Key Points
- Esophageal manometry remains the gold standard for the assessment of esophageal motor activity. However, it is not a primary investigation and should be performed only when the diagnosis has not been achieved by careful history, barium radiology, or endoscopy.
- The most rewarding indication for esophageal manometry is dysphagia.
- There are two main types of manometric recording systems: perfused and solid state. Both have strengths and weaknesses, and the choice of any particular system depends on how these strengths and weaknesses are viewed.
- Optimal recording of either pharyngeal or esophageal motility requires an array of multiple recording points that span the whole region of interest in order to provide an integrated picture of motor function.
- Performance of accurate and high-fidelity manometric recordings requires a thorough understanding of how the manometric system operates as well as careful attention to technique. Poor-quality recordings inevitably lead to erroneous interpretation.
- The major elements of the analysis of pharyngoesophageal manometry are the degree of upper esophageal sphincter relaxation, the integrity of pharyngal peristalsis, and intrabolus pressure. The major elements of the analysis of esophageal motor function are the integrity of esophageal peristalsis and the degree of lower esophageal relaxation. A structured and systematic assessment of these elements should lead to a manometric diagnosis
- Achalasia, diffuse esophageal spasm, and nonspecific motor disorders have distinct manometric features.
Introduction
Disordered pharyngeal and esophageal motor function is a common cause of symptoms, particularly dysphagia, chest pain, and those associated with gastroesophageal reflux. Motor function can be assessed by a variety of recording techniques including radiology, scintigraphy manometry, and most recently intraluminal electrical impedance monitoring. Some of these are complementary. The gold standard, however, for the assessment of motor disorders remains manometry. Manometric measurement of esophageal pressure is the most direct method for assessment of motor function. Only manometry can give information on the strength of contractions. But manometry also has its disadvantages. In assessing the consequences of motility, the movement of intraluminal content is only by inference. However, when the diagnosis requires information about intraluminal flow, this can be obtained by complementary measurement of transit by radiology, scintigraphy, or intraluminal impedance monitoring.
Over recent years, esophageal manometry has become a remarkably sophisticated technique. Technical advances have led to the development of a variety of recording equipment and approaches to manometric measurements and their analysis. The development of powerful computerized acquisition systems, along with high-fidelity multichannel perfusion pumps and manometric catheters, has enabled high-resolution measurement and display of esophageal motility.
Performance of technically adequate manometric recordings and interpretation of the findings requires considerable background knowledge. There have been four technical reports on esophageal manometry.1, 2, 3, 4 This review describes the principal elements of the manometric system, the approaches to the measurements themselves, the clinical application of the techniques, and the manometric features of common pharyngeal and esophageal motor disorders.
Manometric Methods
Manometric Equipment
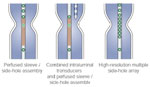
A number of options are available for the performance of esophageal manometry. All are capable of obtaining adequate recordings, and the choice of any particular system depends ultimately on how these strengths and weaknesses are viewed. There are two major choices: systems that depend on assemblies that incorporate intraluminal solid-state transducers, and those that use perfused assemblies connected to external transducers. Both methods when used properly give accurate measurements of esophageal pressures. However, only intraluminal transducers can record accurately the amplitude of pharyngeal pressures although the clinical value of these has yet to be demonstrated.
Perfused Manometric Systems
Perfused manometric systems rely on the transmission of the intraluminal pressures to external pressure transducers along manometric assemblies perfused usually with distilled water.5 They have a number of advantages over assemblies composed of intraluminal transducers. They can readily incorporate a large number (currently up to 21) of recording points in order to provide high-resolution pressure recordings.6, 7, 8 These recording points can be arranged in a wide variety of configurations that can be tailored to specific recording applications, for example, pharyngeal, esophageal, gastroduodenal, and anorectal motility. They can also incorporate specific sensors for sphincter manometry, for example, the sleeve sensor.9 Perfused assemblies are robust and, in particular, some may be autoclaved. In an era of infection control, this is important, as it is the only method of reliably cleaning the assembly. Last, perfused assemblies are substantially less expensive than solid-state assemblies, enabling a laboratory to purchase a range of assemblies for specific purposes.
Micromanometric assemblies 2 to 3 mm in diameter have been developed.10, 11, 12, 13, 14, 15 These are particularly suitable for recordings in neonates and children, but can also be used in adults in order to minimize the discomfort of the assembly.
Perfusion manometry also requires special equipment, such as a low-compliance perfusion pump.5 Although these pumps are robust and will last for many years, they require careful and regular maintenance. The pressure recordings are influenced by the fluid dynamics inherent in any perfusion system. They are subject to hydrostatic effects and differences in the resistance to flow among the manometric channels, both of which influence the actual pressures sensed by the transducer. Once these factors are understood, however, they need not be a handicap. Perfused point sensors, for example, side holes, can track a pressure rise of >300 mmHg.sec-1 , which exceeds the pressure rise rate generated by contractions in the esophageal body.16 Although they are unable to track accurately the extremely rapid pressure increases caused by pharyngeal contractions, they are able to record adequately the timing of these contractions, which is adequate for clinical purposes.
