Key Points
- Peptic stricture was traditionally looked upon as an irreversible end stage of acid–peptic damage.
- Patients with peptic stricture are characterized by advanced age, male gender, and vastly abnormal acid reflux into the esophagus, which often contains significant amounts of duodenal juice components.
- Owing to reasons that are not fully understood, fewer of these patients are presenting at the endoscopy units.
- Modern medical therapy is based on profound acid inhibition combined with endoscopic balloon dilatation.
- Surgical therapy means usually endoscopic dilatation combined with fundoplication to control reflux.
- It is uncommon that complex surgical procedures are required such as resection or esophageal lengthening reconstructions.
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common diseases of the alimentary system of the Western adult population. Indirect evidence has been presented to indicate that the disease is affecting an expanding proportion of the population.1, 2, 3, 4, 5 Moreover, it seems as if the disease is expressed in increasing numbers also in other parts of the world as well, where traditionally very few patients have been affected.6, 7, 8, 9, 10 During the last decades, it was noted that GERD manifested itself in different ways, with a dominant proportion of patients presenting without macroscopically recognizable lesions at the time of diagnostic endoscopy. It is a challenging possibility, related to the many enigmas surrounding the disease, that an increasing number of patients suffering from the disease will not have esophagitis and hypothetically will not even develop it, even though they report the same duration and intensity of symptoms. Despite these many uncertainties, it is obvious that GERD is a chronic disease that requires sustained and maintained therapy in order to control the symptoms and thereby normalize patients' quality of life. Traditionally, chronic GERD patients were seldom seen by the general practitioner but rather by the gastroenterologist. Indeed they presented the specialist with a variety of therapeutic problems, not the least of which was that the actual disease state was often considered to represent the end stage of the disease severity spectrum.1, 11, 12, 13, 14 Accordingly, patients with Barrett's esophagus and/or peptic stricture were met with both frustration and respect.15, 16 The diagnosis of a peptic stricture was frequently associated with the presence of a shortened esophagus (brachyesophagus). This situation often led to further referrals to a specialist surgical center for treatment. In those days fairly complicated operative procedures had to be endorsed, even though they entailed substantial morbidity and significant mortality.15 This historical perspective contrasts profoundly with the situation today, where peptic strictures are seldom diagnosed, and if they are, can be effectively treated by conservative means incorporating aggressive proton pump inhibitor (PPI) therapy and endoscopic balloon dilatation. Consequently, the management strategies relevant for patients with peptic strictures have changed dramatically during recent decades. This review focuses on the epidemiology and pathophysiology of the condition and on modern diagnostic and therapeutic management perspectives.
Clinical Presentation and Diagnostic Features
Epidemiologic Aspects
There are good reasons to look upon peptic strictures as representing severe end stages of reflux disease.15, 16, 17 Not only do peptic strictures develop in 4% to 20% of patients with reflux esophagitis, but as many as 25% to 50% of stricture cases have a concomitant columnar metaplasia of the squamous epithelium (Barrett's esophagus).16, 18 Based on similar preconditions, it has to be recognized that peptic stricture patients suffer not only from absent or very low lower esophageal sphincter (LES) pressures but also from deficient clearance function of the esophagus and large hiatal hernias.19, 20 Altogether, these deficiencies result in massive amounts of gastric juice regurgitating in the oral direction, a refluxate that contains substantial amounts of noxious duodenal components. In old endoscopic series it was reported that peptic strictures were seen in 10% to 15% of cases having chronic GERD. Indeed, similar observations were made in the Western adult population with a massive male predominance and an advanced mean age at the time of referral for endoscopy. Although dysphagia is a common symptom in GERD and a frequent complaint among those who are referred for endoscopy, the prevalence of newly diagnosed strictures has declined dramatically during recent years.21 This is a challenging and somewhat contradictory observation, because the number of hospital admissions for peptic strictures seems to have increased during corresponding time periods.22 Are these two findings compatible with each other? It is quite reasonable to propose that GERD is steadily shifting toward less severe disease manifestations, at least when it comes to the endoscopically visible counterparts of the disease. Moreover, clinicians now have available very effective medical therapies, and their widespread use might well affect even the presenting features of the disease. Still, those few GERD patients who present with transmural fibrosis and decompensated esophageal transport capacity not only remain fairly stable but also need to be referred to a hospital for further evaluation and management. As a consequence of the continuous change in the age profile of the Western population, the end results might well be that the total referral numbers due to peptic strictures may in fact increase.
