Key Points
- Gastroesophageal reflux (GER) and asthma are common in the general population, with GER existing together with asthma in up to 80% of asthmatics.
- The frequency with which GER actually triggers asthma is not known.
- No clinical test at present can reliably confirm the diagnosis of GER-triggered asthma.
- A patient-recognized association of GER with respiratory symptoms may be a reliable predictor of GER-triggered asthma.
- Proton pump inhibitors (PPIs) may improve respiratory symptoms in some asthmatics (perhaps nocturnal?).
- Antireflux surgery improves respiratory symptoms as shown in some studies.
Introduction
The relationship between GER and asthma has been pondered for millennia. The 12th century physician-philosopher, Moses Maimonides, strongly warned against overeating.1 Writing of an association between eating, lying down, and wheezing, Maimonides suggested in his Treatise on Asthma, that sleep was dangerous during an attack:
One should not sleep face downwards, nor on one's back, but lying on the side; at the beginning, on the left side, and the close of one's rest, on the right side. One should not go to sleep immediately after a meal, but only when three or four hours have elapsed. One should not sleep during the day.
Asthma is a disease characterized by bronchoconstriction, which is partially reversible.2 Airway hyperresponsiveness is present.2, 3 Airway inflammation is also a key feature of asthma and results in alterations in the mucosa and submucosa that over time lead to airway remodeling.2, 3 There are many triggers and contributing conditions that can initiate bronchospasm and airway inflammation.2, 3 Most people with asthma have multiple triggers and contributing conditions.4 Genetic factors also predispose to asthma development.2 Asthma varies in severity over time in individual asthmatics.
Gastroesophageal reflux (GER) is a potential trigger or contributing factor in selected (not all) asthmatics.5 Although GER may be a trigger in an individual asthmatic, GER therapy does not "cure" asthma. Therefore, treatment of GER in asthmatics treats a potential contributing condition, not asthma itself. This fact is why the interactions between the two disease states are very complex and asthma outcomes with GER therapy are difficult to interpret. Asthma is very heterogeneous over time in individual asthmatics and is heterogeneous in different asthmatics.
History of Disease
During the 18th century, Nicholas Rosen von Rosenstein,6 the First Physician to His Swedish Majesty, discussed in his 1776 textbook, The Diseases of Children and Their Remedies, what he terms the stomachic cough of children: "Such a cough is caused by the natural proclivity of children to ingest huge quantities of disgusting food, that cannot be digested or changed as it ought." Twenty-six years later, in his 1802 textbook, The History and Cure of Diseases, William Heberden7 wrote, "In most persons, the breath is shorter and more difficult after a meal."
Almost a century later, in 1892, Sir William Osler8 published The Principles and Practice of Medicine in which he prepared for the 20th century with an emphasis on eating habits: "Diet, too has an important influence and in persons subject to this disease severe paroxysms may be triggered by overloading the stomach, or by taking certain articles of food." In the same chapter, Osler again suggested that particular attention be paid to the diet of the asthmatic: "A rule of which experience generally compels them to make is to take the heavy meal in the early part of the day and not retire to bed before gastric digestion is completed."
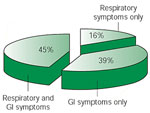
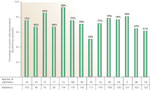
Despite the emphasis on diet and the strong references to a relationship between food, eating habits, and asthma, the GER/asthma concept remained difficult to swallow. For the next 50 years, only a few scattered wheezes were heard amidst the competing voices of subspecialty medicine's bellowing burps and clamoring coughs. One of these scattered wheezes was G.W. Bray,9 who reported that dietary indiscretion in some patients could lead to asthmatic attacks. Bray's comments, however, went relatively unnoticed until Belsey,10 in a review on pulmonary complications of esophageal disease, reported that patients with GER were liable to severe, progressive, and disabling pulmonary damage. Soon after, Urschel and Paulson11 reported in 1967 their experience in patients who were referred for hiatal hernia repair. Figure 1 shows the results of their 5-year experience (from 1961 to 1966) in 636 patients ranging in age from 7 months to 94 years who were referred for surgical correction of GER: 39% had classic reflux symptoms consisting of heartburn, indigestion, and postural aggravation without respiratory symptoms; 45% had both reflux symptoms and respiratory symptoms; and 16% had respiratory symptoms only. Thus, more than 60% of these patients, who were being referred for surgery to correct a gastroesophageal abnormality, actually had respiratory symptoms.
Figure 1: Prevalence of pulmonary diseases in patients referred for hiatal hernia surgery.
Although the authors were from a large surgical practice and the 636 patients had severely symptomatic gastroesophageal reflux (GER) in need of surgical repair, the results of the survey cannot be ignored: 84% had classic GER symptoms comprising heartburn, indigestion, and postural aggravation (88%), whereas 61% had respiratory symptoms comprising cough (47%), bronchitis (35%), asthma and wheezing (16%), pneumonitis (16%), hemoptysis (13%), and hoarseness (12%). More than 60% had symptoms of pulmonary disease coexisting with the GER.
Epidemiology
Asthma is a worldwide problem and in the United States in the year 2000 an estimated 17 million Americans were reported to have asthma.
Prevalence of Gastroesophageal Reflux Disease in Asthmatic Adults
Gastroesophageal Reflux Disease Defined as Reflux Symptoms
Figure 2 shows the prevalence of GER symptoms in adult asthmatics in four studies with sufficient interpretable data. In the first study, Perrin-Fayolle et al.12 found evidence of reflux symptoms in 65% of 150 consecutive (enrolled as they were identified; not referred or selected for the study) asthmatics. In the second study, Sontag et al.13 reported that 72% of 189 consecutive asthmatics had heartburn. Almost half of the 189 had supine nocturnal heartburn and 18% had nocturnal burning in the throat. In the third study, Field et al.14 studied 109 asthmatics and 135 controls in a questionnaire-based, cross-sectional analytic study; 77% of the asthmatics had heartburn, 55% had regurgitation, 24% had difficulty with swallowing, whereas 37% of the group required at least one antireflux medication and 41% had reflux-associated respiratory symptoms during the prior week. Pulmonary symptoms occurred significantly more frequently in the asthmatics than in the controls.
In the fourth study, Kiljander and Laitinen15 randomly selected every 14th patient from a multicenter group of 2225 asthmatics. Of the 90 asthmatics who joined, 51% had GER, which was defined as the presence of GER symptoms.
The results of these four studies, which together comprise a group of 538 asthmatics from France, Canada, Finland, and the United States, are remarkably similar. Taken as a group, 365 of the 538 patients had reflux symptoms, indicating that 68% of asthmatics have reflux symptoms. Despite some weaknesses in the patient selection methods in all three studies, these reports present the most reliable data in the medical literature on GER symptoms in consecutive asthmatics.
Gastroesophageal Reflux Disease Defined as Abnormal Acid Reflux
Figure 3 shows the prevalence of abnormal acid reflux in adult asthmatics in eight studies comprising 718 patients from 13 centers in five countries (France, Chile, Great Britain, Finland, and the United States). The prevalence of abnormal acid reflux, as determined by pH testing, ranged from 33% to 90%.16, 17, 18, 19, 20, 21 In two of the studies, short-term acid reflux testing was used to determine the presence of GER.17, 21 These studies were conducted in the 1980s before the availability of ambulatory 24-hour pH testing. The remaining six studies, comprising 718 patients, utilized ambulatory 24-hour pH testing to determine abnormal GER. In the first seven studies,16, 17, 18, 19, 20, 21, 22 recruitment of consecutive asthmatics was suboptimal in that all depended on referral centers to enroll their patients. Nevertheless, the studies represent the most reliable data found in the literature on pH testing and the prevalence of GER in asthma. In the eighth study, Kiljander and Laitinen15 performed 24-hour pH testing in randomly selected asthmatics. This study, which is the only large study of its kind in which asthmatic patients were randomly selected, showed abnormal acid reflux in 36% of the 90 participating asthmatics.