Intraluminal Transducers
Manometric assemblies can also be constructed that incorporate a series of miniature pressure transducers that sense intraluminal pressure directly. They avoid the need for a perfusion system. This is an advantage for ambulatory manometry, although ambulatory perfusion systems have been developed.17, 18 Intraluminal transducers also have a relatively small diameter, although this is less of an advantage with the advent of microperfusion assemblies. They have a substantial higher frequency response, >5000 Hz and therefore can record an almost simultaneous pressure rise. This capability, however, is necessary only for recording pharyngeal pressure wave amplitude. In the past, a limitation of intraluminal transducers was the smaller number of recording points that could be incorporated relative to perfused assemblies. However, recent developments have led to the construction of assemblies with up to 21 transducers.19
The major disadvantages of intraluminal transducers are cost, fragility, and difficulty of cleaning. The risk of transducer failure is relatively high and may substantially compromise the performance of the assembly. Importantly, in an era of infection control, they cannot be sterilized easily. Currently, there are no reliable sensors for accurate recording of sphincter relaxation. The only currently available sensor for this purpose, the sphinctometer, does not measure sphincter pressure directly, but rather an integral of pressure and length.20 However, the development of closely spaced (0.5- to 1-cm intervals) transducers may overcome this problem.
Recording Demands of Specific Regions
Differences in the anatomy and motor patterns of the various regions of the pharynx and esophagus impose differing demands on the method of recording motor activity.
Pharynx and Upper Esophageal Sphincter
Pharyngeal manometry The major demand of pharyngeal manometry is related to the nature of the pharyngeal musculature. The pharynx is composed of striated muscle with an extremely rapid rate of contraction. It can produce pressure waves with a pressure rise rates up to 600 mmHg.sec-1.21, 22 Such rise rates are beyond the fidelity of perfused systems and require intraluminal transducers for accurate recording of wave amplitude, although as yet there is no clinical need for such accuracy. Perfusion manometry, however, can detect accurately the occurrence and onset of pharyngeal pressure waves as well as the intrabolus pressure, which is a measure of the obstruction to flow across the pharyngoesophageal junction during swallowing.
Pharyngeal pressure recording is commonly performed during esophageal manometry in order to detect swallowing. For this purpose, an air-perfused side-hole sensor will suffice. Typically air perfusion rates of around 2 to 8 mL.min-1 are required. Air perfusion is particularly useful during prolonged esophageal manometry in order to minimize the effects of the perfusate on lower esophageal sphincter (LES) function.11
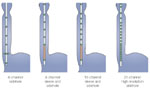
Upper esophageal sphincter The upper esophageal sphincter (UES) has several attributes that impose special demands on recording techniques. First, the anatomic structure of the UES, with the sling-like attachment to the cricoid cartilage, results in substantial radial asymmetry with pressure values in the anteroposterior directions that are considerably higher than in the lateral direction. Thus the sensors have to be oriented in the anteroposterior direction in order to assess the maximal UES pressure. This can only be achieved with an assembly that has a flattened cross section. Second, the high-pressure zone of the sphincter is narrow; the segment with pressure <50% of the maximal is only 1 cm in length.23 The UES is also mobile, making approximately a 0.5-cm excursion during swallowing. This movement will displace a focal sensor from the high pressure zone. Although sample measurements can be made by pulling a focal sensor through the sphincter, such movement of the assembly irritates the pharynx and artifactually increases UES pressure.
Accurate recording of UES pressure, therefore, requires a sensor that is tolerant of sphincteric movement. There are two options available: the perfused sleeve sensor and an array of closely spaced focal sensors, either perfused side holes or intraluminal transducers. Specially constructed sleeve sensors with a flattened cross section have been developed.24 They can record basal UES pressure for prolonged periods and record the degree of UES relaxation with swallowing accurately. Sleeve sensors have been criticized as being unsuitable for UES manometry because they artifactually shorten the duration of UES relaxation. This shortening occurs because the sleeve projects into the pharynx, and the recording of UES relaxation is terminated prematurely by the oncoming pharyngeal peristaltic wave. However, the actual degree of shortening of UES relaxation is small relative to the total duration of relaxation, and appropriate normal values for UES relaxation can be derived for the sleeve sensor. Although an array of closely spaced (0.5-mm intervals) focal sensors can also theoretically tolerate a degree of sphincteric movement, as yet no normal values for this approach have been published. Moreover, the use of multiple side holes increases the amount of water to the pharynx, which may be a problem in patients with poor pharyngeal clearance.
Options for pharyngoesophageal manometry are depicted in Figure 1. The characteristic pattern of pharyngeal peristalsis and UES relaxation are illustrated in Figure 2.
Esophageal Body
Motility of the esophageal body is characterized by peristalsis—a propagated wave of contraction that sweeps down the esophagus at a rate of 2 to 4 cm.sec-1 (Figure 3). Although peristalsis might appear to be a single continuous sequence, recent analyses with high-resolution manometry indicate that it is actually composed of four discrete pressure segments coordinated into what appears to be a smooth uninterrupted sequence.25
Figure 3: Normal esophageal motility.
Each swallow, as detected by the pharyngeal channel, is followed by a propagated sequence of pressure waves that traverses the entire esophageal body. Lower esophageal sphincter (LES) relaxation occurs with each swallow.
The success of peristalsis is dependent on the integrity of propagation and the wave amplitude of individual sequences. Adequate recording requires an array of sensors along the entire esophageal body in order to map the entire peristaltic sequence. The minimum spacing required to avoid missing focal defects appears to be around 4 cm, therefore necessitating seven recording points.8 Although the esophagus can be mapped by moving a smaller array of sensors stepwise up the esophageal body,26 this is cumbersome, uncomfortable for the patient, and does not provide a complete display of individual responses.
