Key Points
- When reflux disease involves the larynx or pharynx, it is referred to as laryngopharyngeal reflux (LPR) or extraesophageal reflux, rather than gastroesophageal reflux disease (GERD).
- Laryngopharyngeal reflux and GERD are two related, yet different, disease states with different risk factors, pathophysiology, treatment, and outcomes.
- Patients with LPR infrequently have significant heartburn, and most commonly present with laryngeal symptoms such as hoarseness, globus sensation, throat clearing, sensation of postnasal drip, difficulty swallowing, chronic cough, and laryngospasm.
- Serious complications of LPR include obstructive pathology, such as laryngeal granulomas, subglottic/glottic stenosis, laryngospasm, and even laryngeal carcinoma.
- Although the complications of LPR may require surgical management, aggressive perioperative reflux therapy should be initiated if the patient is to achieve an optimal outcome.
Laryngopharyngeal Reflux (LPR)
Gastroesophageal reflux involves the backflow of stomach contents into the esophagus. In some cases the gastric refluxate reaches the larynx or pharynx, which is called laryngopharyngeal reflux (LPR). Other synonyms that have been used for LPR include extraesophageal reflux, "atypical" reflux, gastropharyngeal reflux, laryngeal reflux, pharyngoesophageal reflux, and supraesophageal reflux. Using prolonged pH monitoring, the diagnosis of gastroesophageal reflux disease (GERD) is typically not made unless a patient exhibits over 45 reflux episodes or has elevated acid exposure times at the esophageal probe (Table 1). Esophageal acid exposure times below this are considered normal or physiologic esophageal reflux. In contrast, one or two episodes of acid exposure (pH <4) at the proximal probe located in or above the upper esophageal sphincter (UES) is accepted as diagnostic of LPR. Although these diagnostic criteria for GERD and LPR may vary across institutions, the contrasting pH probe criteria for LPR and GERD is a common theme, because laryngeal and pharyngeal mucosa are much more susceptible to acidic injury than esophageal mucosa.
Although some patients meet pH probe criteria for both GERD and LPR, many have LPR or GERD alone. Over recent decades, studies have shown LPR and GERD to be two unique but related disease entities, with different risk factors, symptoms, pathophysiology, and responses to therapy.1, 2, 3, 4, 5, 6 Furthermore, the diagnostic modalities for the evaluation of LPR are different from those for GERD.
Patient risk factor profiles and complaints differ greatly from LPR patients to GERD patients. For example, obesity is not associated with isolated LPR; however, it has a strong association with GERD and it is an independent risk factor for GERD symptoms and erosive esophagitis.4, 5 Patient complaints also differ from LPR to GERD patients. One landmark study demonstrated that 100% of otolaryngology patients with reflux complained of hoarseness, but only 6% of them reported heartburn.6 In contrast, the same study demonstrated that 89% of gastroenterology patients with reflux reported heartburn, and none of them complained of hoarseness.
Esophageal motor function has also been shown to differ between LPR and GERD patients. In one study, esophageal acid clearance time was significantly longer in patients with GERD (isolated or in combination with LPR) than in patients with isolated LPR.2 In addition, the difference in acid clearance between LPR patients and normal individuals was not statistically significantly different. This suggests that differences in pathophysiology between LPR and GERD patients are part of the basis for the contrasting symptomology. Not only is esophageal motility generally different between LPR and GERD patients, but reflux patterns on pH probe studies demonstrate that LPR patients are more likely to have reflux in an upright position whereas GERD patients are more likely to reflux in a supine position.1
Both LPR and GERD can be evaluated by a variety of diagnostic tests, including barium esophagography, radionucleotide scanning, the Bernstein acid perfusion test, esophagoscopy with biopsy, impedence testing, and pH probe monitoring. In addition, the clinical evaluation of LPR generally involves a flexible (or rigid) laryngoscopy examination, and laryngeal sensory testing may be added. Although dual pH probe testing is reasonably sensitive and specific for reflux events, the other diagnostic modalities, such as barium esophagography, radionucleotide scanning, and the Bernstein acid perfusion test, have a low sensitivity and therefore have been largely abandoned as diagnostic tests of choice for reflux. Multichannel intraluminal impedance testing is a newer modality that allows detection of both acidic and nonacidic reflux events, and has shown excellent sensitivity in GERD populations, but its role in LPR patients remains to be determined.7
Perhaps the greatest difference in diagnostic testing for GERD and LPR patients is the role of esophagoscopy with biopsy. Although esophagitis is found in only 12% patients with LPR,3 it is considered the sine qua non of GERD. In fact, most uncomplicated GERD patients never require pH probe testing or esophagoscopy for diagnosis and are simply followed empirically for symptom improvement.8 Because of the low rate of significant esophagitis among LPR patients, either trials of empiric antireflux therapy or pH probe testing are generally warranted as the initial diagnostic steps. Esophagoscopy in LPR patients [whether transnasal esophagoscopy (TNE) or "traditional"] has been reserved for patients with symptoms of both LPR and GERD, patients with other risk factors for esophageal pathology such as Barrett's esophagus, patients requiring chronic antireflux therapy, and patients with pulmonary manifestations such as chronic cough. However, a provocative report from Reavis et al.9 suggests that esophageal screening for Barrett's metaplasia is important for patients with LPR and chronic cough. In this retrospective paper, individuals with LPR symptoms had a greater prevalence of esophageal dysplasia and cancer than patients with "classic" GERD symptoms.