Figure 3: Prevalence of GERD in adult asthmatics: GERD defined as abnormal acid reflux.
On average, 70% of adult asthmatics have GER as defined by abnormal esophageal pH testing.
When the results of these eight studies are combined, 453 of the 718 (63%) enrolled patients had evidence of acid reflux, suggesting that almost two thirds of asthmatics have GER as defined by abnormal acid pH testing
Gastroesophageal Reflux Disease Defined as the Presence of Esophageal Mucosal Disease
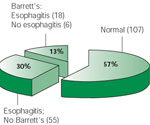
Figure 4 shows the prevalence of esophageal mucosal disease in adult asthmatics.23 Esophageal erosions or ulcerations as seen on endoscopy were present in 39% of consecutive asthmatics and 13% had Barrett's esophagus. In this study, Sontag et al.23 limited the recruitment to consecutive asthmatics who were referred for endoscopy in an approved study on the prevalence of GER abnormalities in consecutive asthmatics. The authors eliminated from their study any patients who were referred for workup because of gastrointestinal symptoms and who were not part of the consecutive asthmatic protocol. Thus, this study appears to be one of the few that reports the prevalence of GER as it relates to esophageal mucosal disease in consecutive asthmatics.
Gastroesophageal Reflux Disease Defined as Presence of Hiatal Hernia
Figure 5 shows three studies that used the presence of hiatal hernia as indirect evidence of the presence of GER.23, 24, 25 In the first study, Mays24 reported on 28 patients with severe asthma, 64% of whom had hiatus hernia and 46% of whom had barium reflux. These studies were criticized by Chernow and Castell26 because of the lack of data demonstrating aspiration, the reliance on upper gastrointestinal series to determine GER, and the abnormally low prevalence of hiatus hernia and barium reflux in the control group. Despite the criticisms, however, they concluded that the postulated relationship between GER and asthma most likely existed, but that it was premature to suggest cause and effect. In the second study, which included 15 patients with nocturnal asthma, Rodriguez-Villarruel et al.25 reported a 73% prevalence rate of reflux based on a combination of barium x-ray studies, endoscopy, and technetium scintigraphy. In this report, all 11 of 15 patients had abnormal barium x-rays, indicating a 73% prevalence rate of reflux in asthmatics. In the third study, Sontag et al.23 showed that hiatal hernia was present in 58% of consecutively chosen asthmatics using predetermined endoscopic criteria for hiatal hernia and esophagitis. In this study, all endoscopies were performed by one of two endoscopists using the same criteria for esophageal mucosal disease and the presence of hiatal hernia. In asthmatics with esophagitis, the hiatal hernia occurred seven times more frequently than in asthmatics without esophagitis, indicating that hiatal hernia in asthmatics is associated with more severe esophageal disease. When all three studies are combined, the prevalence of GER as defined by abnormal barium reflux on fluoroscopy or the presence of a hiatal hernia was 50%.
Figure 5: Prevalence of GERD in adult asthmatics: GERD defined as the presence of hiatal hernia.
On average, 66% of adult asthmatics have GER as defined by the presence of a hiatal hernia.
Taken together, the results of the retrospective studies, with their highly selected referral patterns, agree to a great extent with the results of the prospective epidemiologic and cross-sectional studies, which clearly demonstrate that GER is highly prevalent in asthmatic patients.
Gastroesophageal Reflux Disease Defined as Any Abnormal Reflux Parameter
Figure 6 summarizes three prospective studies performed at the same institution. Approximately 75% of the asthmatics have heartburn and almost 20% awaken with nocturnal burning in the throat13; 80% of consecutive asthmatics have pathologic acid GER in either the upright or supine position and 75% have increased frequency of reflux episodes.16 In addition, almost 60% of consecutive asthmatics have hiatal hernias, and almost 40% have esophageal mucosal damage from reflux.23 These data demonstrate that when all GER parameters are considered, 90% of asthmatics have at least one parameter needed to be considered as having gastroesophageal reflux disease (GERD): reflux symptoms, esophageal mucosal disease, or abnormal acid reflux. It is clear that GER is highly prevalent in adult asthmatics.
Incidence of Gastroesophageal Reflux Disease in Asthma and Asthma in GERD
In one of the few studies to address the development over time of GERD in asthmatics and the development over time of asthma in patients with GERD, Ruigomez et al.27 studied the incidence of each condition using the United Kingdom General Practice Research Database. The investigators identified a large cohort of patients with a first diagnosis of GERD and another large cohort of patients with a first diagnosis of asthma. More than 15,000 patients with a first diagnosis of GERD or asthma were matched with more than 17,000 controls. At a mean follow-up of 3 years, a nonsignificant number of GERD patients were diagnosed with asthma, whereas a significant number of asthmatics were diagnosed with GERD. Such a large study, naturally, has its built-in difficulties, but the authors wisely concluded that the importance of this study lay in its ability to educate primary care physicians in the association between asthma and GERD.
Pathophysiology
Mechanisms
There are many mechanisms by which esophageal contents can alter airway reactivity and result in bronchoconstriction. Neurogenic inflammation plays a role in many of these mechanisms. Mechanisms include a vagally mediated reflex, whereby acid or nonacid contents in the esophagus trigger airway responses, a direct axonal reflex whereby the central nervous system (CNS) is not required to complete a reflex arc, heightened bronchial reactivity, and microaspiration. Esophageal acid also increases minute ventilation without altering pulmonary function. More than one mechanism may be involved. This section also discusses predisposing factors that may lead to the development of GER in asthmatics.
Vagal Reflex
Several investigators have shown that a vagal-esophageal-bronchial reflex is active. The tracheobronchial tree and esophagus share common embryonic foregut origins with autonomic innervation through the vagus nerve.28 Preliminary evidence show that a transcription factor is expressed in the distal esophagus, implying that the distal esophagus is a respiratory-derived structure.29 This finding supports the hypothesis that the esophagus and the respiratory tract are closely related from a developmental standpoint. These vagal airway reflexes may be protective in order to avoid distal lung exposure to orally or inhaled noxious agents.30 In a dog model, Mansfield and colleagues31 first investigated this reflex. They noted that esophageal acid increased respiratory resistance, which was ablated with bilateral vagotomy. They also examined 15 asthmatics with GER, noting that GER symptoms were associated with an increase in respiratory resistance and a decrease in airflow at 25% of vital capacity.32 These pulmonary function findings returned to baseline after GER symptoms were relieved with antacids. The same group examined asthmatics who had a positive Bernstein test and found that esophageal acid infusion caused a 10% increase in total respiratory resistance.33 Wright and colleagues34 confirmed that a vagally mediated reflex is present in studying 136 individuals measuring airflow and arterial oxygen saturation both before and after esophageal acid infusions. There were significant reductions in airflow and oxygen saturation with esophageal acid. Atropine pretreatment abolished these findings and thus blocking the vagal influence.
Harding's35 laboratory did a series of experiments utilizing esophageal acid infusions in asthmatics with and without GER, and in subjects with GER but without asthma, and in normal controls. Peak expiratory flow (PEF) rate decreased with esophageal acid infusions in all groups. Esophageal acid clearance improved PEF rates in all groups except for the asthma with GER group who had further deterioration in their PEF rates. Airway resistance also increased with esophageal acid infusions. These effects did not require microaspiration, as evidenced by proximal esophageal acid, or a positive Bernstein test. To examine the potential role of microaspiration, Harding's36 laboratory performed the same experiment with subjects staying in the supine position during esophageal infusions. Again, esophageal acid infusions caused a decrease in PEF rates and an increase in specific airway resistance that did not improve despite esophageal acid clearance in the asthma with GER group. Furthermore, there was a delayed improvement in airway function that took at least 40 minutes. In a third study, vagolytic doses of intravenous atropine partially ablated this bronchoconstrictive response to esophageal acid, further underscoring the implication of a vagally mediated reflex.37 The temporal association between distal esophageal acid and respiratory symptoms also supports a vagal reflex. One hundred ninety-nine asthmatics were examined for respiratory symptoms while undergoing 24-hour esophageal pH testing; 78% of respiratory symptoms correlated with esophageal acid events, including 90% percent of coughs.38
Conflicting data exist from other investigators who have not found significant bronchoconstrictive responses during esophageal acid infusions. Tan and colleagues39 examined 15 nocturnal asthmatics, measuring respiratory flow, tidal volume, and airflow resistance during sleep and found no significant differences in airflow resistance with esophageal acid. This vagal mechanism may not be significantly active in all subjects.
