Key Points
- Presbyphagia refers to age-related changes in the oropharyngeal and esophageal swallowing of healthy adults.
- Sarcopenia is age-related loss of skeletal muscle mass, organization, and strength.
- Good health is maintained in the presence of disease-free presbyphagia.
- Healthy persons depend on a highly automated neuromuscular sensorimotor process that coordinates chewing, swallowing, and airway protection.
- Central and peripheral nervous system changes with age affect swallowing.
- Oropharyngeal swallowing changes with healthy aging:
- Slower
- Delayed onset of airway protection and upper esophageal sphincter (UES) opening
- Bolus adjacent to airway longer
- Reduced lingual pressures
- Esophageal swallowing changes with aging:
- Duration of esophageal peristalsis is prolonged and amplitude decreases (60–80 years).
- Esophageal contraction amplitude diminishes but function remains intact (80-90 years).
- Reduced frequency of secondary peristalsis
- Increased reflux events in elders
- Although compensatory interventions are traditional, exercise is promising to remediate and perhaps prevent decline in function.
Introduction
Although age-related changes place older adults at risk for dysphagia, an older adult's swallow is not inherently impaired. In the 1960s the term presbyesophagus was coined for what was thought to be deterioration in muscular structure and function within the esophagus of elderly adults. As our diagnostic methods have become increasingly sophisticated, more studies have addressed the effects of aging on normal esophageal function leading to conflicting data owing to differences in testing techniques, confounding variables including various comorbidities common in older people, and a wide range of mean age defining the elderly population. General consensus has evolved to the opinion that presbyesophagus in its original meaning does not exist but that these changes were largely a consequence of the comorbidities common in older age.
Presbyphagia refers to characteristic changes in the mechanism of oropharyngeal/esophageal swallowing of healthy older adults. Clinicians must be able to distinguish among dysphagia, presbyphagia, and other related diagnoses such as globus hystericus to avoid overdiagnosis and overtreatment of dysphagia. Older adults appear to be more vulnerable in transitioning from a healthy older swallow to experiencing dysphagia, especially with additional stressors such as acute illness or certain medications.
With the above in mind, this review discusses the normal swallow, changes related to presbyphagia, and promising management strategies for dysphagia rehabilitation in the elderly. These strategies reflect the concept that at least part of the decline in the elderly swallowing mechanism may be related to sarcopenia, the age-related loss of skeletal muscle mass, organization, and strength.1
The Impact of Dysphagia
It is estimated that 15% to 40% of individuals over 60 years have dysphagia. The prevalence depends on the specific populations sampled, with community-dwelling and independent individuals having rates near 15%.2 This figure is in agreement with the prevalence rates of a number of other geriatric syndromes. Upward of 40% of people living in institutional settings, such as assisted living or skilled nursing facilities, are dysphagic.3 Based on the more optimistic prevalence rate of 15% and the 1998 U.S. census data, it is estimated that six million adults have dysphagia. These numbers mirror the prevalence in European countries as well.4 The projected growth in the number of individuals living in skilled nursing facilities underscores the need to address dysphagia not only in ambulatory and acute care settings but also in long-term-care settings.
The consequences of dysphagia vary from social isolation owing to the embarrassment associated with choking or coughing at mealtime, to physical discomfort (e.g., food sticking in the chest), to potentially life-threatening conditions. The more ominous sequelae include dehydration, malnutrition, and both overt and silent aspiration. For the purposes of this review, aspiration is defined as the entry of material into the airway below the level of the true vocal folds. Silent aspiration refers to the circumstance in which the bolus comprising saliva, food, liquid, or any foreign material, enters the airway below the vocal folds without triggering the overt symptoms such as coughing or throat clearing. Both overt and silent aspiration may contribute to or result in pneumonia, exacerbation of chronic lung diseases, or even asphyxiation and death. To gain a better understanding of the impact of these consequences on an older adult and the impact of dysphagia interventions, research has aimed to develop more meaningful outcome measures. Assessments focused on pathophysiology, function, and health services are now being conducted to create more evidence-based practice in dysphagia care.
Reflecting the biomechanical nature of the normal and abnormal swallow, the precise visualization of both normal and disturbed bolus flow using videofluoroscopy has been well detailed.5, 6 This includes (1) the duration, direction, and completeness of the bolus flow; (2) the duration and extent (range) of anatomic structural movements; and (3) the relationships among bolus flow and structural movements.
Other clinical outcomes of dysphagia have become important end points to assess interventions that aim to make it possible for patients to eat and drink adequately and safely. These include measures of hydration, nutrition, and aspiration episodes. Additionally, pneumonitis, overt aspiration pneumonia, and additional forms of evidence of pulmonary damage are monitored. Nonetheless, it has been difficult to attribute mortality directly to dysphagia because it often is a secondary rather than a primary diagnosis.