Response to antireflux therapy is another major contrast between LPR and GERD patients. The GERD symptoms and esophagitis are often well controlled with once-daily proton pump inhibitor (PPI) therapy with rapid relief of symptoms usually in less than 2 weeks. Daily PPI therapy has been shown to last for less than 14 hours if given in the morning, and an evening dose may last less than 10 hours.10 Although this coverage may be adequate for esophageal healing, the laryngeal mucosa is much more susceptible to refluxate injury, and therefore often requires twice daily (24-hour) PPI coverage.11, 12 Furthermore, GERD symptoms may improve rapidly after initiating PPI therapy, whereas LPR symptoms often take several months to resolve.1, 13
History of Laryngopharyngeal Reflux
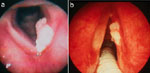
With the introduction of ambulatory pH monitoring, manometry, and flexible fiberoptic esophagoscopy in the 1960s, evidence-based research regarding GERD rapidly expanded. In 1968, acid-related laryngeal ulcerations and granulomas (Figure 1) were first reported in the otolaryngology literature. Subsequent studies suggested that acid reflux might be a contributory factor in hoarseness, globus pharyngeus, dysphagia, chronic cough, otalgia, and laryngospasm. Acid reflux also was implicated as a potential etiology in various laryngeal diagnoses such as posterior laryngitis, laryngeal stenosis,14 and laryngeal carcinoma.
The landmark study by Koufman1 in 1991 demonstrated abnormal acid reflux on dual pH probe testing in 62% of 182 patients with laryngeal carcinoma, stenosis, laryngitis, globus pharyngeus, dysphagia, and chronic cough. Only 43% of the patients had symptoms of heartburn or regurgitation, whereas most of them had laryngopharyngeal symptoms. Many of the patients (n = 128) also underwent barium esophagography with videofluoroscopy, with dysmotility being detected in 12% and spontaneous gastroesophageal reflux (GER) in only 9%. After 6 months of antireflux therapy with behavioral modification and aggressive H2-antagonist therapy, 85% of the patients available for follow-up (n = 123) had resolution of their LPR symptoms. More important, the study included an animal model that demonstrated that more severe inflammation resulted when a preexisting subglottic mucosal injury was repeatedly treated topically with pepsin and acid (up to pH 4) when compared with acid alone or controls. Thus, it was made clear that acid alone was not the sole source of injury in reflux disease, but rather acid and active pepsin.
Epidemiology
Although the prevalence of LPR in the general population is not known, the prevalence of reflux among patients with laryngeal and voice disorders has been estimated to be about 50%.15 In contrast to GERD patients, patients with isolated LPR are not obese.4, 5 Unfortunately, the remaining risk factors and patient profiles for LPR are not well established because epidemiologic studies in this area are lacking.