There is also evidence of autonomic nervous system dysfunction in asthmatics with GER. Harding's40 laboratory noted that 73% had a hypervagal response during a deep breathing maneuver, 31% had a hypervagal response during the Valsalva maneuver, and 6% had a hypervagal response during a tilt test. These data suggest that asthmatics with GER have heightened vagal responsiveness. This hypervagal responsiveness may be partially responsible for the airway responses to esophageal acid. All of these data support the possibility that a vagally mediated reflex is active.
Local Axonal Reflexes
Local axonal reflexes without CNS intervention may also play a role. Fischer and colleagues41 noted a direct neuronal connection between the esophagus and the lung with nitric oxide containing neurons. Stimulation of this axonal reflex results in airway edema in the lung. Daoui and colleagues42 noted that in a guinea pig model, that esophageal acid results in a threefold increase in airway edema, and that tachykinin NK-1 and NK-3 receptor antagonists prevented this response. This experiment verifies that nonvagal peripheral pathways acting through the autonomic ganglia are also active.
Heightened Bronchial Reactivity
Another potential mechanism is heightened bronchial reactivity. Esophageal acid has the potential to prime the respiratory system so that when asthmatics are exposed to another asthma trigger, they have a heightened response. Herve and colleagues43 noted that esophageal acid markedly potentiated the bronchoconstrictive effects induced by voluntary isocapnic hyperventilation and methacholine provocation tests when compared to the airway responses with normal saline infusions. The total dose of methacholine required to reduce the forced expiratory volume in 1 second (FEV1) by 20% (PD20) was significantly lower when esophageal acid was infused compared to normal saline. Furthermore, the vagus nerve is important for this mechanism because this response to esophageal acid was abolished with atropine pretreatment. Thus, esophageal acid-sensitive receptors impact cholinergic bronchial tone by a vagally mediated reflex, and GER may aggravate asthma by increasing bronchomotor responsiveness to other stimuli.
Vincent and colleagues44 also noted heightened bronchial reactivity in asthmatics with GER. In 105 consecutive asthmatics, they noted that PD20 correlated with the number of acid episodes during esophageal pH testing. Wu and colleagues45 examined seven mild asthmatics who did not have GER or esophagitis and performed methacholine challenge tests while infusing saline or acid into the esophagus. The dose of methacholine required for a 35% fall in respiratory conductance decreased significantly during esophageal acid infusion.
Heightened bronchial reactivity with GER is also noted during sleep. Cuttitta and colleagues46 monitored esophageal pH and respiratory resistance in seven asthmatics with moderate to severe GER during sleep. Both long and short duration acid GER episodes were associated with increases in lower respiratory resistance. There was a direct correlation between GER duration and increases in lower airway resistance. The sleep-wake state had no impact on this GER-induced bronchoconstriction.
Microaspiration
Microaspiration is another important pathophysiologic mechanism where microaspiration of esophageal contents elicit significant airway responses. One of the most convincing studies was performed by Tuchman and colleagues47 in a cat model. Esophageal infusion of 10 mL of acid resulted in a 1.5-fold increase in total lung resistance compared to nearly a fivefold increase when 50  L of acid was instilled into the trachea. Furthermore, this esophageal acid response only occurred in 60% of animals compared to 100% of animals when given tracheal acid. This response is also vagally mediated because the effect of tracheal acidification on total lung resistance was abolished with bilateral cervical vagotomy. Examining humans, Varkey and colleagues48 placed a pH probe in the pharynx 2 cm above the upper esophageal sphincter (UES) and in the distal esophagus in 19 asthmatics and seven normal controls. They found that 5% of GER episodes were also associated with a pH drop at the pharyngeal probe, documenting that microaspiration does occur and does so more frequently in asthmatics compared to normal controls. Jack and colleagues49 monitored pH simultaneously in the esophagus and trachea of four patients with severe asthma. Thirty-seven episodes of esophageal reflux lasting more than 5 minutes were observed, and in five of these episodes a fall in tracheal pH also occurred. The PEF rate decreased 84 L/min when esophageal acid and tracheal acid were present compared to 8 L/min when esophageal acid alone was present. This study verifies that tracheal microaspiration of acid is associated with more significant pulmonary function deterioration compared to esophageal acid without tracheal microaspiration.
L of acid was instilled into the trachea. Furthermore, this esophageal acid response only occurred in 60% of animals compared to 100% of animals when given tracheal acid. This response is also vagally mediated because the effect of tracheal acidification on total lung resistance was abolished with bilateral cervical vagotomy. Examining humans, Varkey and colleagues48 placed a pH probe in the pharynx 2 cm above the upper esophageal sphincter (UES) and in the distal esophagus in 19 asthmatics and seven normal controls. They found that 5% of GER episodes were also associated with a pH drop at the pharyngeal probe, documenting that microaspiration does occur and does so more frequently in asthmatics compared to normal controls. Jack and colleagues49 monitored pH simultaneously in the esophagus and trachea of four patients with severe asthma. Thirty-seven episodes of esophageal reflux lasting more than 5 minutes were observed, and in five of these episodes a fall in tracheal pH also occurred. The PEF rate decreased 84 L/min when esophageal acid and tracheal acid were present compared to 8 L/min when esophageal acid alone was present. This study verifies that tracheal microaspiration of acid is associated with more significant pulmonary function deterioration compared to esophageal acid without tracheal microaspiration.
Esophageal Acid Increases Minute Ventilation
Respiratory symptoms with esophageal acid exposure may be attributed to factors other than bronchoconstriction. Field and colleagues50 noted in normal subjects that minute ventilation increased with esophageal acid infusions and decreased with esophageal acid clearance. Respiratory rate also increased with esophageal acid infusions. Chest discomfort correlated with the increases in minute ventilation, which may explain how esophageal acid worsens respiratory symptoms without necessarily altering pulmonary function.
Airway Inflammation with Esophageal Acid
Asthma is characterized by smooth muscle contraction, increased mucus production, and release of inflammatory mediators resulting in airway narrowing and airway remodeling.2 Cellular and molecular mechanisms leading to airway inflammation have been examined in a guinea pig GER model. Hamamoto and colleagues51 noted that esophageal acid caused the release of substance P in the lung, which was associated with airway edema. This airway edema was inhibited by a substance P receptor antagonist. The investigators controlled for microaspiration by ligating the esophagus at the proximal end. Both the lung and the esophagus are innervated by tachykinin-containing afferent nerves that have fibers in the airway epithelia.52 Ricciardolo and colleagues53 examined acid microaspiration and neuroinflammation in a guinea pig model using inhaled citric acid. Inhaled citric acid resulted in a dose-dependent increase in total pulmonary resistance. This response was mediated by the activation of sensory nerves and the release of tachykinins from peripheral nerve terminals. Again, this bronchoconstrictive response was abolished with tachykinin NK-1 receptor antagonists. Furthermore, the release of nitric oxide, a bronchodilator, also occurred, which may explain the paradox that esophageal acid does not always cause a bronchoconstrictive response.53, 54 Nitric oxide is also a marker of airway inflammation. Silvestri and colleagues54 measured exhaled nitric oxide levels in children, noting that these levels were lower in allergic asthmatic children with GER compared to asthmatic children without GER. Correlations were also noted between exhaled nitric oxide levels and esophageal pH data, suggesting that inhalation of acid gastric contents may interfere with nitric oxide production in the airways.