Recently, high-resolution esophageal manometry, using a larger array of more closely spaced (1–1.5 cm intervals) has been developed.8, 27 This allows manometric data and thereby patterns of motility to be displayed topographically, either as a color or contour plot (Figure 4). This approach has yielded useful insights into the physiology of esophageal motility. Topographic displays are visually easier to interpret than conventional line plots. However, it has yet to be demonstrated that this approach provides greater diagnostic accuracy than a more conventional array with 4-cm intervals.8
Figure 4: An example of a normal esophageal response to a water swallow displayed as a conventional line plot (left panel) and as a colored topographical plot (right panel).
In this example, lower esophageal sphincter (LES) pressure was recorded with a series of side-hole sensors that straddled the LES segment. In the topographic display, each pressure point has been converted into a color (see pressure color chart at right). The pressure profile between actual esophageal recording points has been calculated by interpolation in order to provide for a continuous display. (See refs. 8 and 27 for further description of the method.)
Options for manometric assemblies to record esophageal motility are illustrated in Figure 5. The choice of assembly is determined by the number of recording channels in the system. The more recording sites, the greater detail about esophageal motor function will be obtained.
Lower Esophageal Sphincter
Measurement of LES function involves assessment of basal pressure and relaxation. Of these, relaxation is the more important; basal pressure is of limited diagnostic value. As with UES manometry, mobility relative to the recording assembly due to peristalsis-related esophageal shortening28 and radial asymmetry29 impose special demands on measurement of LES pressure. The clinical and functional significance of radial asymmetry is unclear and overestimated. Although it influences the recorded value for basal pressure, it does not appear to influence recorded patterns of LES relaxation.
The most important aspect of LES pressure measurement is that of LES relaxation. Incomplete relaxation is the principal diagnostic feature of achalasia. A sleeve sensor effectively records both basal pressure and relaxation. As with the UES, projection of the sleeve into the esophagus leads to artifactual premature termination of LES relaxation through occlusion of the sleeve by the oncoming peristaltic pressure wave. However, the degree of shortening of LES relaxation is small and is not a problem if appropriate control values are available.30, 31 A single focal sensor tends to overestimate the degree and duration of LES relaxation because of displacement of the sensor into the stomach during peristalsis-associated esophageal shortening. However, an array of closely spaced (0.5-cm) intervals is an acceptable alternative. A recording sensor in the stomach is necessary to provide a reference intragastric pressure.
Recording Devices
All modern manometric recording systems rely on computers for data acquisition display and analysis. They all share common elements. However, there are substantial differences among systems with respect to the user-friendliness of the software, the "appearance" of the display, and the capacity for manual as well as computerized analysis. In this author's view, current computerized analysis of pressure recordings is suboptimal and should not be relied on entirely for the assessment of the study. It is essential that the person analyzing the manometric study, do so on the basis of careful perusal of the actual recording. However, the computer software can be a valuable adjunct to manual analysis, and the availability of "tools" within the software that allow for manual analysis is essential. Overall, the technical adequacy of systems currently offered by established suppliers is comparable, and probably the most important considerations that should determine the choice of a system are the user-friendliness of the software and the level of technical support offered by the supplier.
Manometric Technique
The performance of accurate and high-fidelity perfusion manometric recordings depends on paying adequate attention to a number of details. Equipment should be properly cleaned and well maintained. Perfusion systems are susceptible to accumulation of debris and colonization by bacteria,32 which can block the minute lumina of the flow-resistors and manometric assemblies. Care should be taken with the calibration of the system.
A thorough understanding of the factors that contribute to the recorded pressure is required. The pressure registered by the transducers is a combination of the luminal pressure, the perfusion resistance (the pressure required to push the perfusate down the assembly), and the hydrostatic pressure differences between the transducer and the patient. Changes to flow in an individual recording channel because of a blocked resistor or manometric lumen will artifactually alter the recorded pressure. Hydrostatic pressure is effectively negated if studies are performed with the subject recumbent, as it will be the same for all pressure channels. However, if recordings are made with the subject upright, then appropriate recording baselines need to be determined with the assembly in a vertical position. At the start and end of the study it is helpful to record the atmospheric zero with the transducers open to atmosphere, and the perfusion baseline with the assembly attached and perfused. This process enables the detection of any changes in baseline pressures related to performance of the perfusion system and for appropriate adjustments to be made during analysis.
Pharynx and Upper Esophageal Sphincter
Manometric assessment of the pharynx and UES is best performed with the patient sitting. Monitoring of UES pressure requires a dedicated manometric assembly with either a specially designed sleeve sensor or an array of closely spaced side holes. The sleeve should be positioned such that its proximal margin is approximately 2 cm above the proximal margin of the UES. This can be facilitated by having a side hole at the back of the sleeve, positioned 2 cm distal to the proximal margin of the sleeve, which can be positioned at the midpoint of the UES. This sleeve position minimizes the length of the sleeve exposed to the pharynx but ensures that the sleeve remains within the UES at its maximal oral excursion during swallowing.
Basal UES pressure should be recorded for around 2 to 3 minutes with the subject sitting quietly in a chair. In this circumstance there is minimal extraneous influence on UES pressure.