Pathophysiology
The etiology of reflux events that occur with LPR are largely unknown, although UES dysfunction has been hypothesized as a possible factor. Although esophageal dysmotility and lower esophageal sphincter dysfunction play important roles in GERD, they appear to have less of a role in LPR. Furthermore, manometry, including the pharynx and UES, demonstrates that patients with LPR often have normal esophageal motility.1, 2 Unlike GERD, which primarily involves supine (nocturnal) reflux events, LPR reflux occurs frequently in the upright position. The reflux events in LPR also tend to be brief relative to the prolonged events that occur with GERD.1, 6
The laryngeal damage that occurs in LPR is not caused by acid alone, but it requires both acid and activated pepsin, and it must be remembered that pepsin remains active over a pH of 5.0.1, 16, 17 When compared to the esophageal mucosa, the laryngeal mucosa is injured with much lower levels of acid/pepsin exposure. It has been accepted that the extrinsic defense mechanisms between the laryngopharynx and the esophagus are markedly different, with the latter having much more resistance to acid peptic exposure. In fact, the intrinsic defense mechanisms of the laryngeal and esophageal mucosa are different as well. For example, one of the carbonic anhydrase (CA) isoenzymes, CA III, has been shown to have increased expression in the esophageal mucosa in response to refluxate exposure, whereas the larynx demonstrates a depletion of CA III after chronic reflux exposure. Furthermore, although esophageal mucosal response to acid/pepsin exposure appears to often be readily reversible, laryngeal mucosa can easily be damaged irreversibly.16, 17
History and Symptoms
Patients with LPR commonly present with complaints such as hoarseness, globus sensation, throat clearing, sensation of postnasal drip, difficulty swallowing, chronic cough, choking episodes/laryngospasm, and (occasionally) heartburn. These items have been included in the reflux symptom index (RSI), which is a validated clinical tool for LPR18 (Table 2). Often patients report a long history of LPR symptoms, gradually worsening with time. Occasionally an event such as endotracheal intubation, vocal abuse, or an upper respiratory infection leaves the patient dysphonic, with the patient having increased awareness of the full spectrum of LPR symptoms. Such an injury may be slow to heal in a patient with LPR, and make the patient aware of what had previously been clinically silent LPR. In these situations, it is important to rule out an underlying vocal fold mucosal injury or paresis with a careful evaluation often including laryngeal electromyography. The LPR patients who do not symptomatically improve with antireflux therapy often have another underlying laryngeal disorder such as a vocal fold paresis. Laryngeal examination reveals subtle bowing or hypomobility of one or both vocal folds. If the paresis fails to improve with time, treatment involves augmentation with injection laryngoplasty or medialization laryngoplasty. In some patients, pH probe testing while on antireflux medications is warranted to confirm an appropriate response to medication.
Physical Findings
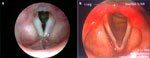
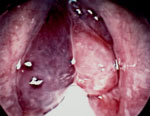
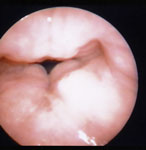
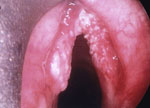
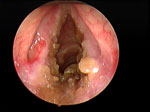
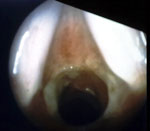
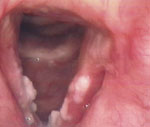
Although LPR has been associated with multiple otolaryngologic disorders, the most common physical findings of LPR are related to laryngeal mucosal edema and injury. Laryngeal mucosal injury can result in serious pathology ranging from ulcerative disease, granulomas, subglottic stenosis, and possibly laryngopharyngeal cancer. However, the most common findings during laryngoscopy are related to chronic inflammatory changes. The first such finding is pseudosulcus vocalis, which refers to infraglottic edema that passes posterior to the vocal process of the arytenoid cartilage (and can thereby be differentiated from true sulcus vocalis) (Figure 2). As an independent finding, pseudosulcus has been strongly associated with LPR.19, 20, 21 The second principal finding in LPR is ventricular obliteration, which refers to edema of the true and false vocal folds appearing to obliterate or obscure visualization of the laryngeal ventricle (Figure 3a), in contrast with the wide open laryngeal ventricles seen in Figure 3b. Interarytenoid and laryngeal erythema is also an LPR finding. However, erythema can vary greatly based on the light source, endoscope, and video monitor used, so this finding alone tends to hold less importance. True vocal fold edema is another important finding with LPR, which can vary from mild to severe polypoid degeneration. Figure 4 demonstrates such severe edema in a patient undergoing surgical voice rehabilitation. In addition to true vocal fold edema, diffuse laryngeal edema and posterior commissure hypertrophy are also common findings in LPR (Figure 5). Granulation tissue or granuloma formation may also be present.