Healthy Swallowing
In the following sections, the effects of aging on swallowing are discussed with reference specifically to the oropharynx and the esophagus. Although characteristics of normal swallowing change with age, the major constant is that normal swallowing at any age is healthy swallowing. That is, deleterious health outcomes including pneumonia, malnutrition, and dehydration are not associated with age-related swallowing changes. Good health is maintained in the presence of disease-free presbyphagia.
Normal Oropharyngeal Swallowing
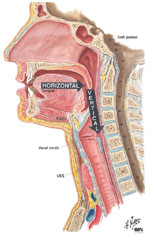
A basic understanding of the relationship between the anatomic components and functional dynamics of the normal swallowing mechanism is essential to understanding the effects of age and age-related diseases. Swallowing is an integrated neuromuscular process that consists of a combination of volitional and relatively automatic movements. Although normal swallowing is usually conceptualized as a continuous sequence of events, the process of deglutition has been variously subdivided into two, three, or four phases or stages.6 Moreover, the system engaged in swallowing may be divided into two basic structural subsystems: horizontal and vertical (Figure 1). This mirrors the direction of bolus flow as well as the potential for gravitational influence on it.7
Figure 1: Oropharyngeal swallowing mechanism.
The mechanism may be divided into two basic structural subsystems, horizontal and vertical, that mirror direction of bolus flow. (Source: Netter medical illustration used with permission of Elsevier. All rights reserved.)
The horizontal subsystem is largely volitional, anatomically comprising structures within the oral cavity. Within this subsystem, food is accepted, contained, and manipulated. Labial, buccal, and lingual actions, in combination with enzyme-rich intraoral fluids from salivary glands, allow manipulation of the texture of food to ultimately mechanically formulate a bolus. The cohesive bolus is moved posteriorly (and horizontally when the subject is in a normal upright seated posture) to the inlet of the superior aspect of the pharynx (Figure 2). To accomplish this, the intrinsic and extrinsic tongue muscles change the shape and the position of the tongue, and stimulate oropharyngeal receptors that trigger ensuing portions of the swallow sequence.7, 8, 9
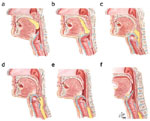
Figure 2: Lateral view of bolus propulsion during swallowing.
a: Voluntary initiation of the swallow by tongue "loading." b: Bolus propulsion by tongue dorsum and UES opening anticipating bolus arrival. c: Bolus entry into the pharynx associated with epiglottal downward tilt, hyolaryngeal excursion, and UES opening. d, e: Linguapharyngeal contact facilitating bolus passage through (d) the pharynx and (e) the UES, and completion of oropharyngeal swallowing. Then the entire bolus is on the esophagus. (Source: Netter medical illustration used with permission of Elsevier.)
The pharyngeal and laryngeal components, in conjunction with the tongue dorsum, comprise the superior aspect of the vertical subsystem where gravity begins to assist in the transport of the bolus. The anatomic juxtaposition of the entrance to the airway (laryngeal vestibule) and the pharyngeal aspect of the upper digestive tract demand biomechanical precision to ensure simultaneous airway protection and bolus transfer or propulsion through the pharynx. As lingual-palatal contact sequentially moves the bolus against the posterior pharyngeal wall, the contact contributes to the positive pressures imparted to the bolus propelling it downward.10, 11 Simultaneously, the pharyngeal constrictors begin contracting in a descending sequence,10, 12 first elevating and widening the entire pharynx to engulf the bolus (Figure 2d,e). A descending peristaltic wave then cleanses the pharynx of residue. The tongue is the primary propulsive mechanism responsible for plunging the bolus into the vertical subsystem, but other mechanisms, such as velopharyngeal closure, also contribute to pressure gradients facilitating the bolus transfer.
Afferent nerve endings detect the sensation of a food bolus, transmitting this sensation to the swallowing center, which in turn activates vagal efferents to first relax the upper esophageal sphincter (UES) and then stimulate vagal efferents along the length of the esophagus to sequentially fire. This process triggers a peristaltic wave that consists of a circular contraction that travels distally at 2 to 4 cm/sec and transverses the entire esophagus in approximately 10 seconds. This act is termed "primary peristalsis." At the initiation of peristalsis, the lower esophageal sphincter (LES) reflexively relaxes to allow the bolus to pass into the stomach.
Secondary peristalsis occurs when distention of the esophagus in the absence of oropharyngeal stimulation initiates a peristaltic wave. This wave usually begins immediately superior to the level of distention. For example, if a large food bolus is not fully cleared by primary peristalsis, secondary peristalsis would then clear the esophagus of the bolus remnants.
In striated muscle—the oropharynx down to the mid-intrathoracic esophagus—the coordination of peristalsis is clearly subject to control from the swallow center. In smooth muscle—the distal intrathoracic esophagus—peristalsis is likely controlled both intrinsically as well as extrinsically. Studies have shown that vagotomy reduces the smooth muscle segment amplitude of peristalsis but that secondary peristalsis persists.13, 14 Peristalsis in the absence of extrinsic innervation has been termed autonomous peristalsis.