Some investigators hypothesize that esophageal acid, especially with proximal migration, results in the accidental inhalation of acid, which alters airway homeostasis.55 Neuroinflammation occurs with activation of capsaicin-sensitive sensory nerves. Protons can activate these nerves, resulting in the release of tackykinins that, in conjunction with kinins, nitric oxide, oxygen radicals, and proteases, modulate airway inflammation. Airway protective mechanisms may not be able to neutralize the acid load in the airway epithelium, thus exposing it to injury. Furthermore, Stein56 hypothesized that esophageal contents may damage upper airway epithelium, resulting in the release of cytokines and adhesion molecules leading to further inflammation and initiation of other inflammatory pathways.
Sacco and colleagues57 examined airway inflammation associated with asthma-like symptoms in children with abnormal esophageal pH tests. Bronchoalveolar lavage (BAL) examined inflammatory indices. Compared to controls, children with GER and respiratory symptoms had higher amounts of neutrophils, lipid-laden macrophage indices, and higher concentrations of interleukin-8 (IL-8), myeloperoxidase, and elastase in their BAL fluid. Furthermore, neutrophil proportions correlated with the lipid-laden macrophage index as well as IL-8 and myeloperoxidase levels. These investigators concluded that IL-8 may be important in the recruitment and activation of neutrophils in the airway of children with asthma and GER. The presence of lipid-laden macrophages suggests that microaspiration may be an important inducer of airway inflammation.57
In conclusion, all of these pathophysiologic mechanisms may be important. These mechanisms lead to airway inflammation and partially explain how GER triggers asthma in selected asthmatics.
Predisposing Factors to Gastroesophageal Reflux Development in Asthmatics
Many factors can lead to GER development in asthmatics, including the presence of autonomic dysregulation as previously noted by Lodi and colleagues.40 Other potential predisposing elements include the presence of an increased pressure gradient between the thorax and the abdomen and altered crural diaphragmatic function leading to GER episodes. Airway obstruction also triggers transient LES relaxations. Medications used in asthma therapy may also potentiate GER. Furthermore, asthmatics have lifestyle issues that may predispose to GER.
During periods of increased respiratory effort, as in asthma attacks, asthmatics have an increased pressure gradient between the thorax, reflecting pleural pressure, and the abdominal cavity that could override lower esophageal sphincter (LES) pressure.58 The crural diaphragm also participates in LES pressure generation.59 Hyperinflation associated with bronchospasm has the potential to place the crural diaphragm at a functional disadvantage because of geometric flattening.60 Moote and colleagues60 noted that methacholine-induced airflow obstruction was associated with longer esophageal acid contact times. Singh and Jain61 noted that therapy directed toward asthma reduced GER symptoms from approximately 5 days a week to less than 2 days a week. All of these studies point to the possibility that asthma exacerbations can predispose to GER development.
Bronchoconstriction can result in transient LES relaxations. Zerbib and colleagues62 monitored esophageal manometry using a Dent sleeve and esophageal pH in eight asthmatics and eight controls while inducing airflow obstruction with inhaled methacholine. Airflow obstruction significantly increased the number of transient LES relations and the number of esophageal acid episodes. Reversal of airflow obstruction using inhaled salbutamol, a  -agonist, decreased the number of transient LES relaxations to near baseline values. These data confirm that there is an airflow obstruction-GER cycle so that bronchospasm may induce transient LES relaxations and thus GER episodes.
-agonist, decreased the number of transient LES relaxations to near baseline values. These data confirm that there is an airflow obstruction-GER cycle so that bronchospasm may induce transient LES relaxations and thus GER episodes.
Another predisposing factor to GER development is the high prevalence of hiatal hernia found in asthmatics. Mays and colleagues24 examined 28 asthmatics and 468 control subjects with barium esophagrams and found that 64% of asthmatics had a hiatal hernia compared to 19% of control subject. This high prevalence was also noted by Sontag and colleagues,23 whose investigations showed that 58% of consecutive unselected asthmatics had a hiatal hernia. Transient LES relaxations are more likely to be followed by GER episodes if a hiatal hernia is present.63
Medications utilized to treat asthma may also predispose to GER development. For instance, theophylline increases gastric acid secretion and decreases LES pressure.28 Ekström and Tibbling,64 in a placebo-controlled trial, noted that GER symptoms increased 170% and daytime reflux increased 24% with theophylline. Other investigators found that theophylline may not be an important factor. Hubert and colleagues65 did not find any differences in 24-hour esophageal pH variables regardless of whether asthmatics were on theophylline or placebo. Nonacid reflux using impedance monitoring has not been adequately assessed in asthmatics with GER.
Inhaled  -agonists may also impact esophageal function. Crowell and colleagues66 performed a prospective randomized, double-blind, placebo-controlled crossover trial evaluating the effects of sequential doses of inhaled albuterol (2.5 to 10 mg), a
-agonists may also impact esophageal function. Crowell and colleagues66 performed a prospective randomized, double-blind, placebo-controlled crossover trial evaluating the effects of sequential doses of inhaled albuterol (2.5 to 10 mg), a  -agonist, on esophageal manometry in nine healthy volunteers. Inhaled albuterol produced a dose-dependent decrease in LES pressure. The amplitude of esophageal contractions at the mid-esophagus also decreased. The authors did not note changes in the number of transient LES relaxations with albuterol. Repeated doses of
-agonist, on esophageal manometry in nine healthy volunteers. Inhaled albuterol produced a dose-dependent decrease in LES pressure. The amplitude of esophageal contractions at the mid-esophagus also decreased. The authors did not note changes in the number of transient LES relaxations with albuterol. Repeated doses of  -agonists are given during acute asthma exacerbations.
-agonists are given during acute asthma exacerbations.
Oral corticosteroids are also used during asthma exacerbations. Harding's67 laboratory performed a prospective placebo-controlled, crossover trial and noted that 60 mg a day of oral prednisone for 7 days resulted in significant increases in esophageal contact times at both the proximal and distal pH probe positions.
Asthmatics also have lifestyle behaviors that predispose to GER development. Sontag and colleagues13 noted in 261 asthmatics and in 218 controls that 60% of asthmatics and 44% of controls ate right before bedtime and this bedtime eating was associated with awakening during sleep because of GER symptoms.
In conclusion, there are multiple physiologic alterations associated with asthma that may promote GER.
Clinical Features
Clinical features of GER-triggered asthma are very similar to clinical features of asthma.5 Asthma commonly presents with dyspnea, chest tightness, and cough. Some patients may present with cough alone.2, 68, 69 Furthermore, patients may notice sputum production. They may also notice wheezing, which can occur during inspiration and expiration. They may have nocturnal awakenings associated with respiratory symptoms. Many asthmatics notice specific symptoms when exposed to triggers. Allergic triggers include cat dander and dust. Nonallergic triggers include irritants, strong perfumes, cigarette smoke, and fumes from petrochemicals. Some patients notice pulmonary symptoms with acute weather changes. Exercise can also induce asthma. Anxiety and strong emotions can also trigger bronchospasm. Gastroesophageal reflux is also a potential trigger of asthma.5 Acute asthma symptoms are relieved with inhaled  -agonists. Asthma results in bronchospasm and airway inflammation. Airway inflammation leads to airway narrowing and remodeling.2
-agonists. Asthma results in bronchospasm and airway inflammation. Airway inflammation leads to airway narrowing and remodeling.2
Clinicians may wish to consult the Guidelines for Diagnosis and Management of Asthma: Expert Panel Report 2,68 and also guidelines on selected topics, developed by the National Institutes of Health,69 for further guidance concerning the clinical features of asthma.
Because GER is a potential asthma trigger, all asthmatics should be questioned about the possibility of esophageal GER symptoms.5 Patients may have heartburn, regurgitation, or worsening cough/bronchospasm associated with foods that lower the LES pressure. Patients should be asked about dysphagia. The frequency and severity of heartburn and other esophageal symptoms should be quantitated because patients with chronic persistent symptoms should be evaluated with endoscopy to rule out the possibility of Barrett's esophagitis or other GER complications.