Upper esophageal sphincter relaxation is assessed in response to water swallows. In patients with pharyngeal dysfunction, it may be helpful to turn off the perfusion pump between swallows to minimize the amount of perfusate delivered to the pharynx. The standard bolus volume is 5 mL. In patients with incomplete UES relaxation or opening, such as that associated with a cricopharyngeal bar or Zenker's diverticulum, the pressure required to push the bolus through the UES is increased.33 This pressure is transmitted to the sleeve and may give the appearance of incomplete relaxation. In such circumstances, use of a smaller bolus volume such as 1 or 2 mL may be useful. The number of swallows needed to assess UES relaxation adequately has not been determined, but at least five at each bolus volume tested would seem appropriate.
Esophageal Body and Lower Esophageal Sphincter
As esophageal motor function involves coordination of both esophageal body peristalsis and LES relaxation, it is both logical and convenient to assess these activities concurrently. This requires a manometric assembly with an array of recording sites at 4-cm intervals or less along the esophageal body and either a sleeve sensor or an array of closely spaced side holes straddling the gastroesophageal junction. A swallow marker should be used to accurately record the timing of swallowing as well as the occurrence of second swallows (which may cause deglutitive inhibition), and to provide evidence of failed peristalsis. This is most readily and possibly most accurately achieved using a perfused pharyngeal side-hole sensor. However, adequate results can be obtained from a sensor belt or microphone on the neck or with submental electromyographic recordings. If LES pressure is to be monitored continuously, rather than by a pull-through technique, a side hole in the stomach is required to provide a gastric reference pressure.
Studies are typically performed with the subject recumbent; the lateral recumbent position is more comfortable for the patient. Peristalsis in the upright position is more variable.34
Basal LES pressure can be assessed either by performing a "pull-through" of a focal sensor or by making a short 3- to 5-minute continuous recording of LES pressure using a sleeve sensor or side-hole array. Continuous recordings have several advantages over pull-though techniques: they provide a more representative sample of basal LES pressure, which varies from minute to minute, and they are more comfortable for the patient. The use of radial array of focal sensors to create a radial profile of the LES during a pull-through maneuver has been proposed. Computer software can use these data to create a three-dimensional vector plot.35, 36 However, this adds little clinically useful information to that gained from either a single sensor.
Peristalsis and LES relaxation are normally assessed in response to 5-mL water swallows. Dry swallows are more commonly associated with nonperistaltic responses37, 38 and shorter and less complete LES relaxation.30 Because peristaltic responses to swallows vary within subjects, and because peristaltic abnormalities may be intermittent, at least 10 swallows should be tested to provide an adequate sample.39, 40 The swallows should be spaced at least 15 seconds apart to avoid disturbance of the peristaltic response by the adjacent swallow.41, 42 Some studies have assessed the value of assessing esophageal motility in response to swallowing solid boluses.43, 44, 45 Solid boluses may reproduce symptoms such as dysphagia and chest pain. However, as yet only qualitative information can be obtained from this approach as there has been no standardisation of this technique.
Manometric Analysis
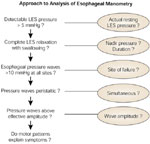
Analysis of manometric recordings is relatively straightforward so long as certain basic principles are followed. Manometric diagnoses are established primarily by assessment of the integrity of peristalsis and UES and LES relaxation. Basal sphincter pressure is of relatively little diagnostic importance.
Pharynx and Upper Esophageal Sphincter
The major elements of the analysis of pharyngoesophageal manometry are assessment of UES relaxation, the integrity of pharyngeal peristalsis, and intrabolus pressure (Figure 6). All these goals can be achieved using a perfused manometric system. The use of miniature intraluminal transducers to measure pharyngeal peristalsis is probably not justified for routine clinical use. Adequate clinically useful information about pharyngeal motor function can be obtained from high-quality videofluoroscopic examination.
Recorded values for pharyngeal peristaltic amplitude are highly dependent on the type of transducer used and its radial orientation within the pharynx. Using radially sensitive intraluminal transducers, wave amplitudes are generally within the range of 100 to 150 mmHg.46 Wave amplitudes recorded by perfused sensors are substantially lower (30 to 60 mmHg) but are sufficient to determine the integrity of propagation.
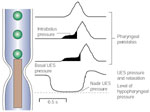
An important element of pharyngeal pressure recordings is the intrabolus pressure. This is the pressure imposed on the bolus by the pharyngeal pressure wave as the bolus is pushed through the relatively narrow UES opening.47 This pressure manifests as a relatively low-level (8 to 10 mmHg) increase in basal pharyngeal pressure ahead of the pharyngeal wave. A major determinant of intrabolus pressure is the resistance at the UES during relaxation and opening.48 Impairment of opening, for example, cricopharyngeal bars33 or Zenker's diverticulum,49 or relaxation, for example, cricopharyngeal achalasia,50 will increase intrabolus pressure (Figure 7). Intrabolus pressure is also directly related to bolus volume. Increasing the volume of the swallowed bolus to 10 or 20 mL can amplify the magnitude of the intrabolus pressure, particularly in circumstances where UES opening is impaired.
Figure 7: Recording of pharyngoesophageal motility in a patient with dysphagia due to a cricopharyngeal bar.
Responses to 1- and 5-mL water boluses are shown. Note that with the smaller 1-mL bolus, UES relaxation is complete and there is only a small intrabolus pressure (shaded). With the larger 5-mL bolus, UES relaxation appears to be incomplete. However, this is an artefact due to the effects of the higher intrabolus pressure on the sleeve recording.