The term deglutitive inhibition describes the phenomenon whereby a second swallow is initiated before the first peristaltic contraction transverses the entire esophagus. This second swallow results in termination of the first peristaltic wave. In esophageal striated muscle, deglutitive inhibition terminates the initial peristaltic wave. If a second swallow is initiated while the first peristaltic contraction is progressing in the smooth muscle esophagus, the first wave diminishes progressively over 1 to 2 seconds.
Airway protection is ensured during the swallow by three levels of sphincteric closure: (1) aryepiglottic folds, (2) the false vocal folds, and (3) the true vocal folds. The hyolaryngeal complex is also lifted upward and forward by the combined contraction of the suprahyoid and thyrohyoid muscles, and pharyngeal elevators. This hyolaryngeal elevation and anterior movement, coupled with tongue base retraction, covers the laryngeal vestibule, diverts the bolus laterally around the airway with the epiglottis assuming a more horizontal position providing cover of the laryngeal vestibule. The biomechanical effects of hyolaryngeal excursion also, very importantly, provide the traction pull on the cricoid cartilage moving it anteriorly, an important aspect of UES opening.11, 15, 16 It must be emphasized that the UES opening via active traction pull on the hyolaryngeal mechanism upward and forward, together with the centrally mediated neural relaxation, results in distention of the pharyngeal lumen. Timely relaxation and opening of the UES permits continuous vertical passage of the bolus into the esophagus, and the pharyngeal transport stage of the swallow terminates when the UES returns to its hypertonic, closed "resting state."
Normal Esophageal Swallowing
When viewed in its simplest form, the esophagus is a hollow muscular tube with sphincters at each end that functions as a conduit to transport food from the oropharynx to the stomach. However, normal esophageal function involves complex interactions between the musculature of the oropharynx, the esophagus, and multiple neurologic reflexes that as yet are not fully understood.
The esophagus is an approximately 20- to 24-cm hollow muscular tube that is anatomically defined as the area between the distal portion of the UES and the proximal portion of the LES. It is composed of an inner circular muscle layer and an outer longitudinal muscle layer. In between the two layers lies a nerve network called the myenteric plexus or Auerbach's plexus.
The esophagus comprises both striated and smooth muscle. The upper esophagus, including the UES, is composed of striated muscle. The middle esophagus contains a mixture of both smooth and striated muscle, with the proportion of smooth muscle increasing distally. The distal third of the esophagus and the LES exclusively contain smooth muscle.17
The UES is composed of the inferior pharyngeal constrictor, the cricopharyngeus, and adjacent portions of the cervical esophagus. This complex is innervated by the pharyngeal branch of the vagus nerve. At rest, the UES is tonically contracted owing to a constant excitatory neural discharge. The pressure of this contraction is asymmetric with higher pressures generated anteriorly and posteriorly as opposed to the lower pressures laterally. The UES pressure is increased with inspiration, Valsalva maneuver, stress, secondary peristalsis, gagging, and slow distention in the upper esophagus, and is reflexively decreased with belching, vomiting, or any mechanism that results in rapid esophageal distention. It also decreases during sleep. With oropharyngeal initiation of a swallow, the neural discharge to the UES ceases, briefly permitting UES relaxation. In its relaxed state, opening of the sphincter is then facilitated both by the traction pull on the cricoid cartilage forward as well as by the upward hyolaryngeal mechanism. This results in a lumen through which food passes when propelled adequately by the tongue and related musculature.
Traditionally, the LES was thought to be a physiologic structure without corresponding anatomy. However, in the late 1970s, a discrete area of asymmetrically thickened circular smooth muscle was identified within the diaphragmatic hiatus.18 The resting pressure of the LES is between 10 and 30 mmHg greater than intragastric pressure. Unlike the UES, the mechanism of LES resting contraction appears related to an intrinsic muscular component.
Neurophysiology of Oropharyngeal Swallowing
Historically, swallowing was viewed as a sequence of reflex arcs first involving the pharynx and subsequently the esophagus. Findings from both quantitative temporospatial studies related to normal swallowing16 in the last two decades, as well as the current knowledge of underlying neural substrates, have provided new insights into oropharyngeal swallowing mechanisms showing that it is a patterned response rather than a traditional reflex.19
Sensorimotor control of swallowing requires coordinated activity between both the cranial and spinal nerve systems, including the peripheral nerves, their central nuclei, and their neural centers. More specifically, the neural control of swallowing involves five major components: (1) afferent sensory fibers contained in cranial nerves, (2) cerebral and midbrain fibers that synapse with the brainstem swallowing centers, (3) paired swallowing centers in the brainstem, (4) efferent motor fibers contained in cranial nerves and the ansa cervicalis, and (5) muscle and end organs. Thus this neural network spans all levels of the neuraxis from the cerebrum superiorly to brainstem and spinal nerves inferiorly and muscles and end organs at the periphery. This relatively diffuse network is designed to integrate and sequence both the volitional and the more automatic activities of swallowing.