Patients may also notice respiratory symptoms associated with esophageal symptoms. For instance, Field and colleagues14 noted that 41% of asthmatics noted reflux-associated respiratory symptoms, and 28% used their inhalers while experiencing GER symptoms. Ask patients if they use their inhaler when experiencing GER symptoms. Obesity and nocturnal GER symptoms are linked to asthma onset as noted by Gunnbjörnsdôttir and colleagues.70 These investigators examined 5- to 10-year follow-up of the European Community Respiratory Health Survey involving 16,191 participants from five northern European countries. Asthma and nocturnal GER symptoms increased in prevalence along with body mass index (BMI). Nocturnal GER and obesity were independent risk factors for asthma onset, wheeze, and nighttime symptoms. If asthmatics are obese, they are put at increased risk for developing GER.
Asthmatics may also develop GER symptoms only during acute asthma exacerbations. Asthma medications, including theophylline, systemic  -agonists (that lower LES pressure), or repeated inhalations of
-agonists (that lower LES pressure), or repeated inhalations of  -agonists and/or oral prednisone can worsen GER.66
-agonists and/or oral prednisone can worsen GER.66
Many asthmatics with GER do not have esophageal symptoms. In these patients, GER is clinically silent. These asthmatics are difficult to identify because demographic features do not predict silent GER.6 Twenty-four-hour esophageal pH testing in asthmatics without esophageal GER symptoms revealed abnormal esophageal acid contact times in 62% of these patients.71
Patients with GER-triggered asthma may also have other extraesophageal manifestations of GER, including laryngitis, sore throat, globus, postnasal drip, and hoarseness.5 Often esophageal GER symptoms are not present in patients with extraesophageal manifestations of GER.
History and Symptoms
History and symptoms of GER-triggered asthma are very similar to clinical features of asthma and GER, as previously discussed. Potentially, asthma symptoms can be temporally related with GER symptoms, and patients should be questioned about this relationship.38 Special attention to sleep-time awakenings owing to bronchospasm may point toward the possibility of GER-triggered asthma.13 The use of rescue inhalers associated with GER symptoms should also alert the clinician. Asthma symptoms may be more severe after eating a high-fat meal that delays gastric emptying or foods that lower LES pressure such as chocolate, peppermint, caffeine, or alcohol.5 Medications such as theophylline, systemic  -agonists, and repeated inhalations of
-agonists, and repeated inhalations of  -agonist and oral corticosteroids may also promote GER-triggered asthma.64, 65, 66, 67 Eating or drinking acidic foods including tomatoes and tomato products, citrus, and any carbonated beverage (pH –1) may trigger symptoms.5 Patients may also have regurgitation or regurgitation of liquid onto their pillow during the night. They may have more symptomatology while sleeping on their back or after consuming a large meal just prior to bedtime.7 For instance, Sontag and colleagues13 examined 261 asthmatics and noted that 50% of them had nocturnal awakening from heartburn, with 33% of them noticing suffocation, cough, or wheezing preceded by heartburn or regurgitation awakening them during sleep. As previously mentioned, GER in asthmatics may be clinically silent, and esophageal pH testing may be needed to identify GER.71
-agonist and oral corticosteroids may also promote GER-triggered asthma.64, 65, 66, 67 Eating or drinking acidic foods including tomatoes and tomato products, citrus, and any carbonated beverage (pH –1) may trigger symptoms.5 Patients may also have regurgitation or regurgitation of liquid onto their pillow during the night. They may have more symptomatology while sleeping on their back or after consuming a large meal just prior to bedtime.7 For instance, Sontag and colleagues13 examined 261 asthmatics and noted that 50% of them had nocturnal awakening from heartburn, with 33% of them noticing suffocation, cough, or wheezing preceded by heartburn or regurgitation awakening them during sleep. As previously mentioned, GER in asthmatics may be clinically silent, and esophageal pH testing may be needed to identify GER.71
Physical Findings
Gastroesophageal reflux–triggered asthma does not have specific physical exam findings. Patients may be obese with high BMIs. They may have evidence of extraesophageal manifestations of GER as previously mentioned. They may have a cushingoid appearance from oral corticosteroid therapy. They may have hoarseness related to extraesophageal manifestations of GER or from use of inhaled corticosteroids. They may have an increased respiratory rate with accessory muscle use if an acute asthma attack is present. Lung exam may show bronchospasm and wheezing on inspiration and expiration. Careful attention should be paid to air movement volume to ensure adequate airflow. Other physical examine findings of asthma include a prolonged expiratory phase and wheezing on end expiration.2 Physical exam findings for GER are nonspecific. Examine for wheezing after exposing the patient to a potential trigger. Cough may be noted with deep respiratory efforts. Sputum production may also be present.
Laboratory Findings
Laboratory findings in GER-triggered asthma are similar to those of asthma alone. Pulmonary function testing including spirometry should be done in all asthmatics.2 Spirometry reveals a decreased FEV1/forced vital capacity (FVC) ratio as well as decreases in minute forced expiratory flow rates (FEF25%/75%) and PEF rates.2 Patients may have hyperinflation with an increase in residual volume. They also may have coughing while undergoing pulmonary function tests. The hallmark of asthma is airflow reversibility, and this can be documented by pre- and postbronchodilator spirometry with a 10% improvement in FEV1 with inhaled  -agonists.2, 68, 69 Furthermore, methacholine challenge or other provocative challenge testing can be performed. This documents the presence of airway hyperreactivity.
-agonists.2, 68, 69 Furthermore, methacholine challenge or other provocative challenge testing can be performed. This documents the presence of airway hyperreactivity.
Esophageal testing can be performed to document GER. Esophageal pH testing allows asthma symptoms to be correlated with esophageal acid events.71 It can also be used to assess adequacy of GER therapy, and is useful in diagnosing GER in asthmatics who do not have esophageal GER symptoms.5 Identifying GER is especially important in asthmatics who have difficult-to- control asthma.72, 73, 74, 75 Esophageal pH testing utilizing a pH probe 5 cm above the LES and at the level of the UES has a sensitivity and specificity of approximately 90% in diagnosing GER in asthmatics.5 Many asthmatics have abnormal esophageal acid contact times. Sontag and colleagues16 performed esophageal pH testing in consecutive asthmatics and reported that abnormal esophageal acid contact times were present in 80% of subjects. Correlation between respiratory symptoms and esophageal acid events can be performed. Our laboratory noted that of 151 respiratory symptoms reported during 24-hour esophageal pH testing, 119 (79%) were associated with esophageal acid.38
Minimal data are available on the usefulness of wireless pH probe (Bravo pH Probe, Medtronics, Minneapolis, MN) and esophageal impedance in asthmatics.76, 77 Esophageal manometry can document motility disorders in asthmatics. For instance, Kjellen and colleagues78 found that 38% of 97 consecutive asthmatics had esophageal dysmotility, and 27% had LES hypotension. Sontag and colleagues16 found that asthma patients compared to control subjects had significantly lower LES pressures. Another report showed that 68% of asthmatics had esophageal dysmotility.79 Upper endoscopy is useful in detecting esophageal mucosal injury including esophagitis, strictures, webs, Barrett's esophagus, and esophageal tumors.80 Endoscopy has poor sensitivity that limits its usefulness as a diagnostic test for GER-triggered asthma. Similar findings are true with barium esophagram, which has a sensitivity approximating 40%.79 Other testing including radiolabeled technetium sulfur colloid, scintiscanning, and Bernstein test have very low sensitivities in the asthma setting.79, 81, 82 Another useful test to examine GER-triggered asthma is an empiric GER treatment trial using a proton pump inhibitor (PPI).5 This simple technique is definitive for both diagnosing and assessing GER as a potential asthma trigger. Response to PPI therapy can also ensure a cause-and-effect relationship between GER and specific asthma symptoms.15 A minimum 3-month empiric trial should be used to assess asthma symptom improvement.83 Note that asthma symptoms, PEF rate monitoring, and pulmonary function tests should be performed during this empiric PPI trial.83
Differential Diagnosis
Asthma and GER have specific diagnostic criteria.2, 67, 68, 71 Asthma's differential diagnosis includes laryngospasm, wheezing owing to other causes including pulmonary embolism, foreign body in the airway, and endobronchial or tracheal tumors. Other causes of wheezing include left ventricular dysfunction with pulmonary edema. Other intrinsic pulmonary diseases including chronic obstructive pulmonary disease (COPD), cystic fibrosis, sarcoidosis, and interstitial lung diseases can also present with bronchospasm. Full clinical evaluation with pulmonary function studies (with flow volume loop), computed tomography (CT) scanning with high-resolution sections, and bronchoscopy can help differentiate these disease states. Echocardiogram and other means of assessing cardiac function and cardiac blood can rule out cardiac or ischemic causes of bronchospasm.