Figure 2: Infraglottic edema: a finding highly sensitive but not specific for laryngopharyngeal reflux (LPR).
(Source: Belafsky et al.19, with permission from Lippincott, Williams and Wilkins.)
Figure 3: a: Ventricular obliteration is secondary to edema of the true vocal folds and false vocal cords.
b: Wide-open laryngeal ventricles in a patient who also has mild presbylaryngia.
Analogous to the RSI, these laryngeal findings have been used to create the reflux finding score (RFS), which is a validated tool for LPR19 (Table 3). Although the RSI and RFS are not diagnostic for LPR and are not a replacement for pH monitoring, they do assist greatly in the diagnosis of LPR and in following the patient's clinical response to treatment.
Laboratory Findings
Diagnostic modalities such as barium esophagography, radionucleotide scanning, and the Bernstein acid perfusion test have all been shown to have a low sensitivity and therefore have been largely abandoned as diagnostic tests for LPR. Recently, impedance monitoring has been introduced as a modality that allows detection of both acidic and nonacidic reflux events. Impedance may play a role in detecting nonacidic reflux events in symptomatic patients who have had negative pH probe studies, or in LPR patients who are refractory to antireflux therapy. Its most significant role may be in the evaluation of pulmonary patients such as those with chronic cough and refractory reactive airways diseases and chronic obstructive pulmonary disease (COPD). However, because of the newness of the technology, there is currently little information available on the application of impedance testing to LPR patients. Although dual pH probe monitoring has several disadvantages, with the greatest being patient discomfort, it remains the most sensitive and specific diagnostic test available for LPR.22, 23
Dual pH probe testing is typically done in an outpatient setting with the patient being monitored over a 18- to 24-hour period. Using manometry, or endoscopic guidance, the lower probe is placed approximately 5 cm above the lower esophageal sphincter, with the upper probe placed just above the UES. This placement of the upper or proximal probe is essential, because the UES functions as the final barrier against LPR. Thus, proximal esophageal reflux does not accurately reflect extraesophageal or pharyngeal reflux events owing to the protective function of the UES.24, 25
The pH probe criteria for LPR remain controversial owing to the lack of strong normative data. A recent meta-analysis by Merati et al.26 demonstrated that both pharyngeal probe acid reflux events (pH <4) and acid exposure times were significantly lower in normal subjects when compared to patients with LPR symptoms. However, nearly 20% of the normal, asymptomatic subjects had at least one episode of acid exposure at the pharyngeal probe when it was placed above the UES, and the percentage was even higher when the probe was at the UES. Thus, although asymptomatic, normal subjects may have some laryngopharyngeal acid exposure, the threshold for when acid exposure meets the criteria for clinically significant LPR is not well established. We define LPR as one or more pharyngeal reflux events (pH <4) over a 24-hour period, although some normal individuals will be included in this group. An event is only counted as a pharyngeal reflux event if it is a rapid, sharp drop in pH that is immediately preceded by esophageal acid exposure and does not occur with swallowing. This criterion may change as more normative data are analyzed (currently in progress). Furthermore, studies have demonstrated that pepsin-induced laryngeal mucosal injury can occur up to a pH of 5.0, and therefore pH <5 may more appropriately define extraesophageal acid reflux than the current standard of pH <4.16, 17
Clinically one finds that the previous two diagnostic modalities (physical examination and pH monitoring) are complementary in nature, and when used together are often powerful tools. Oelschlager et al.27 grouped patients clinically by positive or negative pH testing and normal or abnormal RFSs. Proton pump inhibitor therapy was effective in 88% of individuals in whom both studies were abnormal, whereas only half that number responded in those with a normal RFS and pH study.