Healthy persons depend on a highly automated neuromuscular sensorimotor process that seamlessly coordinates the activities of chewing, swallowing, and airway protection. To accomplish a normal swallow in 2 seconds or less, the muscles of chewing interact with 26 pairs of striated pharyngeal and laryngeal muscles. The muscles involved in chewing include the masseters, temporalis, and pterygoids (all innervated by cranial nerve V); the lip and buccal musculature, the orbicularis oris, and the buccinator (all innervated by cranial nerve VII); and the intrinsic and extrinsic lingual muscles (all innervated by cranial nerve XII). Optimal structural integrity and precise neural mediation result in continuous, rapid bolus flow from the mouth to the esophagus that accommodates variation in bolus size, texture, temperature, and the individual's intent to swallow, chew, or just hold the bolus in the mouth
Neurophysiology of Esophageal Swallowing
Sensory information for the entire esophagus, including both sphincters, is carried via the parasympathetic and sympathetic nervous systems. The parasympathetic involvement is via the vagus nerve whereas the sympathetic spinal afferents travel via C1 through L3 preganglions. Both networks ultimately enter the nucleus tractus solitarius (NTS), which processes the information and activates the motor system response. These afferents also pass to higher brain centers.
Within the esophageal muscle fiber, free vagal nerve endings known as mechanoreceptors are stimulated by esophageal distention and modulate esophageal contraction. Nocireceptors, which originate through the spinal afferent pathway, respond to noxious stimuli such as acid or heat. Importantly, evidence exists that vagal afferents can modulate spinal afferents and vice versa.
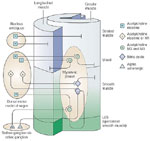
Parasympathetic control through the vagus nerve regulates esophageal peristalsis. The STN activates progressive sequential firing of motor neurons that ultimately carry out the swallow response. Although the esophagus is often described as simple, Figure 3 is a diagrammatic representation of its inherent complexities.
Figure 3: Diagrammatic representation of the intrinsic and extrinsic innervation of the esophagus.
(Source: Castell DO, ed. The Esophagus, 2nd ed. Boston: Little, Brown, 1995:17, with permission from Lippincott, Williams & Wilkins.)
The somatic vagal efferent neurons that innervate the striated muscle portion of the esophagus including the UES originate in the medulla's nucleus ambiguus motor nuclei. The visceral motor neurons that innervate the esophageal smooth muscle, including the LES, originate in the dorsal motor nucleus of the vagus and synapse on the myenteric plexus. Myenteric plexus neurons then directly innervate smooth muscle. Therefore, in the smooth muscle function, the myenteric plexus serves as a relay between the vagus nerve and the esophageal muscle.
There are two major groups of effector neurons that innervate the smooth muscle of the esophagus. The first are excitatory neurons that mediate cholinergic contraction of circular and longitudinal muscle through muscarinic M2 and M3 receptors. The second are inhibitory nonadrenergic, noncholinergic neurons that inhibit circular muscle. There is evidence that nitric oxide may be the neurotransmitter responsible for inhibition effect.20 Many other neuropeptides have been identified in esophageal neural tissue, although their exact roles remain unknown.
In striated muscle, efferent neurons contact nicotinic cholinergic end plates directly on muscle fibers that contain the neurotransmitters acetylcholine (ACh) and calcitonin gene-related peptide (CGRP). Although myenteric plexi are present in areas of both smooth and striated muscle, their role in striated muscle function is unclear.
Senescent Swallowing
Oropharynx
Traditional thinking suggests that causes of dysphagia are always disease related, with direct or indirect damage to effector end-organ systems of swallowing. More recent research, however, suggests that swallowing changes occur even with healthy aging. This work, focused primarily on anatomy and physiology of the oropharyngeal swallowing mechanism, describes a progression of change that may put the older population at increased risk for dysphagia. This research is particularly relevant when an older healthy adult, whose functional reserve (ability to adapt to stress)21 is naturally diminished with age, is faced with increased stressors such as central nervous system (CNS)–altering medications, mechanical perturbations (e.g., nasogastric tubes or tracheostomy), or chronic medical conditions (e.g., frailty) that might not elicit dysphagia in a less vulnerable individual. Translation of this work into clinical practice should provide safeguards against overdiagnosis and overtreatment of dysphagia in the elderly population.
Healthy swallowing in the elderly occurs more slowly. The increased time of swallowing in this population results from a longer horizontal component. Delays related to the vertical component also occur. In those over age 65, the initiation of laryngeal and pharyngeal events, including laryngeal vestibule closure, maximal hyolaryngeal excursion, and UES opening, are significantly slowed relative to durations recorded in adults younger than 45 years old.22, 23, 24, 25 In older healthy adults, it is not uncommon for the bolus to spend a greater length of time adjacent to an open airway, by pooling or pocketing in the pharyngeal recesses, than in younger adults. This senescent change may be associated with greater risk for airway penetration or aspiration.