Diagnosis
Numerous methods have been utilized to support the diagnosis of GERD-related pulmonary disease. These methods (Table 1) include (1) inspection of sputum for lipid-laden alveolar macrophages, (2) scintigraphic monitoring to document pulmonary aspiration of gastric contents, (3) ambulatory esophageal pH monitoring and acid infusion of the esophagus to provoke vagally mediated bronchoconstriction, and (4) surveys on the prevalence of GER symptoms. Unfortunately, none of these techniques has been proved reliable to predict which patients may have GER-triggered or GER-associated pulmonary disease.
Sputum Inspection
The finding of lipid-laden alveolar macrophages in children84 and adults85 reportedly demonstrates aspiration of gastric material into the pulmonary tree. Specificity, however, appears to be unacceptably low, making the test clinically impractical.
Scintigraphic Monitoring
Technetium-99m isotope-sulfur colloid scintigraphic monitoring has been used to document pulmonary aspiration of gastric contents. Reich and coworkers86 examined seven patients with alleged GER; one had questionable aspiration, two showed traces of isotope in the lungs, and four patients had normal-appearing scans. Potential explanations for negative scans include the following: (1) patients really did not aspirate or they refrained from eating before bedtime and reflux did not occur; (2) patients had reflux, but aspiration was infrequent; and (3) aspiration did occur but the material was cleared during the 8-hour interval.
Ghaed and Stein87 found no evidence of pulmonary aspiration during 20 scans in 10 asthmatic patients.
Chernow et al.88 studied six patients with suspected nocturnal aspiration while they concomitantly monitored overnight intraesophageal pH. Three patients had prolonged episodes of acid GER and positive lung scans and three patients had prolonged episodes of decreased LES pressures, rapid clearing of the esophageal acid, and negative scans. The investigators suggested that good esophageal motility with rapid clearance was important in preventing pulmonary aspiration.
Thus, for the following reasons, technetium scanning appears limited in its usefulness to detect aspiration: (1) the quantity of aspirated gastric contents necessary to cause bronchospasm or cough may be small enough to escape detection with current scanning methods; (2) aspiration of gastric contents may occur but the pulmonary mucociliary apparatus may rapidly clear the technetium, leaving only the resulting bronchospasm; (3) aspiration may occur only sporadically and thus multiple scans on successive days might be necessary to obtain a positive result; and (4) factors other than aspiration may be causing the bronchospasm.
Esophageal pH Monitoring and Acid Infusion
Ambulatory esophageal pH monitoring has been used in studies to both support and reject an association between GER and pulmonary disease. Martin and colleagues90 demonstrated the onset of clinical wheezing during an episode of GER in three of eight children with nocturnal asthma. In 25 children they observed a highly significant correlation between the frequency of nocturnal asthma and the severity of GER. Esophageal pH testing, however, could only suggest reflux as a cause of wheezing, but could not predict which patients were at risk.
Similarly, Jolley and coworkers91 used pH testing to show that respiratory distress was preceded by acid reflux in seven of 17 children who were thought to have reflux-triggered respiratory symptoms.
Euler and associates89 used pH testing to study 49 infants and children with GER, 11 of whom had a history of recurrent pulmonary infections: six had multiple coughing spells associated with episodes of acid reflux and five had neither reflux nor coughing spells. Although chronic pulmonary changes were present on chest radiographs in all six of the children with reflux-associated pulmonary symptoms, the pulmonary changes also were present in two of the five children with no reflux-associated pulmonary symptoms. It is possible, therefore, that pH testing failed to identify some patients with reflux-triggered pulmonary symptoms. With no follow-up confirmation, the investigators could only suspect that pH testing successfully identified the patients with reflux-triggered pulmonary symptoms.
Donald and colleagues92 used pH testing in 13 patients with respiratory symptoms thought possibly to be owing to GER. Five of the 13 patients had nocturnal choking following an episode of acid reflux. Four of these five patients were greatly improved with medical or surgical treatment of the reflux, indicating that pH testing was successful in identifying some patients with GER-triggered respiratory symptoms. In the eight remaining patients who had no nocturnal choking after an episode of acid reflux, the clinical outcome, unfortunately, is not reported.
Pellegrini and associates93 studied 100 patients with GER. Although 48 had pulmonary symptoms suggesting aspiration, only eight had evidence of aspiration during the pH test and nine had evidence of potential aspiration. Five of the eight patients with aspiration improved markedly with surgical correction of the reflux. The authors suggest that aspiration occurs less frequently than would be suspected by the symptom history. Follow-up information would be required, however, to determine whether patients with reflux-triggered pulmonary symptoms went undetected as a result of false-negative pH tests.
In a follow-up study by the same group, Patti and colleagues94 studied 23 consecutive patients with respiratory symptoms of unexplained etiology. Upper and lower esophageal sphincter pressures were obtained along with upper and lower pH measurements. Aspiration was diagnosed when respiratory symptoms occurred during or within 3 minutes after a reflux episode recorded at both levels of the esophagus. Of the 23 patients, 11 were considered to be aspirators. Most importantly, aspirators had lower LES pressures, decreased amplitude of peristalsis in both the lower and upper esophagus, and lower UES pressures. Thus aspirators had a dysfunction of all three barriers to aspiration.
The results of the esophageal pH studies have been cited as evidence in support of the cause-and-effect theory of reflux-triggered pulmonary disease symptoms. The occurrence together, however, of a respiratory symptom and an episode of acid reflux is not always helpful in establishing cause and effect. Wiener and coworkers95 attempted to devise a symptom index to determine whether certain chest symptoms should be considered as related to the episode of GER: If the chest symptoms and the acid reflux episode occur together less than 25% of the time or more than 75% of the time, the causal association is considered to be low and high, respectively. These indexes, unfortunately, do not take into consideration the importance of the total number of chest symptom episodes. For example, a 100% causal association would apply equally to a person with one chest symptom during one reflux episode and a person with 50 chest symptoms during 50 separate reflux episodes. Furthermore, whether the results from patients with chest symptoms owing to GER can be applied to patients with asthma symptoms from GER is unknown.
It is more than likely that the rigid restrictions placed on patients during the esophageal pH testing result in less than the usual amount of acid reflux and actually lead to an underestimation the amount of reflux that occurs at home, especially before bedtime, when patients are under no dietary restrictions. Therefore, the many published reports on pH testing may be seriously underestimating the amount of GER actually occurring, and may not be useful to predict which patients have reflux-triggered or reflux-associated pulmonary disease.
In a sophisticated attempt to clarify the GER/asthma relationship, Avidan et al.96 analyzed 128 24-hour ambulatory pH tracings that had been obtained in asthmatics during routine conduct of pH testing. A series of elegantly conducted sensitivity analyses demonstrated that half of all coughs and wheezes in asthmatics were associated with acid reflux into the esophagus. Although occasionally a cough episode led to acid reflux, it was, in the vast majority of instances, the acid reflux episode that led to the cough.