Upper esophageal sphincter pressure is referenced to hyopharyngeal pressure. Basal UES pressure is highly variable and influenced considerably by the state of wakefulness, emotional stress, and respiration. Values obtained by the pull-through technique are higher than those obtained by continuous recording because of the irritation caused by the pull-through. Values are also directly related to the diameter of the assembly.14 Thus normal values for basal UES pressure vary widely, and it is not possible to define a meaningful normal range. However, because basal pressure is not essential for clinical diagnosis, this variation is not a major obstacle to the interpretation of pharyngoesophageal manometric recordings.
The most critical element of the analysis of pharyngeal manometry is the assessment of UES relaxation and its timing in relation to pharyngeal peristalsis. Upper esophageal sphincter relaxation starts as the pharyngeal peristaltic wave sweeps through the hypopharynx. Relaxation is usually complete with nadir pressures of approximately 1 to 2 mmHg above hypopharyngeal pressure.23, 51 Upper esophageal sphincter relaxation extends until the arrival of the pharyngeal peristatic wave. The duration of relaxation is approximately 0.4 to 0.5 seconds.23, 47, 51
Esophagus and Lower Esophageal Sphincter
Evaluation of esophageal motor function involves determination of esophageal body and LES function. Structured assessment of these elements should lead to a manometric diagnosis (Figure 8).
Esophageal Peristalsis
Esophageal body motor function is judged by the amplitude and propagation of the pressure waves. From these two variables, the presence and success rate of peristalsis can be determined (Figure 9). For reasons discussed above, only responses to single swallows separated from adjacent swallows by at least 15 seconds should be analyzed. Peristaltic wave amplitude is measured from the end-expiratory pressure to the peak of the pressure wave. Propagation is determined by the timing of the major upstroke of the pressure wave, which has been shown by videofluoroscopy to correlate with lumen occlusion.52
Wave amplitude is a major determinant of esophageal clearance. A minimum wave amplitude of 30 mmHg in the distal esophagus and 12 mmHg in the proximal esophagus is required for consistently effective clearance.52, 53 Wave amplitudes <30 mmHg are generally scored as hypotensive. Wave amplitudes <10 mmHg are usually scored as failed, as they are usually not associated with lumen occlusion. Wave amplitudes <180 mmHg are termed hypertensive. However, the significance of such waves is unclear.
Esophageal pressure waves are typically single-peaked, although double-peaked waves are not uncommon. Waves with three or more peaks, however, are rare and generally judged to be abnormal.37 By convention, a pressure wave is scored as multipeaked if the drop in pressure between the peaks is  10 mmHg less than, and occurs
10 mmHg less than, and occurs  1 second after, the preceding peak.54 The duration of pressure waves is normally <7 seconds.37 Prolonged, often multipeaked, pressure waves have been described in patients with chest pain.55
1 second after, the preceding peak.54 The duration of pressure waves is normally <7 seconds.37 Prolonged, often multipeaked, pressure waves have been described in patients with chest pain.55
Propagation velocity is determined by the timing of the initial upstroke at adjacent recording points and the distance between these points. Normal peristalsis propagates at a rate of 2 to 4 cm.s-1. Propagation rates >6 cm.s-1 are associated with poor esophageal clearance.56 The propagation rate at which pressure waves are deemed to be simultaneous has not been defined, but the value of 6.25 cm.s-1 has been suggested.1 However, whether this value is consistent with earlier definitions of manometric disorders characterized by the presence of excessive simultaneous contractions is unclear.
Simultaneous pressure waves can be classified into isobaric and nonisobaric patterns. Isobaric pressure waves, usually of low amplitude, have similar amplitudes and configurations and are recorded from adjacent sites within a single open-lumened esophageal segment. Nonisobaric pressure waves have different amplitudes and configurations. This is an important distinction, as the mechanisms and implications of the two patterns are different. Nonisobaric pressure waves represent true simultaneous lumen-occluding contractions at different sites, whereas isobaric pressure waves represent nonlumen occluding contractions.57
Lower Esophageal Sphincter
Analysis of LES function entails determination of its axial location, basal pressure, and degree of relaxation. The axial location of the LES is measured from the nares. The proximal margin of the LES is defined as the distance at which intraesophageal (as opposed to intragastric or intrasphincteric) pressure is first recorded. This distance is needed for positioning of traditional wired esophageal pH electrodes.58, 59, 60
Lower esophageal sphincter pressure is measured relative to gastric pressure. When using a pull-through technique, the gastric pressure at the start of the pull-through is used. When using continuous sphincter pressure monitoring with a sleeve sensor or side-hole array, gastric pressure is monitored concurrently and LES pressure referenced to intragastric pressure at that moment. Traditionally, LES pressure is measured at end-expiration at which time the effects of the crural diaphragm on pressure measured within the LES are minimal. End-expiration can be identified from the changes in either esophageal or gastric pressure induced by respiration. However, some manometrists use mean pressure throughout the respiratory cycle, as this can more readily be determined by some computer-based systems.31 Lower esophageal sphincter pressures measured at end expiration are lower than those measured as mean pressures and therefore require different normal values. Normal values for each method have been published.1, 30, 31, 61 Lower esophageal sphincter pressure is referenced to intragastric pressure. Usually several samples are taken with the pull-though technique, whereas with continuous monitoring an average is taken over a 3- to 5-minute interval, excluding swallow-induced relaxations and contractions. Basal LES pressure can vary widely within individuals and according to the method of recording and analysis. Consequently, normal values also vary. However, it is generally accepted that basal LES pressures <5 mmHg are abnormal.