Although pharyngeal events are slowed (such as delayed UES opening relative to bolus entry) in the elderly, the critical component of the range of UES opening is also diminished.26 Scintigraphic studies have shown increased pharyngeal residue with age, possibly related to the limited UES opening, resulting in greater exposure of the laryngeal vestibule to the swallowed bolus in older individuals.27 Aspiration and airway penetration are believed to be the most significant adverse clinical outcomes of misdirected bolus flow. In older adults, penetration of the bolus into the airway occurs more often and to a deeper and more severe level than in younger adults.22, 28
When the swallowing mechanism is functionally altered or perturbed in older people, such as with the placement of a nasogastric tube, airway penetration can be even more pronounced. A study examining this issue found that liquid penetrated the airway significantly more frequently when a nasogastric tube was in place in men and women older than 70 years.22 Thus, it appears that under stressful conditions or system disturbances, older individuals are less able to compensate and thus are at greater risk for airway penetration or aspiration.
Age-related changes in the generation of lingual pressure also define presbyphagia. Healthy older individuals have reduced isometric tongue pressures compared with younger individuals.29, 30 In contrast, the generation of maximal lingual pressure during swallowing (which requires submaximal pressures), remains "young" in magnitude. Because the peak lingual pressures used in swallowing are lower than those generated isometrically, healthy older individuals manage to achieve pressures necessary to effect a successful swallow. The difference between maximum isometric pressure and peak swallowing pressures can be used as an indicator of the functional reserve available for swallowing. As people get older, the slower swallowing mechanics may actually be used as a benefit as it can allow greater time to recruit the necessary number of motor units for pressures critical for adequate bolus propulsion through the oropharynx.30 Thus, therapeutically speeding up an elderly patient's swallow may be detrimental, as it may result in insufficient swallow pressures and therefore may be contraindicated as a therapy technique. This is only one of the issues that may generate experimental questions warranting study for improving treatment of dysphagia and prevention of potential untoward consequences of presbyphagia.
On occasion, the perturbations or functional alterations in the swallowing mechanism, or perhaps general age-related or disease-related frailty, may not allow safe swallows in older adults. That is, the compensation of slower swallowing may not be enough for these individuals in whom presbyphagia crosses over into dysphagia. At this point, compensatory interventions or other rehabilitation efforts to promote safe swallowing will be required.
Neurophysiologic correlates of senescent oropharyngeal swallowing Not only are fine and gross motor functions slowed with age, the reaction time to sensory stimuli also decline.31, 32 Magnetic resonance imaging (MRI) is highly sensitive in detecting periventricular white-matter hyperintensities (PVHs) in the cerebral white-matter tracts. Neuroimaging studies using cranial MRIs in normal adults show a relationship between slower swallowing and both the increased number and severity of PVHs in the brain, supporting the concept that voluntary control of swallowing is mediated by corticobulbar pathways that travel within the periventricular white matter.33 The appearance of and the degree of these PVHs increase with age and may explain, at least in part, the relatively asymptomatic decline in oropharyngeal motor performance observed in older people. Cerebral atrophy, blood flow changes, and other age-related conditions must also be factored into the process of presbyphagia development.
Changes in the peripheral nervous system as well as those occurring centrally are documented with age and may be related to sarcopenia in muscles of the head and neck. Structurally, sarcopenia is associated with reductions in muscle mass and cross-sectional area (CSA), as a result of a reduction in the number or size of muscle fibers, as well as a transformation or selective loss of specific muscle fiber types.34, 35, 36, 37 One study reported on the reduction in tongue muscle fiber diameter in the superior longitudinal muscle in 50 human subjects.38 Reductions in fiber diameters began at age 40 in males and age 30 in females.38 In addition, an increase in fatty and connective tissue and increased amyloid deposits in the blood vessels of the tongue have been reported.39 In another study, changes in human laryngeal muscle specimens (thyroarytenoid) from autopsy or laryngectomy show a tendency for decreasing type II (fast) muscle fibers and increasing type I (slow) fiber composition with increasing age.40 Additionally, there are reports of sarcopenia-like changes in muscles of the upper aerodigestive tract. However, most of the work in the area of sarcopenia has been performed in the limb musculature. Further work in cranial muscle change is needed.
Muscle disuse, which may be better termed as "reduction" or "alteration" in use,41 has been proposed as the cause of muscular atrophy observed in the aged. This hypothesis has intuitive appeal, owing to the assumption that elderly people are less active. However, the fact that muscles of speech, swallowing, and respiration continue to be used by the elderly in approximately the same degree as by younger adults argues against this hypothesis.42 Further, "disuse" has not been proven as a simple causative factor in animal studies.34, 41, 43 Again, it is clear that mechanisms underlying sarcopenia in muscles of the head and neck must be defined to allow the design and implementation of appropriate treatments.