Differential Diagnosis
Laryngopharyngeal reflux is a chronic, relapsing condition that results from repeated extraesophageal exposure to gastric refluxate. Other conditions that cause chronic laryngitis should be included in the differential diagnosis, depending on the patient's clinical presentation. If the patient presents with fever, lethargy, and a more abrupt onset of symptoms, infectious causes should be considered, such as supraglottic bacterial infection (group A streptococcus, Haemophilus influenzae), or viral infection (parainfluenza, influenza, rhinovirus, adenovirus, herpes simplex). Acute onset of laryngeal edema without fever/lethargy is more suggestive of an anaphylactic or angioedema reaction. However, immunocompromised patients may mount little response with infectious laryngitis, and are susceptible to a wide variety of infectious etiologies, so immune status should always be taken into consideration. In more chronic forms of laryngitis, the differential should include allergy, granulomatous diseases, autoimmune diseases, inhalant injury, and radiation therapy (Figure 6). Allergies can produce severe, acute laryngeal edema in the cases of anaphylaxis, or low-grade inflammatory changes in the case of chronic antigenic stimulation. Granulomatous diseases include infectious diseases such as tuberculosis (Mycobacterium tuberculosis), leprosy (Mycobacterium leprae), scleroma (Klebsiella rhinoscleromatis), actinomycosis (Actinomyces bovis or israelii), tularemia (Francisella tularensis), glanders (Pseudomonas mallei), and syphilis (Treponema pallidum). Granulomatous fungal infections can also affect the larynx, including candidiasis (Candida albicans), blastomycosis (Blastomycosis dermatitidis), histoplasmosis (Histoplasma capsulatum) (Figure 7), aspergillosis (Aspergillus fumigatus), coccidioidomycosis (Coccidioides immitis), and sporotrichosis (Sporothrix schenckii). Noninfectious granulomatous diseases of the larynx that should also be considered include sarcoidosis and Wegener's granulomatosis (Figure 8). Autoimmune diseases that produce laryngeal inflammation most commonly have coexistent systemic symptoms. Examples include rheumatoid arthritis, systemic lupus erythematosus, cicatricial pemphigoid, relapsing polychondritis, and amyloidosis.
Figure 7: a: Histoplasmosis mimicking a squamous cell carcinoma.
The lesion resolved with antifungal therapy. b: Squamous cell carcinoma of the right true vocal fold at surgery.
Figure 8: Crusting granulation tissue seen in the larynx and subglottis of a patient with Wegener's granulomatosis.
Similar findings are often seen in the sinonasal region, trachea, and bronchi.
Inhalation injuries are most commonly related to tobacco use, but can include pollution and thermal (burn) injuries. In some cases chronic steroid inhaler use can also cause laryngeal inflammation that can mimic LPR. Finally, radiation therapy for head and neck malignancy often results in chronic laryngeal edema, which may be mistaken for LPR. Of course, in all these cases of chronic laryngitis, patients should also be tested for coexistent LPR or empirically treated because response to any therapy will be suboptimal if LPR is not also adequately addressed. In evaluating any patient with chronic laryngitis, it must be a priority to rule out an underlying laryngeal malignancy, which can be masked in cases of severe laryngeal inflammation.
Clinical Course and Complications
If inadequately treated, LPR can result in multiple pharyngeal and laryngotracheal manifestations. The most concerning of LPR complications are those that can result in airway obstruction, and include laryngospasm, paradoxical vocal fold motion, granuloma, stenosis, polypoid degeneration, and even laryngeal carcinoma.1, 15, 28 The motion disorders associated with LPR (laryngospasm and paradoxical vocal fold motion) can often be managed with aggressive antireflux therapy combined with speech therapy. Laryngeal lesions such as granulomas and subglottic stenosis often show drastic improvement or resolution with antireflux therapy alone, so, in the case of a safe airway, initial medical management of reflux is generally warranted. Even patients who require surgical intervention for obstructive airway lesions such as polypoid degeneration, laryngeal carcinoma, granulomas, and stenosis, should be started on aggressive antireflux therapy preoperatively and maintained on it postoperatively. Not only do all of these disorders have an association with LPR, but in our experience if the LPR is not adequately treated perioperatively, the recurrence and complication rates are higher.27
Granulomas
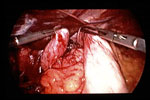
The etiology of laryngeal granulomas is multifactorial, but LPR should always be suspected as playing a role. Granulomas may result from the combination of acute mucosal ulceration of the vocal process, LPR, and chronic vocal trauma owing to throat clearing or a hard speaking style. By itself, chronic vocal trauma can lead to vocal fold ulcers and granulomas; however, in the majority of cases LPR is a cofactor. The clinician should consider each of the possible factors and address each if therapy is to be effective. In the case of granulomas, effective antireflux therapy is sufficient to allow healing in the majority of patients as long as vocally abusive behaviors also are corrected (Video 1).
Video 1: Left vocal process granuloma, which responded well to antireflux therapy and speech therapy.
Note the granulomas bilobed appearance, which is very commonly seen.