The Aging Esophagus
The impact of age has many dimensions, as reflected by the presence of esophagitis in 81% of patients over the age of 60, compared to 47% among younger patients despite similar frequency and severity of heartburn. Others have reported the corresponding figures to be 25% and 15%, respectively.17, 20 Brunnen and coworkers23 reported a dramatic increase in the frequency of the peptic strictures in patients who had passed the age of 50 years. The complexity of the situation is also illustrated by the fact that elderly people more frequently take medications known to decrease the sphincter tone, which may promote reflux. It is also well documented that the frequency of sliding hiatal hernias, which affect the clearance of reflux material from the distal esophagus, increase with age.24 Furthermore, the clearance capacity may be impaired in the elderly as a consequence of disturbances in esophageal motility and saliva production. If adjustments are made for concomitant disorders such as diabetes mellitus and rheumatologic disorders, which by themselves may alter esophageal motility, it has been found that the amplitude of the peristaltic pressure wave decreases with age but not the duration and propagation velocity. On the other hand, an increased frequency of nonpropulsive, often-repetitive, contractions has been reported. Slight but insignificant decreases in saliva volume and bicarbonate concentration have been reported in older subjects.20 More importantly, however, is that older subjects may show a decreased salivary bicarbonate response to acid perfusion challenge to the distal esophagus. Another important contributing factor that has to be recognized is the age-related decrease in esophageal pain perception.
Involved Mechanisms
The mechanisms for strictures formation in reflux disease are complex. Initially an inflammatory process with edema and inflammatory reaction develops and then progresses into the depositions of connective tissue, eventually resulting in fibrosis. In the vast majority of cases, strictures develop as a consequence of severe mucosal peptic damage, as represented by Los Angeles grade C and D lesions, at the time of diagnostic endoscopy. If similar lesions remain unrecognized or untreated, the mucosal damage continues and subsequently affects deeper layers of the esophageal muscular wall. Initially esophageal narrowing results from edema and spasm, and is reversible when treated adequately with, for example, profound acid-suppression therapy. Fibrosis of the muscularis mucosa results as a consequence of continued uncontrolled reflux injury and may lead to the deposition of type 3 collagen and reversible fibrotic narrowing of the esophagus. In conjunction with these destructive processes, reparatory mechanisms are operational with the appearance and expansion of connective tissue, which leads to fibrous tissue formation. In a situation when the latter predominates in the esophageal wall, even affecting the periesophageal tissue, we eventually have the development of a transmural stricture.25, 26, 27
Associated Concomitant Conditions
There are numerous associated conditions other than GERD that may present with esophageal strictures. Such conditions include Barrett's esophagus, scleroderma, Zollinger-Ellison syndrome, Schatzki's rings, postachalasia treatment, and previous treatment with prolonged nasogastric intubations.28, 29, 30, 31 In the older literature, Barrett's esophagus was frequently suggested to be associated with peptic strictures, although more recent data would suggest a weakening of the association. Schatzki's ring is almost always associated with a hiatal hernia and commonly progresses to a peptic stricture. It has been demonstrated that more than half of patients with Schatzki's ring concomitantly have reflux disease when studied by 24-hour pH monitoring. Furthermore, the natural history of these rings suggests that a substantial proportion may progress to a complete stricture within 1 to 5 years.
Scleroderma is associated with esophageal symptoms in the majority of patients, and almost half of them have peptic strictures. These strictures are particularly problematic in that the underlying esophageal defect, aperistalsis, and low or absent lower esophageal sphincter (LES) pressure all cause prolonged acid damage to the esophagus.
Aspirin and nonsteroidal antiinflammatory drugs (NSAIDs) have frequently been closely associated with esophageal stricture formation.32, 33 It has been shown that almost 75% of the stricture patients consume similar drugs compared to only one fourth of the controls. Others have shown that almost 50% of patients with benign esophageal strictures had taken NSAID within the 12 months preceding the diagnosis, compared to only 10% among controls. Therefore, a history of drug use is very important to obtain.
Prolonged use of nasogastric tubes may be associated with the development of long and complex esophageal strictures due to the impairment in LES function and prolonged acid exposure to the esophageal mucosa. Similar strictures may be particularly difficult do deal with and may require multiple sequential dilatations.
Diagnostic Workup
The history can often facilitate diagnosing the cause of complications of esophageal stricture in the majority of cases. The typical presentation of esophageal stricture includes the insidious and sometimes sudden occurrence of dysphagia to solid food with antecedent pyrosis. However, in up to 25% of cases there is no prior history of heartburn and other acid-related symptoms. In fact, some patients present a history in which reflux-related symptoms might even resolve over time secondary to progression of fibrosis and esophageal narrowing, only to return after therapeutic dilation.34, 35, 36, 37, 38, 39, 40 Over time both solid and liquid dysphagia may develop due to progressive narrowing of the stricture and associated inflammation affecting esophageal motility. Intermittent dysphagia, separated by long periods of no symptoms, is most probably suggestive of a Schatzki's ring.