The morphologic changes observed in aged muscle and at nerve-muscle connections may have physiologic or functional consequences,44 such as a diminished ability to sustain synaptic transmission,45, 46 reduced force generation per unit of muscle fiber CSA,43 and altered or disorganized patterns of muscular contraction during complex movements.47, 48 Reduced muscle forces and altered temporal properties have been reported in human and animal studies. These indicate that senescent laryngeal muscle contractions are generally of reduced force, require more time to reach peak contraction, and also need more time for recovery prior to initiation of the next contraction.49, 50 These factors could contribute to reductions in motor performance required for swallowing actions.
In summary, the differences in swallowing function for elderly versus young individuals appear dependent on age-associated changes of both central and peripheral mechanisms. It can be hypothesized that slowed swallowing seen in most healthy elderly individuals which remains coordinated and effective, may not reflect CNS deterioration as much as it represents a compensatory strategy for achieving pressure-generation values needed for successful bolus propulsion. Nonetheless, further clarification of neural underpinnings of age-related changes in swallowing will undoubtedly provide directions for development of effective intervention and prevention techniques.
Esophagus
Effect of aging on primary esophageal peristalsis In the 1960s, two studies from the same institution were published that used cineradiography and manometry to study esophageal motor function in nonagenarians, many of whom had concomitant neurologic disease or diabetes that may have influenced their swallow function. Both studies found this patient population to have numerous repetitive, nonpropulsive peristaltic contractions as well as tertiary contractions, esophageal dilation, and delay of esophageal emptying. The term presbyesophagus was coined to describe this phenomenon.51, 52 In 1974, Hollis and Castell53 published a manometric study comparing esophageal function of elderly men without such comorbidities to younger controls. They reported a significant decrease in peristaltic amplitude in men older than 80 but found no significant change in the speed or duration of peristalsis and no increase in dysmotility. They proposed that aging results in weakened esophageal muscle but that innervation remained intact.
As technology has improved and more diagnostic studies have become available, evaluations of geriatric swallow function continue to yield contradictory results. Richter et al.54 performed manometry on 75 healthy adults ranging from 22 to 79 years of age with the primary goal of establishing normal pressure ranges. His group found that mean contractile amplitude in the esophagus increases distally in all groups and that, surprisingly, distal contractile amplitude became significantly greater with each increasing decade until it peaked in the sixth decade. Duration of contraction was similar in all age groups. In contrast, Ren et al.55 found that in comparing young and old subjects, primary peristaltic amplitude was similar; however, at all sites of the esophagus, the peristaltic wave duration was longer in the elderly population. This finding corroborates Aly and Abdel-Aty's56 work, which demonstrated that normal esophageal transit time is longer in patients older than 40, with the increased duration involving transit across the LES.
More recently, a manometric study involving 79 elderly subjects found a significant decrease in peristaltic wave amplitude, a decrease in both LES and UES pressures, as well as an increase in the duration of peristaltic contractions and an increase in the number of failed contractions.57 The authors of this study proposed adjusting manometric normal pressure ranges for age based on these data. Likewise, another manometric study of healthy Japanese volunteers found that the elderly population (>60 years) had decreased peristaltic contraction amplitude compared to the young volunteers (<49 years). The elderly subjects were more likely to have failed peristaltic contractions as well.58
However, a similarly large, albeit retrospective, study comparing older (>65 years) and younger (<45 years) patients with dysphagia did not find any significant difference in peristaltic amplitude, duration, or LES pressures.59
Several studies have demonstrated an increase in both the number of failed peristaltic events as well as synchronous contractions.57, 58, 60 Ferriolli's group60 used manometry in conjunction with scintigraphy to evaluate healthy asymptomatic old and young volunteers' response to varying bolus viscosities. Failed or synchronous peristaltic contractions and incomplete esophageal emptying occurred more often in the elderly patients. The frequency of failed and synchronous contractions was more commonly observed with the more viscous substance (in this case sugar cane syrup). However, in contrast to prior studies, no significant differences were observed in peristaltic amplitude or duration.
In older healthy subjects, studies using impedance planimetry combined with intraesophageal balloon distention allowing simultaneous assessment of biomechanical properties and visceral sensation have shown that older healthy subjects have a significantly larger esophageal lumen in the areas of smooth and striated muscle. The smooth muscle segment of the esophagus was less compliant in older subjects, but there was no significant difference in compliance of striated esophageal muscle between the groups.61 These changes could lead to the impairment of peristalsis noted in the elderly.
