consistent with deep tissue edema and lack of tissue compliance.1 In a study in which high-resolution ultrasound was used to compare esophageal tissue between 11 children with eosinophilic esophagitis and eight normal controls, we determined that children with eosinophilic esophagitis developed significant esophageal wall expansion, with thickening of the total wall, mucosa/submucosa, and muscularis propria.35 Stevoff et al.34 report an 85-year-old with a midesophageal stricture. Endoscopic ultrasound demonstrated circumferential but asymmetric wall thickening of the distal esophageal muscularis propria. The mucosal appearance was normal. Because of concern for malignancy, a resection of the esophageal stricture was performed, and the muscularis propria showed more than 100 eosinophils/HPF, whereas the mucosa had less than 5 per HPF. No malignancy was found.34
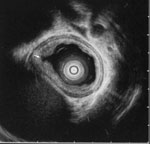
Figure 5: Esophageal furrow in a 15-year-old boy with dysphagia.
Endosonographic image (20-MHz miniprobe) showing esophageal furrow (arrow) flanked by folds of thickened mucosa and submucosa. (Source: Endoscopic ultrasound courtesy of Victor Fox, M.D., Boston. Figure adapted from Fox et al.,3 with permission from the American Society for Gastrointestinal Endoscopy.)
Diagnosis
Eosinophilic esophagitis needs to be considered in patients with food impaction, or unexplained feeding refusal, esophageal symptoms, or dysphagia that has not responded to PPI administration. When characteristic histologic features are found in this setting and a patient responds to antiallergic treatment, the diagnosis is made.1, 3
However, exact diagnostic criteria applicable to all patients that can be used to compare different results from different centers are not yet available. One of the main problems in the literature is that the criterion to decide which number of eosinophils represents an excessive number varies from center to center. In general it is now accepted that the presence of more than 15 or 20 eosinophils/HPF is diagnostic.3
The second area of confusion is the tools used by different centers to decide that the eosinophilic infiltration is not secondary to GERD. Because GERD is much more common than eosinophilic esophagitis, and we have observed dense eosinophilia in patients with reflux esophagitis, it is our practice to treat all patients with significant esophageal eosinophilia with high-dose proton pump inhibition for 4 to 8 weeks. If symptoms persist, a repeat endoscopy is performed to assess the impact of PPI treatment. If eosinophilia persists, this provides confirmatory evidence of the diagnosis of eosinophilic esophagitis. Alternatively, pH monitoring of the distal esophagus is performed to assess for the absence of pathologic acid reflux.
Differential Diagnosis
When the clinician is faced with an esophageal mucosal biopsy that contains large numbers (more than 15 or 20) eosinophils per HPF), a number of other diseases need to be ruled out such as GERD, inflammatory bowel diseases, collagen vascular disease, drug hypersensitivity reaction, parasitic infection, malignant conditions, hypereosinophilic syndrome, and autoimmune diseases.47 The following are the main conditions that need to be considered in the differential diagnosis.
Reflux Esophagitis
The presence of eosinophils in the esophageal mucosa became identified with the presence of gastroesophageal reflux in the early 1980s.48 Those initial studies demonstrated that prolonged acid exposure of the esophageal mucosa correlated with the finding of intraepithelial eosinophils, and from that moment the presence of esophageal eosinophils was thought to be secondary to gastroesophageal reflux, in both adults and children. Most patients with peptic esophagitis respond to aggressive antireflux therapy. Generally patients with peptic esophagitis have less than five eosinophils per HPF, but we have observed children with abnormal pH monitoring with esophageal mucosa containing more than 20 eosinophils per HPF who have responded to PPI administration. Therefore, GERD needs to be excluded by providing aggressive antacid treatment, and if necessary with the performance of pH monitoring of the distal esophagus.
Eosinophilic Gastroenteritis
Eosinophilic infiltration of the esophagus can be part of eosinophilic gastroenteritis. This is a condition in which there is generalized eosinophilic infiltration throughout the GI tract, often accompanied by mild peripheral eosinophilia. In patients with eosinophilic esophagitis, the eosinophilic infiltration occurs only in the esophagus, and multiple studies have shown that biopsies from the antrum and duodenum are normal. In comparison, patients with eosinophilic gastroenteritis have demonstrated eosinophilic infiltration in other areas of the GI tract, particularly the antrum. Basic studies suggest that the chemokine eotaxin is critical in the pathogenesis of eosinophilic inflammation of the stomach and small intestine.