The first recommended diagnostic procedure for patients with dysphagia is endoscopy, if not otherwise contraindicated. It has the sensitivity to detect even subtle mucosal lesions, such as esophagitis, and is superior to any alternative approach. Furthermore, endoscopy enables the use of various therapeutic devices such as the passage of guidewires through the endoscope to perform balloon dilatation. The appearance of a peptic stricture is characterized by a smooth narrowing of the distal esophagus with decreased mucosal vascular pattern, which is difficult to distend with air insufflation. Peptic strictures are usually located at the squamocolumnar junction; if another location is found, the stricture might not be peptic. Biopsies from the stricture are mandatory to rule out any associated neoplasm. Biopsy does not preclude subsequent dilatation because the combination has not been associated with an increased perforation risk.
In patients with repeated strictures, and particularly in those with more complicated strictures, barium swallow is indicated. A barium investigation reliably identifies the location, diameter, and length of the peptic lesions. It should be kept in mind that barium swallow is more sensitive than endoscopy for detection of relatively "open" strictures, that is, those that are  10 mm in diameter. Furthermore, a barium swallow has the advantage of more accurately identifying the presence of a Zenker or epiphrenic diverticulum or paraesophageal hernia. It is, however, reasonable to propose that a barium swallow should be a secondary step in the investigation algorithm of these patients.
10 mm in diameter. Furthermore, a barium swallow has the advantage of more accurately identifying the presence of a Zenker or epiphrenic diverticulum or paraesophageal hernia. It is, however, reasonable to propose that a barium swallow should be a secondary step in the investigation algorithm of these patients.
Therapeutic Considerations
Pretreatment Classification
A benign peptic esophageal stricture should always be looked upon as a serious complication resulting from persistent and chronic gastroesophageal reflux, the magnitude of which is far above what is considered to be normal.21, 41, 42 Moreover, it has to be recognized that esophageal strictures may have different degrees of severity, with a variety of clinical complications not the least regarding the responses to medical or surgical treatment involving esophageal dilatation.18 Few methods of classification of esophageal strictures have been proposed that would offer a basis for choosing the most effective and appropriate treatment as well as a tool for clinical research purposes. Successful management depends on accurate preoperative evaluation, preoperative and pretreatment assessment of the patient, as well as the character of the stricture. The choices between medical or surgical treatment, incorporating more conservative or sometimes more aggressive operative procedure such as esophageal resection, remain difficult and controversial. A stricture classification that could eventually be very useful, based on assessment of the severity and anatomic extent, has been launched by Csendes's group,18 whereby the stricture is classified according to its internal diameter, its length, and the ease with which dilatation can be performed to determine the preferred treatment, incorporating the prognosis relevant for early as well as late results (Table 1). The diameter of the stricture is determined by endoscopic evaluation and radiologic investigation. The length of this stricture is measured radiologically, expressed in millimeters, and categorized into one of three groups (I.to III). The physician who performs the procedure can determine the visibility and ease of dilatation. Findings can be divided into two categories: easy dilatation, where the dilator easily passes trough the stenosis, and difficult dilatation, where it becomes necessary to use guidewires during fluoroscopic guidance to bypass difficult anatomic passages. The anatomic and histologic features are taken into account, such as the presence of fibrous tissue, a long narrow or sigmoid segment of the stricture with lateral blind recesses, or a lateral hole at the point of the stricture.
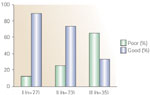
The response to dilatation is considered to be good when the patient experiences complete relief or substantial improvement of the dysphagia, and the patient is now able to swallow solid food, which usually occurs when the internal diameter of the esophageal lumen remains wider than 15 mm (45 French). The response is considered poor when repeated dilatations become necessary due to recurrence of the stricture, when it is difficult to enlarge the internal diameter of the esophagus, and when an enlargement of the esophageal lumen can be obtained but early (within 1 month after dilatation) reappearance of the stricture is observed and the patient can swallow only liquid or semisolid food. It is crucial also to classify these strictures because it is most likely that it may have consequences for the subsequent choice of therapeutic management (Figure 1). It is possible that strictures of type III may best be treated surgically, and in those instances medical treatment with endoscopic esophageal dilation may only be tried initially, with a readiness to promptly switch therapeutic strategy at the time of failure. The lack of standardized classification and characterization of the patient series are likely to constitute the most important reasons behind the divergences in outcomes reported in the literature.
Figure 1: The response to dilatation of peptic strictures by pretreatment classification.