In conclusion, it is difficult to directly compare the above studies owing to differences in subject population average ages and degree of comorbidities as well as differences in manometric and radiographic techniques including bolus size, consistency, and body position. It appears that in subjects older than 90, the majority have comorbidities that would possibly predispose them to an esophageal motility disorder thus it is difficult to distinguish whether dysmotility in this group is due to age plus disease or disease alone. In healthy subjects aged 80 to 90, esophageal muscle weakness exists but the swallow function remains intact. In subjects aged 60 to 80 and perhaps somewhat younger, the duration of peristalsis is prolonged and the amplitude may be lessened, although whether these findings are clinically significant remains unclear.
Effect of aging on secondary esophageal peristalsis As mentioned previously, secondary peristalsis occurs in response to a local esophageal stimulus caused by distention. It is believed to play an important role in clearing substances such as residual bolus and refluxate, and, in conjunction with neurologic reflexes, preventing reflux from penetrating the airway. Only one study has directly evaluated the effect of aging on secondary peristalsis. Ren et al.55 compared secondary peristalsis in healthy elderly subjects (average age 74 years) to that in healthy young controls (average age 35 years). Secondary peristalsis was either absent or significantly less frequent in the elderly compared to the young, whereas primary peristalsis was similar across the groups. The authors speculate that this diminished capacity for secondary peristalsis and therefore inadequate volume clearance may predispose the elderly to complications of reflux disease.
Effect of aging on the lower esophageal sphincter Several studies have demonstrated similar resting LES pressures between healthy young and elderly subjects.54, 59, 62 However, Bardan et al.62 found that the relationship between the high-pressure zone of the LES and the respiratory pressure inversion point (PIP) was significantly different in the elderly compared to the young. The PIP is considered to be a reference point that separates the intrathoracic LES segment from the intraabdominal LES segment. In the Bardan et al. study, although the length of the LES high-pressure zone was similar, the portion of LES proximal to the PIP was longer and the portion distal to the PIP was shorter in elderly subjects. Work by Xie et al.63 on pharyngeal water stimulation effects on LES relaxation also corroborated these results, as did a manometric study of healthy older vs. younger volunteers57 that found that subjects older than 65 had a significantly shorter LES length. As other investigators have found that the PIP could be secondary to respiratory movement alone, the physiologic and clinical significance of this is yet to be determined.64
Xie et al.63 compared the effects of pharyngeal water stimulation on LES relaxation resulting in gastroesophageal reflux in healthy young and elderly subjects. Elderly subjects had significantly more gastroesophageal reflux in the postprandial state compared to the younger subjects. Thus it is possible that the shorter abdominal segment of the LES predisposes the elderly to these reflux events.
Effect of aging on esophageal sensation Much remains to be discovered about the effects of aging on esophageal visceral perception. Various studies have demonstrated that healthy elderly patients require more esophageal stimuli to perceive the disturbance and also have a higher threshold for the sensation of pain. Using graded distal intraesophageal balloon distention, Lasch et al.65 demonstrated that mean balloon volumes required to sense pain were significantly higher in a group of nondiabetic, elderly subjects without esophageal symptoms (mean age 72.5 years) compared to a younger group with the same inclusion criteria (mean age 27 years). In fact, of the older group, five of the 17 individuals experienced no pain at maximum balloon distention, whereas all of the younger patients experienced pain with this degree of distention.
Similarly, Rao et al.61 confirmed that older healthy subjects (mean age 74 years) experienced significantly less distal esophageal (i.e., smooth muscle) pain with graded intraesophageal balloon distention than did younger subjects (mean age 27 years). In addition, Rao's group reported less striated muscle pain sensation in the elderly subjects compared to younger subjects. There was a trend toward a higher volume threshold for perception in the older group.
One theory as to why older patients have decreased visceral sensation involves changes in the peripheral nervous system. Several studies have determined that the number of myenteric neurons in the esophagus decreases with age, whereas no difference was identified in smooth muscle thickness.66, 67 The myenteric plexus serves as a relay between esophageal motor cells and the nerves. Therefore, if the myenteric neurons were significantly decreased as part of the aging process, it makes sense that elderly patients would have less efficient perception of mechanical, chemical, and noxious stimuli. Independently, this decreased perception is not pathologic, although in appropriate circumstances a disease process may occur. An example of this is Barrett's esophagus.