Paroxysmal Laryngospasm
Laryngospasm is an uncommon complaint, but patients who experience this frightening symptom are usually able to describe events in vivid detail.29 Laryngospasm is often paroxysmal, and it usually occurs without warning, even awakening some patients from sleep. In some cases, the attacks may have a predictable pattern, such as during exercise. Some patients are aware of a relationship between LPR and the laryngospasm attacks, others are not. In our experience, the majority of patients with paroxysmal laryngospasm respond to some degree to antireflux therapy, but antireflux surgery (fundoplication) may be necessary in patients who fail medical treatment.
In a canine model, Loughlin et al.30 showed that chemoreceptors on the epiglottis responded to acid stimulation at pH of 2.5 or less by triggering reflex laryngospasm. The afferent limb of this reflex is supplied by the superior laryngeal nerve, and nerve interruption abolished the laryngospasm reflex.
Laryngopharyngeal reflux–induced laryngospasm may also be associated with paradoxical vocal fold movement and even with sudden infant death syndrome (SIDS).31
Laryngeal Stenosis
Laryngopharyngeal reflux is the primary cause of most cases of subglottic and posterior glottic stenosis.1, 14, 28 (Figure 9). It can cause, or indefinitely perpetuate, laryngeal inflammation. Using a canine model, it has been shown that intermittent (three times per week only) applications of acid and pepsin to the subglottic region following mucosal injury results in nonhealing ulceration of the cricoid and subglottic stenosis.28 Laryngopharyngeal reflux documented by pH testing has been found in 92% of stenosis cases.1 In the authors' experience, aggressive antireflux treatment coupled with gentle, precise surgery is highly successful in these patients.
Laryngeal Cancer
The most important risk factors for the development of laryngeal carcinoma are tobacco and alcohol; however, LPR also appears to be an important cofactor, especially in nonsmokers1, 32 (Figure 10). Koufman1 reported 31 consecutive cases of laryngeal carcinoma in which LPR was documented in 84%, but only 58% were active smokers. The exact relationship between LPR and malignant changes remains to be proved, but the available data suggest that most patients who develop laryngeal malignancy both smoke and have LPR. In addition, leukoplakia and other premalignant appearing lesions may resolve or significantly regress with antireflux therapy.
Tobacco and alcohol adversely influence almost all of the body's antireflux mechanisms. They delay gastric emptying, decrease both upper and lower esophageal sphincter pressures and esophageal motility, decrease mucosal resistance, and increase gastric acid secretion, and thus, strongly predispose one to reflux. Antireflux treatment is recommended for all patients with laryngeal neoplasia, with or without other risk factors.
Dysphagia
Laryngopharyngeal reflux can lead to inflammation, edema, and resultant decreased sensation of the larynx and pharynx, which can cause dysphagia with or without globus sensation.33 Treatment of reflux commonly improves or sometimes eliminates these symptoms. Laryngopharyngeal reflux has also been implicated as a possible etiology for Zenker's diverticulum.34
Treatment
Because laryngeal mucosa is much more easily injured with acid/pepsin than esophageal mucosa, the treatment for GERD and LPR are different. Although the esophagus can tolerate exposure of up to 50 episodes of reflux a day without injury, as few as three isolated episodes of laryngeal acid/pepsin exposure per week have been shown to induce injury in experimental models.1, 28 Owing to these differences in defense mechanisms, intermittent treatment of GERD is often successful, although more chronic therapy is necessary for LPR.
Although H2-receptor antagonists were once considered optimal medical therapy, they only inhibit one of several pathways involved in gastric acid production. In the 1980s, PPIs were introduced in the United States as drugs that target the end point of gastric acid production, the H+, K+–adenosine triphosphatase (ATPase) pump. Although PPIs have been shown to be highly effective, daily PPI therapy has been shown to last for less than 14 hours if given in the morning. Furthermore, an evening dose may last less than 10 hours.10 Although a daily dose of PPI may be adequate in the esophagus, the laryngeal mucosa often requires full 24-hour acid suppression (twice daily PPI coverage with the patient instructed to take the first PPI dose approximately 30 to 45 minutes before breakfast and the second approximately 30 to 45 minutes before the evening meal) to prevent further injury, allow mucosal healing, and enable patients to obtain maximal symptomatic relief.11, 12
When treating LPR patients, it is helpful to categorize the severity of the patients' reflux into one of three classes: minor, major, or life-threatening.8 Patients with minor LPR consider their symptoms to be an annoyance but not socially impairing. Patients with major LPR have symptoms that impair their way of life (social or work). Finally, patients with life-threatening LPR have airway obstruction, severe pulmonary disease, or malignancy.