Assessment of LES relaxation is the most important meaningful aspect of LES function. Lower esophageal sphincter relaxation is measured as the residual or nadir pressure. This can be expressed as either a proportion or percentage of basal pressure or as an absolute value. The former is influenced by the level of basal pressure, and patients with very high basal pressures may have normal percentage LES relaxation but an abnormally high nadir pressure. As the nadir pressure is a determinant of flow across the esophagogastric junction, it is more accurate to express LES relaxation in this manner. The measured nadir pressure will also be influenced by the method of analysis, whether at end expiration or as a mean pressure. Normal values for both approaches have been published.30, 31, 61 Normal LES relaxation is commonly believed to be "complete." However, it is usual for there to be a small nadir pressure of up to 7 mmHg even at maximal relaxation.30 With a sleeve sensor, the duration of LES relaxation is usually <8 seconds.30
Clinical Applications
Clinical Indications
Esophageal manometry is indicated in patients whose symptoms and other investigations, for example, endoscopy or radiologic studies, suggest a motor disorder. It is not a primary investigation and should be performed only when the diagnosis has not been achieved by careful history, barium radiology, or endoscopy.
Dysphagia is the most rewarding indication for manometry. Dysphagia associated with motor disorders is typically intermittent, variable, and associated with both liquids and solids, although it is commonly worse with solids. There is a clear indication for manometry in patients with a normal endoscopy who have consistent dysphagia with solids if there is associated regurgitation or weight loss or if there is significant impairment of lifestyle. The likelihood of finding a major motor disorder such as achalasia or diffuse spasm, which may be amenable to treatment, is relatively high. However, not all dysphagia needs to be tested by manometry. Mild intermittent dysphagia due to weak esophageal contractions is relatively common, particularly in patients with gastroesophageal reflux disease. Investigating these patients is usually not indicated except in patients who are anxious to have this investigated as far as possible or perhaps in patients undergoing antireflux surgery.
Noncardiac chest pain is a common reason for referral for manometric studies. However, the diagnostic yield is relatively low, particularly when dysphagia is absent. Achalasia and diffuse spasm account for less than 5% of patients. The clinical significance of other nonspecific patterns of abnormal esophageal motility is unclear.
Gastroesophageal reflux disease: Esophageal manometry has a limited application in patients with reflux disease. There are no diagnostic manometric features. Manometry is useful to define the location of the LES for the purposes of positioning a pH electrode for ambulatory pH monitoring. Manometry is also commonly performed before antireflux surgery to exclude other diagnoses such as achalasia, which may present with reflux-type symptoms, and to exclude major weaknesses in esophageal peristalsis, which may compromise the surgical outcome. Current evidence would suggest that routine preoperative manometry in patients with clearly defined reflux disease is not justified. However, it is reasonable to assess patients suspected of having severely compromised esophageal motor function, such as those with symptoms to suggest scleroderma or with a dilated or poorly motile esophagus on barium swallow.
Relationship to Other Diagnostic Tests
Manometric studies are usually second- or third-line investigations following initial assessment by radiology and endoscopy. The relationship of manometry to other investigations in the assessment of oropharyngeal and esophageal dysphagia has been discussed in technical reviews from the American Gastroenterological Association.2, 4
Radiology
In patients with dysphagia, a careful video-barium swallow is the investigation of first choice, particularly in patients with an oropharyngeal pattern of dysphagia.62, 63 The initial diagnostic concern is whether the patient has a structural abnormality or a motility disorder. Although endoscopy is a sensitive investigation for significant strictures, rings, webs, and esophagitis, minor Schatzki rings, muscular rings, and disordered motility may be missed. Inclusion of solid boluses and views in the prone-oblique posture increase the sensitivity of the radiologic examination. Radiology and manometry are complementary investigations in the assessment of UES function, and manometry should not be used as a stand-alone test. Radiology provides information on UES opening, whereas manometry measures UES relaxation. For example, defective UES relaxation may be missed by radiology because effective pharyngeal peristalsis is able to force the UES open by generating a sufficiently high intrabolus pressure. Conversely, inadequate UES opening in a patient with a cricopharyngeal bar33 or Zenker's diverticulum49 may be radiologically misinterpreted as abnormal UES relaxation.
Endoscopy
Endoscopy is necessary to fully exclude inflammatory or structural lesions, and should normally precede manometric evaluation. In patients with oropharyngeal dysphagia, formal examination by an ear, nose, and throat (ENT) specialist may be required.
Scintigraphy
Radionuclide assessment of esophageal transit is a useful screening tool for esophageal dysmotility in circumstances where manometry is not readily available.64, 65, 66 It is sensitive for the detection of major motor abnormalities such as achalasia and diffuse spasm, which produce characteristic transit patterns. Although it may miss nonspecific abnormalities such as ineffective or hypertensive peristalsis and isolated abnormalities of lower esophageal sphincter function, the clinical significance of these is questionable.
Intraluminal Electrical Impedance Monitoring
Measurement of intraluminal electrical impedance is the most recently introduced technique for the assessment of esophageal motility. In contrast to manometry, it assesses the movement of intraluminal content. Impedance measurement can be combined with either solid state or perfusion manometry,67, 68 and it provides a functional perspective by providing information on the effectiveness of esophageal clearance associated with the underlying motor activity.53 However, the place of impedance monitoring in routine manometric testing has yet to be established.