(Source: Modified from Braghetto et al.18)
Medical Therapy
What implication does the information discussed in this review have on the modern therapeutic treatment choices for peptic strictures with or without the presence of Barrett's esophagus? Data have accumulated to show that most patient with complicated reflux esophagitis will continue to have abnormal esophageal acid exposure, despite complete symptom relief on standard types of medical acid inhibitory treatment.40, 41, 42, 43 In those instances, reflux most often occurs during the night, when the patient is in the recumbent position, with delayed esophageal clearance and the potential for sustained mucosal damage. Recent studies have found that abnormal esophageal acid exposure continues in these patients despite even very high doses of PPIs. Furthermore, there is variability in the response to PPIs among patients, particularly those in the older age groups. It can therefore be argued that PPI therapy has to be individualized, depending on the level of reduction in acid exposure as assessed by 24-hour pH monitoring.
In the era before the introduction of PPIs, peptic strictures were widely regarded as fixed, fibrotic lesions that would respond only to a mechanical therapy aimed at stretching or tearing the fibrous tissue. In support of this notion, clinical trials comparing treatment with histamine H2-receptor antagonist with placebo in patients with peptic esophageal stenosis found an improvement in esophagitis scores in those patients who received active drug but no reduction in the need for dilation.44, 45, 46 However, other studies of similar patients have shown that aggressive acid-suppression therapy with PPIs both improve these features and decrease the need for subsequent esophageal dilatation (Table 2).47, 48, 49, 50, 51, 52, 53 In this context it should be remembered that one reversible component in these patients, with concomitant reflux esophagitis, is the contribution of inflammation to the dysphagia, which promptly reacts to modern therapies.
Endoscopic Dilatation
In addition to treatment with PPIs, patients with dysphagia caused by esophageal peptic strictures are treated with esophageal dilatation. Three major types of esophageal dilating devices are commonly used: (1) mercury field bougies that are passed linearly through the mouth (e.g., Maloney dilators); (2) polyvinyl bougies that can be passed over a guidewire positioned through the stricture using either fluoroscopic or endoscopic guidance (e.g., Savary dilators); and (3) balloon dilators that are passed either over a guidewire or through the working channel of the endoscope. Usually the physician passes a series of dilators or gradually increases the diameter of the balloon to stretch out the stricture. No convincing evidence has been presented to establish the superiority of one type of dilator over the other. Serious complications such as perforation and bleeding occurred in approximately 0.5% of all esophageal dilation procedures.54, 55, 56, 57, 58, 59, 60 Endoscopic management of difficult strictures will develop further, including the use of complementary procedures such as steroid injection and temporary placement of expandable stents.61, 62
Surgical Therapies
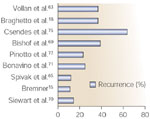
Which surgical procedures can be used in the treatment of peptic strictures? Different techniques have been proposed during the last 20 years but until now there has been no consensus on which one results in the most favorable outcome, even with long follow-up (Figure 2). Conservative antireflux surgery with classic fundoplication has been employed for peptic stricture patients with a long-term success rate ranging from 65 to 90%, depending on a variety of factors, such as the procedure employed, length of follow-up, and the nature of the long-term evaluation.63, 64, 65, 66, 67, 68, 69, 70, 71 Siewert70 reviewed the results of dilatation and antireflux surgery and reported good results in 85% of the cases, with a mortality rate of 2%. Recent studies using the laparoscopic approach report a 12% failure rate, whereas others demonstrate significantly higher recurrence rates (25%). More aggressive procedures such as esophageal lengthening gastroplasty of the Collies-Nissen type or Collies-Belsey Mark IV type have been proposed, in view of the previously reported poor results with classic antireflux surgery. The rationale behind this surgical approach rests on the presence of a short esophagus in virtually all of these patients and the need to ensure an intraabdominal portion of the esophagus to obtain sustained reflux control. The Collies-Belsey repair is associated with success rates ranging from 65% to 85%, with a postoperative mortality varying from 0.4% to 2%. Corresponding figures have been reported after the Nissen type of antireflux repair.72, 73, 74, 75 More mutilating surgical procedures, incorporating partial gastrectomy, vagotomy with or without biliary diversion, or duodenal switch procedures have been introduced.74, 75 These procedures yield good results in a significant proportion of patients, but the morbidity and especially the mortality speak strongly against the use of these quite extensive surgical procedures in the management of, by definition, a benign disease.
Esophageal resection has been proposed in patients with severe stricture, poor contractility, or high-grade dysplasia. Similar procedures are associated with increased morbidity and mortality, and the functional results of esophageal reconstruction with the stomach or colon as a conduit are invariably burdened with some degree of postoperative sequelae. It is therefore important to use these operations only in very selected groups of patient. The introduction of vagal-sparing esophagectomy,76 however, may change this situation so that a somewhat more liberal use of resective procedures in cases of therapy-resistant peptic strictures would be possible.