Esophageal Rings and Strictures
The presence of peptic esophageal strictures, congenital esophageal rings, and Schatzki's rings needs to be excluded as a cause for the symptoms. Radiographic and endoscopic studies may be needed.
Treatment
The initiation of the proper medical treatment of eosinophilic esophagitis requires detailed discussion with the patient and family. A number of treatments are effective in eliminating symptoms and reducing esophageal eosinophilia, each carrying its own risks and benefits and ease of compliance. Dietary elimination is safe and offers lifelong treatment, but compliance can be difficult. Topical steroids offer an easily administered alternative but carries potential side effects of esophageal candidiasis, and this treatment should not be used for prolonged periods.21, 27
There have been no placebo-controlled, blinded comparative studies to determine the optimal treatment for eosinophilic esophagitis. Regardless of the treatment implemented, it is our practice to perform an endoscopy after a course of treatment to ensure that the inflammatory pattern has resolved. This information allows one to proceed with either weaning of medication or reintroduction of hypoallergenic foods, and documents an effective treatment for the next episode of symptoms.
Dietary Management
Following diagnosis, every effort should be made to identify foods that are associated with symptoms and eosinophilic infiltration. Consultation with an allergist for evaluation, and RAST, skin prick, and potential patch testing are mandatory2, 18; if a food can be identified, strict elimination should follow. Some centers recommend that if food elimination leads to symptom resolution, positive foods should be added back at a rate of one new food every 5 to 7 days.18 If food restriction does not improve symptoms, exclusive use of an amino acid–based formula can be used and is effective at resolving clinicopathologic features.11 It has been suggested that the elemental diet should be used for 4 weeks, followed by the addition of individual foods every 5 to 7 days.18 Whenever diet restriction is implemented, it is mandatory to provide consultation with a dietitian/nutritionist to ensure that caloric, protein, and micronutrient requirements are met. Nutritional delivery by a nasogastric tube may be necessary in some patients.
Corticosteroids
Corticosteroids inhibit synthesis and secretion of cytokines known to influence eosinophil growth differentiation and activation. Children who are unresponsive to dietary restriction or who cannot tolerate dietary treatment clearly benefit from systemic and topical corticosteroids. Liacouras et al.30 reported 20 children with eosinophilic esophagitis who responded both clinically and histologically to 4 weeks of methylprednisolone. However, after withdrawal of the steroids, nine of the 20 patients had recurrence of symptoms over a 12-month period.
In an effort to reduce steroid exposure, the use of topical corticosteroids as an alternative treatment for eosinophilic esophagitis was first reported by Faubion et al.17 Fluticasone propionate and betamethasone meter dose inhalers were sprayed in the mouth of patients with eosinophilic esophagitis without a spacer. Patients did not inhale and then swallowed the actuated steroid, thus delivering a topical antiinflammatory product to the esophageal mucosa. Since then, several other studies reported successful treatment with topical steroids in over 70 children and adults.26, 27, 49 This treatment is particularly attractive because only 1% of the drug is absorbed systemically and it undergoes rapid hepatic processing. The main potential side effect is oral/esophageal Candida infection that developed in three of 20 patients in one series.27 In a retrospective series of 20 children, Noel et al.27 found that children with negative allergy testing responded to fluticasone, whereas those with identifiable allergies showed a partial response in 20% and no response in another 20%. The authors concluded that those patients with eosinophilic esophagitis with identifiable allergies who failed dietary elimination may have a blunted response to treatment. These findings are different from the prospective study from Teitelbaum et al.,26 in which all 13 children who completed the treatment with fluticasone had resolution of symptoms, even though they had failed dietary elimination.26 Recently Arora et al.49 described their retrospective experience with 21 adults treated with fluticasone. After 6 weeks, dysphagia resolved for a minimum of 4 months in all patients. No endoscopic data posttreatment were provided. Swallowed fluticasone is therefore an attractive alternative, as it does not involved unpalatable formulas, does not require nasogastric feedings, is effective, and has a good safety profile.