Barrett's esophagus is a complication of long-standing gastroesophageal reflux disease (GERD) whereby the normal stratified squamous esophageal tissue is transformed into intestinal mucosa by metaplasia. Owing to the chronicity required for this transformation to occur, it is typically a disease of the elderly population.68 Patients with Barrett's esophagus have diminished perception to acid infusion and balloon distention, resulting in the implication of Barrett's as a cause of the altered perception. A recent study challenged this perception, hypothesizing that age rather than the presence of Barrett's may be the cause of reduced chemoreception. The study compared the acid infusion perception threshold in older (>65 years old) versus younger (<50 years old) patients with Barrett's epithelium of similar length. Interestingly, elderly patients had a higher threshold for sensory perception as well as a longer lag time to perception, suggesting that age rather than Barrett's may result in decreased pain perception.69
Effect of aging on reflexes Aerodigestive and esophageal reflexes oversee the relationship between the upper gastrointestinal tract and the upper respiratory tract during a swallow. Specific reflexes serve to protect the airway from aspiration through mechanisms such as secondary peristalsis, which clears residual volume from the pharynx and esophagus, vocal cord closure, and prevention of gastric content reflux into the esophagus and pharynx.70 Many of the reflexes have been shown to be unfavorably affected by age.55, 71, 72, 73
Age-Related Conditions
Age-related difficulties and comorbidities can develop throughout the upper aerodigestive tract and have the potential of influencing the integrity of the swallow. In the horizontal subsystem, the food bolus may be inadequately prepared owing to poor or absent dentition,74 periodontal disease, ill-fitting dentures, or inappropriate salivation from xerostomia.75 Musculoskeletal factors such as weakness of the muscles of mastication, arthritis of the temporomandibular joint or larynx, osteoporosis of the jaw, changes in tongue strength related to sarcopenia, and lack of coordination of oropharyngeal events can deter efficient swallowing. Sensory input for taste, temperature, and tactile sensation changes in many older adults,76, 77, 78 and may impair sensorimotor interaction required for proper bolus formation and timely response of the swallowing sequence. All of these factors may detract from the pleasure of eating.
Dysphagia Intervention and Prevention Paradigms
Treatment for dysphagia is usually either rehabilitative or compensatory in approach. Rehabilitative interventions have the capacity to directly improve the dysphagia at the biologic level. That is, muscle or neural circuitry is the target of rehabilitative therapy that may have direct influence on physiology, biomechanics, and bolus flow. Alternatively, compensatory interventions avoid or reduce the effects of the impaired structures or neuropathology. For example, modification of the environment, the patient's position during mealtime, or the texture of the food can help reverse disordered physiology and biomechanics on bolus flow.
Traditionally, interventions for dysphagia in the elderly are most often compensatory in nature and are directed at modifying bolus flow by targeting biomechanical features of the swallow or by adapting the environment. The latter can be accomplished by the introduction of a care provider for a patient who is unable to manage for him/herself. Examples of compensatory strategies for dysphagia treatment are postural adjustment, slowing the rate of eating, limiting bolus size, adaptive equipment, and the most commonly used environment adaptation, diet modification.
Rehabilitative exercises are, by nature, more active and rigorous. During the past 20 years a body of literature has emerged that suggests that loss of muscle strength with age is, to a great extent, reversible through incorporating exercise and strength training into rehabilitative programs.
The research in the area of exercise for elderly people has typically been applied to reducing falls in elderly persons,79 rather than swallowing impairment. However, both clinical and animal studies examining this issue are currently underway with results beginning to appear.80 Studies of progressive resistance exercise in the limbs demonstrate that elderly men and women, even those 90 years or older, can increase muscle size and strength with training.81, 82, 83, 84 According to Evans,85 "There is no pharmacologic intervention that holds a greater promise of improving health and promoting independence in the elderly than does exercise."
There are compelling preliminary data to suggest that increased muscle activation and/or strength and effort training may play an important role in swallowing rehabilitation.86, 87, 88, 89, 90, 91, 92, 93 For example, reports in the clinical literature suggest that improvements in swallowing function follow performance of tongue exercises in combination with other factors.94 In a randomized trial, young, healthy adults significantly increased maximum tongue strength with either standard strength exercises using a tongue depressor or the Iowa Oral Performance Instrument, compared to a group that received no treatment.88 In another study of asymptomatic, healthy elderly subjects, significant increases were found in the magnitude of laryngeal excursion, and the CSA of the UES opening after performance of a head-raising exercise.89 Two other reports, one using a small number of elderly subjects and the other a head and neck cancer patient, showed improved force and temporal characteristics of the swallow after 8 weeks of progressive lingual resistance training.87, 90 These changes translated to improvements in the subjects' nutritional intake and quality of life. Perhaps even more hopeful for the future is a study demonstrating that even healthy elders, after 8 weeks of progressive resistance training, not only increased lingual strength but had direct carryover of the strength training to functional increased pressures during swallowing.29 Thus, although there is substantial literature in limb systems to date, some preliminary data in head and neck systems is pointing to the potential benefits of strength training as a possible intervention for swallowing disorders. Exercise targeting sarcopenia with or without additional imparment warrants further clinical study, because such an approach holds the promise of prevention of age-related dysphagia for individuals who employ exercise regimes focusing on head and neck musculature.
Conclusion
As the population ages, strategies for treating elderly persons with dysphagia will be of crucial importance. Research into the underlying pathophysiology of age-related swallowing disorders is critical in trying to create interventions with sound scientific bases to encourage prevention, slowing, or reversal of these conditions. Certainly the present state of knowledge calls for more research with a need for systematic, carefully controlled studies including randomized clinical trials in this area.