Clinical Course and Complications
Because GER therapy has the potential to improve asthma outcomes in selected asthmatics, all asthmatics should be assessed for GER. If GER is suspected clinically, or the patient has difficult-to-control asthma even in the absence of GER symptoms, an empiric trial of PPI can be initiated.20 An American Thoracic Society workshop on refractory asthma recommended that GER be investigated in refractory asthmatics.97 Because of PPI's superior effectiveness, PPI twice daily, 30 minutes to 1 hour before breakfast and dinner, should be used for at least 3 months. Asthma symptoms, PEF rates, and asthma medication use should be monitored both before and during the empiric PPI trial.5 An empiric PPI trial should also be considered in asthmatics with moderate or severe-persistent asthma, especially in those requiring oral corticosteroids, even in the absence of GER symptoms. If asthma outcomes improve at the end of the 3-month empiric PPI trial, then GER therapy should be continued. Maintenance GER therapy could begin by tapering the PPI to once daily. Gastroesophageal reflux lifestyle therapy should also be implemented at the beginning of the empiric PPI trial. If asthma outcomes are not improved at the end of the 3-month PPI empiric trial, then GER is most likely not to be a trigger of the individual's asthma if GER is found to be under adequate control with esophageal pH testing. The role of nonacid reflux is not clearly delineated in GER-triggered asthma; however, it may potentially play a role. O'Connor and colleagues98 examined the cost-effectiveness of strategies utilized to diagnose GER-triggered asthma, and reported that empiric omeprazole for 3 months followed by 24-hour esophageal pH testing in the nonresponders was the most cost-effective way to include or exclude GER as a trigger in individual asthmatics. There are no published trials examining the cost-effectiveness of medical versus surgical therapy in GER-triggered asthma.
Surgical fundoplication may also be helpful in GER-triggered asthma. Surgery should be reserved for asthmatics who have improvement of asthma with medical GER therapy, especially in those who have normal esophageal motility.
Investigators examined predictors of asthma response with GER therapy. Asthma characteristics include difficult-to-control asthma, nonallergic intrinsic asthma, the presence of nocturnal asthma, and obesity with a BMI of >29.7 kg/m2.21, 74, 99, 100 Characteristics of GER have also been found to be positive predictors for asthma response in GER-triggered asthma. These include reflux-associated respiratory symptoms, regurgitation more than twice weekly, proximal acid on esophageal pH testing, and abnormal amounts of distal acid on esophageal pH testing.83, 100, 101, 102
Long-term follow-up evaluation of GER-triggered asthma was examined by Sontag and colleagues103 for up to 19 years in 16 surgically treated patients. There was an immediate and sustained reduction in acute nocturnal exacerbations of wheezing, coughing, and dyspnea. By 2 years, there was marked improvement in asthma in up to 75% of patients. There are no data examining long-term asthma outcomes in medically treated patients with PPIs.
Long-term management of GER-triggered asthma includes regular evaluation of both pulmonary and GER symptoms while on GER therapy. Further GER diagnostic evaluation should be considered if symptoms worsen. Over time, some patients are able to taper off PPI therapy, although this is rare in our personal clinical experience. It is hoped that further investigations will enable more specific recommendations to be made concerning long-term management of GER-triggered asthma.
Treatment
Prospective Proton Pump Inhibitor Studies
After the first published report of a patient with disabling asthma that dramatically improved with omeprazole therapy, the stage was set for prospective trials.104 Since 1994, there have been at least nine nonsurgical studies in which the PPIs omeprazole and lansoprazole were prospectively studied. Table 2 shows the characteristics and results of these studies.22, 82, 105, 106, 107, 108, 109, 110, 111 Of the eight omeprazole studies, five were of the double-blind, randomized, placebo-controlled, and crossover design with treatment ranging from as short as only 4 weeks to as long as only 12 weeks. There were serious problems with the methodology of these studies. First, two studies enrolled asthmatics whose percent variability at baseline was almost normal.106, 108 Such groups could hardly improve with any treatment. Second, five of the seven studies measured only asthma symptoms as an outcome variable, and only two of the five reported improvement.83, 109 Both these studies warrant comment. The first study used the Asthma Quality of Life questionnaire to determine symptomatic improvement.108 Although the study was a crossover design, it did continue for 8 full weeks. Most important, however, is that the subjective symptom parameters paralleled the objective improvement (although only a trend) of the FEV1. The second study, although not randomized, controlled, or blinded, is almost certainly the most systematic, critical, and illuminating study to date.83 Thirty adult asthmatics with solid baseline asthma data were treated with increasing doses of omeprazole until esophageal pH monitoring demonstrated negligible esophageal acid exposure. The omeprazole dose required to accomplish this goal was then given for 3 months while asthma parameters were measured. With this regimen, two thirds of the asthmatics reduced their asthma symptoms by nearly 60%. In addition, the objective parameters of (1) asthma medication requirements, (2) FEV1, and (3) PEF also improved, all of which parallel the improvement in symptoms.
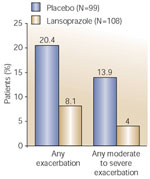
In the one lansoprazole study, Littner et al.111 conducted a double-blind, placebo-controlled trial of 207 moderate-to-severe asthmatics with acid reflux symptoms. Although acid suppression with lansoprazole failed to demonstrate improvement in the diary-recorded daily asthma symptoms, which was the primary outcome variable, acid suppression did significantly decrease the number of mild, moderate, and severe asthma exacerbations (Figure 7) and did significantly improve the overall quality of life. This study was well designed, well planned, and well conducted. Unfortunately, the investigators chose to make the outcome completely dependent on symptoms that may have been better than usual during the very short 14-day baseline.111 In such a case, demonstration of improvement becomes more difficult. Future studies of this kind may benefit from a longer baseline collection period.
Figure 7: Effect of lansoprazole on asthma exacerbations.
(Source: Littner et al.111 with permission)
Overall, the medical management of GER has not provided consistent improvement of asthma control. A recent systematic literature review of 12 relevant randomized controlled trials conducted mostly in adult asthmatics and ranging in duration from 1 week to 6 months reported that treatment of reflux esophagitis did not consistently improve FEV1, PEF rate, asthma symptoms, nocturnal asthma symptoms, or use of asthma medications.112 Indeed, significant improvement in wheeze was reported in only two of the 12 studies.
Uncontrolled Surgical Trials
Uncontrolled Surgical Studies in Adults
Early reports of an association between GER and asthma described a reduction or even a disappearance of the asthmatic state after antireflux surgery.20, 21, 98, 112, 113, 114 In most of these studies, however, pulmonary function tests were not performed before or after surgical repair. Despite the lack of objective data, the dramatic subjective improvement reported in some of the studies (e.g., complete elimination of asthma after 20 years) cannot be ignored. Figure 8 shows the results of the early studies and the more recent uncontrolled studies of antireflux surgery in adults with asthma.
In the earlier uncontrolled adult studies, surgical repair of GER resulted in partial or complete remission of asthma in 18 (75%) of 24 patients113 and 96 (74%) of 129 patients.114 Pulmonary function tests, unfortunately, were not performed before and after surgical repair. It appears, however, that many patients have dramatic subjective improvement in asthma after antireflux surgery.
In three of the studies, objective data were obtained both before and after treatment: 12 (92%) of 13 asthmatic patients improved after antireflux surgery, and most were able to reduce or discontinue completely the pulmonary medications114; seven (70%) of 10 asthmatic patients had at least temporary improvement in pulmonary function115; and 18 (41%) of 44 asthmatic patients who had received surgical correction of GER at least 5 years earlier reported marked improvement or cure and an additional 27% reported moderate improvement.99 In five of the studies, laparoscopic fundoplication was the procedure performed in most of the patients.116, 117, 118, 119 Similar to the open antireflux procedures, the results were dramatic, with improvement or cure of pulmonary symptoms in 50% to 80% of patients.
Controlled Surgical Trials
Only three randomized surgical trials have been reported. In the first, Larrain and coworkers21 conducted an initial 6-month study in 81 patients as well as a 5-year follow-up study. By the end of 6 months, the mean symptom score and medicine score was significantly better in the surgical group and the cimetidine group than in the placebo group. The cimetidine group required less medication, and the surgical group required substantially less medication, but the placebo group required the same or more. By 5 years, only the surgical group had maintained its symptom-free status; the placebo and cimetidine groups were unchanged.