Manometric Features of Common Disorders
Particular symptom patterns can be produced by different esophageal motor disorders or other disease. Usually, referral for manometry is for evaluation of symptoms rather than the evaluation of a single diagnostic possibility. However, it is easier to discuss the diagnostic value of manometry by disease state.
Pharyngeal Motor Disorders
Disorders of pharyngeal motor function may involve pharynx or upper esophageal sphincter. Pharyngeal motility is most effectively assessed radiologically, and manometry is most useful to clarify upper esophageal sphincter function.
Cricopharyngeal Bar
Cricopharyngeal bars are characterized radiologically by a prominent indentation on lateral views of the pharynx. They are commonly attributed to spasm or failed relaxation of the cricopharyngeus and thus described as "cricopharyngeal achalasia." However, careful manometric evaluation has shown that UES relaxation is normal and that the abnormality is one of impaired opening,33 which can be detected manometrically by an increased intrabolus pressure (Figure 7). Histologic examination of the cricopharyngeus from patients with cricopharyngeal bars has shown muscle fiber degeneration and fibrosis69 similar to that seen in patients with Zenker's diverticulum.49 Cricopharyngeal bars are usually asymptomatic. However, they may cause dysphagia if the degree of opening is sufficiently limited, and myotomy has been shown to relieve dysphagia in some patients.69
Cricopharyngeal Achalasia
True cricopharyngeal achalasia associated with impaired UES relaxation is uncommon. It is usually seen with lesions affecting the medullary swallowing center, for example, stroke and head injury, and is usually associated with abnormalities of pharyngeal propulsion and airway protection. Abnormal UES relaxation has also been reported in patients with Parkinson's disease.50
Zenker's Diverticulum
Zenker's diverticulum is primarily a radiologic diagnosis; manometry is diagnostically noncontributory. Premature UES relaxation, premature sphincter closure, and delayed UES relaxation have been variably described.70 However, such observations were made using inadequate manometric methodology. Studies have shown that Zenker's diverticulum is a pulsion diverticulum as a result of impaired UES opening; relaxation is normal.49 Histologic examination of the cricopharyngeus has shown myopathic changes and fibrosis consistent with a loss of muscle elasticity.
Esophageal Motor Disorders
Esophageal motor disorders can be characterised manometrically by abnormalities of LES or esophageal body motor function or both. A variety of abnormalities are encountered in patients with esophageal symptoms. Based on these patterns a number of classifications have been proposed,71 some relatively complex.72 However, only two disorders, achalasia and diffuse esophageal spasm, have sufficiently characteristic patterns and clinical features to be considered as distinct motor disorders. The remainder consist of nonspecific abnormalities whose relationship to the patient's symptoms and thereby the clinical relevance are often unclear.
Achalasia
Achalasia is characterised manometrically by two cardinal features: incomplete LES relaxation and absent peristalsis (Figure 10). Additional features that are often encountered but that are not in themselves diagnostic are an increased basal intraesophageal pressure that is higher than intragastric pressure, and an increased basal LES pressure. Variations in the manometric features occur between patients, which makes manometric diagnosis difficult,73 and manometric findings should always be considered in the context of the clinical radiologic and endoscopic findings.
Figure 10: Representative recording of esophageal motility from a patient with achalasia.
Note the following features: (1) incomplete LES relaxation with swallowing, (2) absent peristalsis—only low-amplitude spontaneous activity is present, and (3) intraesophageal pressure that is higher than intragastric pressure.
Impaired LES relaxation is functionally the most important abnormality. It may be difficult to pass the manometric assembly through the esophagogastric junction. The nadir pressure threshold that defines incomplete relaxation depends on the method of analysis of LES pressure, and whether end-expiration, midexpiration, or average LES pressure is used. Typically there is partial relaxation. Occasionally relaxation may appear to be complete.73, 74 However, in such instances careful inspection of the LES recording usually reveals subtle abnormalities such as a delayed onset and shortened duration of relaxation, and relaxation appears to be functionally inadequate.61, 75 Basal LES pressure may be increased in about 50% of patients.
Aperistalsis is the other cardinal feature of achalasia. Classically, the esophageal body is characterized by only low-amplitude (<30 mmHg) simultaneous isobaric pressure waves. A few patients have wave amplitudes within or above the normal range. Although this pattern has been termed "vigorous" achalasia, clinically these patients do not differ substantially from those with more classical manometric features,76 and management is the same. Occasionally, the pattern of esophageal motility may mimic a diffuse spasm, and overlap between achalasia and diffuse spasm has been reported.77 The aperistaltic segment usually involves the entire esophageal body. However, partial preservation of peristalsis may be present in the proximal esophagus.
Basal intraesophageal pressure may be greater than intragastric pressure, leading to an increased esophageal-gastric pressure gradient.73 This is a consequence of esophageal retention and often disappears if the patient regurgitates retained esophageal contents or excess fluid is aspirated from the esophagus during the manometry.
Idiopathic achalasia must also be distinguished from pseudoachalasia.78, 79 Manometrically the features are the same and the diagnosis depends on clinical, radiologic, and endoscopic features. Achalasia is not the only cause of aperistalsis. Severe peristaltic failure and complete aperistalsis may also be seen in patients with connective issue diseases such as scleroderma, amyloidosis, and diabetes mellitus, and in reflux disease. However, in such patients, basal LES pressure is typically very low, LES relaxation is complete, and there is no increase in basal intraesophageal pressure.