Regardless of the clinical severity, all patients are counseled on dietary (low fat diet, etc.) and lifestyle modifications (avoidance of carbonated beverages, alcohol, tobacco, etc). In general, the medical treatment approach to patients with minor LPR is less aggressive. Dietary and lifestyle modifications with twice-daily H2-receptor antagonist therapy or once-daily PPI treatment is a cost-effective starting point that is effective for many patients. In patients whom this therapy fails, twice-daily PPI therapy is initiated. Therapy is maintained for at least 6 months. If the patient is asymptomatic at that time (RSI <10) and laryngeal inflammation has improved (RFS <5), the medication may be slowly tapered off, often using an H2-receptor antagonist prior to complete discontinuation of antireflux medications. Because of the chronic-intermittent nature of LPR, the patient is counseled that relapses are common, and many patients ultimately need lifetime treatment.
In treating with patients with major LPR, a more aggressive initial medical therapy is implemented beginning with twice-daily PPI treatment. After 2 months of treatment, if the patient does not have symptomatic improvement, the PPI dose may be doubled or an H2-receptor antagonist may be added in the evening. A recent prospective study has suggested that twice-daily PPI with evening H2-receptor antagonist therapy is no more effective than twice daily PPI therapy.11 However, this study did not look specifically at patients with significant nocturnal/supine acid reflux on pH probe study, and in this group of patients, addition of an evening H2-receptor antagonist seems reasonable. As with the minor LPR patients, if the patient has responded well after 6 months of therapy the medication may be decreased. If the patient fails to respond, alternative PPIs are often tried, and the patient may be pH probe tested while on PPI medications in order to test for drug efficacy.35 If the patient fails multiple twice-daily PPI medications, a fundoplication may be warranted if the diagnosis of LPR or non–acid reflux is confirmed.
Life-threatening LPR requires initial reflux therapy to be even more aggressive. If possible, these patients should undergo initial pH probe monitoring to establish the severity of LPR at baseline, and to help individualize treatment. In these patients, initial medical therapy can be as aggressive as three to four times daily PPI regimen, depending on pH probe study results. In younger patients with life-threatening LPR, fundoplication surgery may be an excellent option, rather than a lifetime of antireflux therapy36 (Figure 11). In older patients, the decision about medical treatment versus fundoplication is based on symptom severity and response to medical therapy, severity of the LPR, existing comorbidities, and patient preference.
Outcomes
Patients with LPR often take several months to resolve their symptoms and laryngeal abnormalities once appropriate therapy is initiated. Although many patients have symptomatic improvement after 2 months of therapy, laryngeal examination findings continue to improve for up to 6 months after initiation of antireflux therapy. One study showed that approximately 50% of LPR patients on twice-daily PPI therapy have symptomatic improvement at 2 months, and an additional 22% improve from 2 to 4 months after initiating therapy.13 Even fundoplication surgery does not result in immediate improvement in LPR, and symptoms and examination findings continue to show improvement from 4 to 14 months after fundoplication.
Future Directions
Research into laryngopharyngeal reflux and its various manifestations, treatments, and complications has only recently been gaining momentum. Its very pathophysiology remains uncertain as does its optimal therapy. Further work characterizing what is a normal degree of LPR is ongoing as well as studies into the potential value of using a pH level of 5 as a marker for reflux. Multichannel intraluminal impedance and high-resolution manometric studies of the esophagus, pharynx, and upper esophageal sphincter may shed light on the pathophysiologic mechanisms at work in LPR. In addition, the role of TNE as a safe and effective screening test for Barrett's esophagus remains to be determined.
Improved pharmacologic means to combat acid production continue, as well as adjuvant means of treatment such as liquid alginate and antipepsin drugs that could potentially protect laryngopharyngeal mucosa and cartilage from the deleterious effects of the active proteolytic enzyme.
Finally, progress is being made in the cellular effects of acid and pepsin in the laryngopharynx, which should yield further information into the mechanism of injury, direct tissue diagnosis, and possibly further elucidate the mechanisms of laryngeal carcinogenesis.