Endoscopic Treatment
Endoscopic food disimpaction and esophageal dilation may be necessary in some patients with eosinophilic esophagitis. Dilatation should be conservative, given the risk for perforation.1 Some authors have recommended beginning with small-diameter bougies.1 Given the propensity for mucosal tearing, it is recommended that after one or two bougies an endoscopic evaluation is necessary, to determine if it is safe to continue.1 This tearing can lead to significant pain and esophageal perforation.50, 51
It has been shown, however, that even in cases with a narrow esophagus and strictures, medical treatment should be attempted first. There are no prospective controlled studies compared dilatation with medical therapy, but in general dilatation should be reserved for those who have not responded to medical therapy. If a patient is egg allergic, one should avoid propofol (egg-based emulsion) sedation.
Potential Treatments
Leukotriene Inhibitors
Symptoms such as food impaction and dysphagia are likely related to smooth muscle contraction, making leukotriene inhibitors an attractive potential therapeutic agent. Montelukast treatment reduced symptoms but showed no impact on epithelial eosinophilia in one study.52 High doses (100 mg/d) of montelukast were required to reduce symptoms.
Anti-Interleukin-5 Antibody
Because basic studies determined a key role for IL-5 in the pathogenesis of eosinophilic esophagitis, the targeting of this molecule has gained interest. The use of mepolizumab, a humanized monoclonal blocking anti-IL-5 antibody, brought about significant reduction in symptoms and esophageal eosinophilia in an 18-year-old man with eosinophilic esophagitis.53
Long-Term Outcome
There is limited information available regarding long-term outcome. Straumann et al.19 followed 30 adult patients for up to 11.5 years and found that although the eosinophilic infiltration persisted in all symptomatic patients, the cell numbers decreased (but did not normalize) over time. The inflammatory process did not progress to the stomach or duodenum. No patient developed hypereosinophilic syndromes, malignancy, or a premalignant lesion. There was no impact on nutritional state. In almost two thirds there was no change in the severity of the dysphagia. One patient experienced a major negative effect on social and professional activities, whereas 15 patients had a minor impact from the dysphagia.
Future Questions
Immunohistochemical illuminations will bring more definition to this evolving picture. Do dendritic cells sample antigen from the esophageal lumen? Are there defects in the innate defense mechanisms that predispose the esophageal mucosa to an allergic response? What histologic features distinguish eosinophilic esophagitis from GERD? What is the role of environmental allergens? Is there any role for nonacid reflux in the pathogenesis of this disease? Could nonacid reflux of food particles induce an inflammatory response? Lastly, do proton pump inhibitors increase the incidence of allergic GI disease? Answers to these questions will bring more clarity to the diagnostician and insight into eosinophilic esophagitis's pathogenesis.
Criteria for the diagnosis will have to be better defined, and uniform diagnostic criteria need to be adopted, to allow comparisons between different centers. Given the fact that the response to treatment in this disease is ultimately evaluated by histology, and that repeated endoscopies are necessary, noninvasive methods to assess the status of the esophageal mucosa are needed. It is possible that in the future salivary, serum, or stool analysis for eosinophil mediators will be linked to esophageal inflammation, thus reducing the need for endoscopy and mucosal biopsy.
The exact treatment needs to be defined. Controlled trials are needed. What is the role that food elimination will play? When is it indicated to use steroids? After remission is obtained, how can it be maintained?
Finally, future decisions regarding treatment of eosinophilic esophagitis will be strongly influenced by the natural history of the disease. Patients are often asymptomatic. Thus, is it worth tolerating a severely restricted diet or utilizing toxic corticosteroids when daily life is not significantly affected? If natural history studies determine that a significant number of patients develop strictures or other complications of chronic inflammation, the answer will be yes. As such, if diet management is not acceptable or available, the development of an appropriate maintenance medication will become a high priority. Even though Barrett's esophagus has not been associated with eosinophilic esophagitis, what is the long-term effect of esophageal inflammation?
Taken together, human and murine studies demonstrate the immunologic potential of the esophagus. Future studies will focus on mechanisms that eosinophils impact esophageal function. Microarray analysis of affected tissues will begin to uncover differences between the allergic and nonallergic phenotypes. Which eosinophil-derived mediators impact esophageal motility? Why do eosinophils home specifically to the squamous epithelia as opposed to the columnar epithelium? The combination of in vitro, in vivo, and translational studies will give new insights into the development of novel treatments.