Diffuse Esophageal Spasm
In contrast to achalasia, the manometric criteria for diffuse esophageal spasm are not well defined. The essential feature is that of excessive proportion of simultaneous pressure waves in the presence of peristalsis (Figure 11). A low rate of simultaneous pressure waves occurs in normal subjects, and the presence of simultaneous pressure waves after  20% of water swallows are required for the diagnosis of diffuse spasm.71, 80 However, if all of the pressure waves are simultaneous, the diagnosis of achalasia should be considered. Currently, wave amplitude is not a component of the diagnosis, although contractions that are symptomatic tend to be of higher amplitude and repetitive.81 However, low-amplitude simultaneous pressure waves, seen in disorders such as scleroderma, reflux disease, amyloidosis, pseudo-obstruction, and diabetes, and that are a consequence of non–lumen-occluding contractions, should not be interpreted as diffuse spasm. Other manometric findings less consistently found include prolonged pressure waves, repetitive peaks (three or more), spontaneous pressure waves, and increased basal LES pressure and incomplete LES relaxation.82, 83
20% of water swallows are required for the diagnosis of diffuse spasm.71, 80 However, if all of the pressure waves are simultaneous, the diagnosis of achalasia should be considered. Currently, wave amplitude is not a component of the diagnosis, although contractions that are symptomatic tend to be of higher amplitude and repetitive.81 However, low-amplitude simultaneous pressure waves, seen in disorders such as scleroderma, reflux disease, amyloidosis, pseudo-obstruction, and diabetes, and that are a consequence of non–lumen-occluding contractions, should not be interpreted as diffuse spasm. Other manometric findings less consistently found include prolonged pressure waves, repetitive peaks (three or more), spontaneous pressure waves, and increased basal LES pressure and incomplete LES relaxation.82, 83
Nonspecific Motor Disorders
The majority of abnormal patterns of esophageal motility do not meet the criteria for a motility disorder and generally are classified as nonspecific. This is the largest group of manometric disorders and includes abnormalities of both LES and esophageal body function.
Ineffective motility is characterized by an increased proportion (>30%) of low-amplitude (<30 mmHg) pressure waves in the distal esophagus.84 Commonly this may be associated with failed peristalsis (Figure 12). Functionally, this pattern is associated with impairment of esophageal clearance.53 Many patients with ineffective peristalsis have reflux disease, although it is not diagnostic of this condition.
Figure 12: Representative recording of esophageal motility from a patient with a nonspecific esophageal motor disorder.
In this instance, the motor pattern is one of generally low-amplitude pressure waves consistent with "ineffective" peristalsis.
Hypertensive peristalsis is characterized by a mean wave amplitude of >180 mmHg in the distal esophagus.85 Basal LES pressure may also be increased. The clinical significance of this motor pattern is unclear. Although it appears to be overrepresented in patients with noncardiac chest pain, the relationship between the high-amplitude pressure waves and chest pain remains uncertain.
Isolated incomplete LES relaxation: Occasionally, incomplete LES relaxation is seen in the presence of peristalsis86 (Figure 13). Often it is associated with increased basal LES pressure and a hypercontracting distal esophagus. Inspection of the esophageal body recording usually reveals an exaggerated pressure ramp ahead of the oncoming peristaltic wave as a result of the increased resistance to flow across the incompletely relaxed LES. Although, in some cases, it might represent a forme fruste of achalasia,73, 77 the long-term outcome is usually benign.
Figure 13: Representative recording of esophageal motility from a patient with an isolated abnormality of lower esophageal sphincter (LES) relaxation.
Basal LES pressure is relatively high. Although LES relaxation clearly occurs with swallowing, there is an abnormally high nadir pressure. This results in an increased pressure "ramp" in the distal esophagus cause by increased resistance to the flow of the bolus across the incompletely relaxed LES. Peristalsis is preserved, thereby effectively excluding achalasia. A similar effect can be seen after fundoplication.
Scleroderma and other connective tissue diseases are commonly associated with disordered esophageal motility. The characteristic abnormalities are low to absent basal LES pressure and weak ineffective pressure waves in the smooth muscle segment of the esophagus (Figure 14). Although the abnormalities are more commonly seen in scleroderma, they are not unique to this disorder and can also be found in other connective tissue diseases such as mixed connective tissue disease, rheumatoid arthritis, and systemic lupus erythematosus.87, 88, 89, 91
Other diseases: Disordered esophageal motility has been identified in other systemic disorders, including diabetes mellitus,92, 93, 94 Parkinson's disease,95 chronic alcoholism,96, 97 muscular dystrophies,98 inflammatory myopathies,99 and amyloidosis.100, 101
Conclusion
High-quality esophageal manometry requires a number of important considerations. The operator should have a thorough understanding of how the manometric recording system operates, what factors impair its performance, and how to recognize and correct any problems. Appropriate care and attention should be paid to the details that underlie high-quality recordings so that the best interpretation can be made. The recordings should be analyzed systematically, and interpreted and reported in the context of the clinical problem.



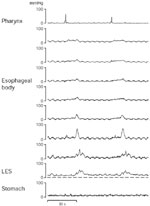
![Figure 2 : Normal upper esophageal sphincter (UES) relaxation recorded by a sleeve|[ndash]|side-hole assembly. Unfortunately we are unable to provide accessible alternative text for this. If you require assistance to access this image, or to obtain a text description, please contact npg@nature.com](/gimo/contents/pt1/thumbs/gimo30-f2.jpg)