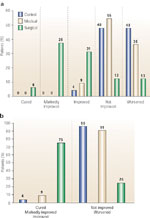
The results of the second study are shown in Figure 9. In this study, 73 patients with both GER and asthma were randomized to receive antacids, ranitidine 150 mg three times daily, or antireflux surgery.103 Follow-up for up to 15 years showed that only surgical correction of reflux significantly improved pulmonary function, decreased the need for bronchodilators and prednisone, and diminished or eliminated the symptoms of asthma.
Figure 9: Overall clinical response of asthma to antireflux therapy.
a: By the end of 2 years, complete cure occurred only in the surgical group and in only 6% of patients. Marked improvement or cure occurred in 44% of surgery patients and in no patients in the control or medical groups. b: Improvement, marked improvement, or cure in the overall asthma status occurred in 74.9% of the surgical group, 9.1% of the medical group, and 4.2% of the control group (p <.001, surgical vs. medical and control). The overall status worsened in 47.8% of the control group, 36.4% of the medical group, and 12.5% of the surgical group. The worsening in the surgical group was attributed entirely to two patients who died within 1 year of their surgery. (Source: Adapted from Sontag et al.103)
In the third study, Spechler et al.124 reported on 151 patients who were enrolled in a multicenter study of therapies for severe GER. The 5-year follow-up demonstrated no improvement in pulmonary function status after 1 year with either the medical or the surgical antireflux treatment. Although these results are consistent with our findings, it is important to note that the 151 patients in the severe GER study were chosen because of GER symptoms, and if pulmonary disease was present, it was incidental and not necessarily apparent. Therefore, no real conclusions can be drawn from this large study.
Outcomes
Interpretation of Studies
The most compelling evidence for the existence of reflux-triggered asthma comes from the results of those clinical studies in which GER was adequately treated.125 In the several studies that report a beneficial response of asthma symptoms to acid reduction therapy with H2-receptor antagonists, the results are not convincing, possibly because the dosage of H2-receptor antagonists used was inadequate to prevent reflux.20, 101, 124 On the other hand, the studies demonstrating improvement or even cessation of wheezing after surgical correction of the reflux provided the strongest evidence yet that GER is either a cause of or contributing factor to the asthma.21, 99, 103, 114 It is reasonable to suggest that the dramatic improvement provided by surgery is related to the prevention not only of acid reflux but also of all gastric reflux.
In conclusion, a number of statements can be made from the current studies:
- Most asthmatics have GER, and the evidence is strong that GER plays an important role in some patients with asthma.
- Despite sophisticated study methods and technologically advanced diagnostic tests, the results of published studies on mechanisms have failed to provide a diagnostic test with a degree of certainty great enough to identify which patients have GER-triggered or GER-exacerbated asthma and which patients will respond to antireflux therapy.
- The difficulties involved in establishing a definite cause-and-effect relationship between GER and asthma are real.
- Even positive results on such direct tests as sputum inspection and scintigraphic monitoring, both of which establish reflux into the tracheobronchial tree, do not necessarily establish cause or effect, and certainly cannot be used to predict outcomes.
- Ambulatory esophageal pH testing can suggest, but cannot prove, the diagnosis of GER-triggered asthma, and we cannot safely rely on pH testing to make our clinical decisions.
- A trial of a PPI is indicated to assess whether asthma improves subjectively and objectively, but the dose must be high enough to prevent even silent esophageal acid exposure, and the duration must be long enough to allow for detection of even subtle trends in subjective and objective respiratory improvement.
- Antireflux surgery, remains a real option and should not be withheld if GER is a reasonable suspect in asthma exacerbations.
- Although strong opinions have been voiced as to whether a good response to PPI therapy predicts a good response to antireflux surgery, the opinions, although logical, are based on personal experience and "gut feelings"; a good PPI response may not necessarily predict a good antireflux surgery response.
- Opinions suggesting that a poor response to PPI predicts a poor response to antireflux surgery may also seem logical, but are not based on clinical data; a poor PPI response may not necessarily predict a poor antireflux surgery response.
- Finally, the only current antireflux therapy that has survived the test of time, even without strong evidence, is the following:
- Avoid eating for 3 hours before bedtime.
- Avoid dietary fat and large meals, which delays gastric emptying.
- Elevate the head of the bed.
- Use PPIs.
- Undergo antireflux surgery.
When the method is found that predicts which patients with GER and asthma will respond to antireflux treatment, the results could be profound: fewer hospitalizations for respiratory complications, less pulmonary morbidity and mortality, less need for pulmonary medications, less time lost from work, fewer visits to a physician's office, and less illness associated with corticosteroid therapy. For the present time, however, clinical judgment and good sense are still our best friends. It is not unreasonable to urge our patients to alter their lifestyle: the huge-volume, calorie-dense, high-fat meals that are eaten before bedtime are not likely to prevent GER or add to our life expectancy.
Gastroesophageal Reflux and Other Pulmonary Diseases
Gastroesophageal reflux is a comorbid condition in other pulmonary diseases including cystic fibrosis (CF) and interstitial lung disease (ILD), such as idiopathic pulmonary fibrosis (IPF) and scleroderma. It may also be a cofactor in bronchitis and COPD. All of these comorbid conditions have only a small body of evidence suggesting a possible relationship between GER and the specific pulmonary disease.
Gastroesophageal Reflux Prevalence in Cystic Fibrosis Patients
Patients with CF have a GER prevalence of approximately 50%126. Furthermore, studies show that CF patients with GER have more severe pulmonary dysfunction compared to those without GER. Although prospective data are not available, aggressive GER therapy may slow the rate of decline in pulmonary function. Gastroesophageal reflux should be treated in CF patients.
Gastroesophageal Reflux Prevalence in Idiopathic Pulmonary Fibrosis Patients
Gastroesophageal reflux may also be a cofactor in IPF. Mays and colleagues127 noted that 54% of IPF patients had GER on barium esophagram compared to 8.5% of normal controls. Furthermore, Tobin and colleagues128 noted that 16 of 17 IPF patients had abnormal esophageal acid contact times compared to four of eight control subjects on esophageal pH testing. Twenty-five percent of these patients had "clinically silent" GER. Raghu and colleagues129 noted that 91% of patients had abnormal esophageal acid contact times, prospectively. A retrospective case series of four patients with IPF showed that aggressive GER therapy stabilized pulmonary function and other clinical outcomes.130 This disease has no proven effective therapy, with most patients dying of respiratory failure within 2 to 5 years of diagnosis.131 It is hoped that further studies will delineate the importance of GER in IPF.
Gastroesophageal Reflux Prevalence in Patients with Systemic Sclerosis
Gastroesophageal reflux is also common in patients with pulmonary manifestations of systemic sclerosis. Johnson and colleagues132 noted that 11 of 13 subjects had abnormal esophageal acid contact times at the proximal probe on pH testing. They also noted a correlation between proximal acid and a decrease in lung diffusion capacity.
Gastroesophageal Reflux Comorbidity in Chronic Obstructive Pulmonary Disease Patients
Gastroesophageal reflux may also be a comorbid factor in COPD. For instance, El-Serag and Sonnenberg133 noted that COPD and esophagitis do coexist with an increased odds ratio of 1.22 in more than 101,0000 veterans with esophagitis. Mokhlesi and colleagues134 noted that COPD patients have an increased prevalence of heartburn or regurgitation and used GER medications more frequently than control groups. More research is needed in this area.
Future Areas for Research and Investigation
Gastroesophageal reflux is a potential trigger in selected asthmatics. There are many unanswered questions about the association between asthma and GER. There are no data examining the role of nonacid GER on airway reactivity. Better diagnostic methods are needed to identify individual asthmatics who have GER-triggered asthma versus individual asthmatics who have GER, but in whom GER is not an asthma trigger. Identifying predictors of asthma response with GER therapy is one way to approach this problem. Furthermore, research is needed on how best to treat GER chronically in the asthma population. Long-term asthma outcomes with GER therapy have not been adequately addressed. It is hoped that future investigations will address these and other areas.