Key Points
- Barrett's esophagus (BE) is condition in which normal esophageal squamous epithelium is replaced by a spectrum of metaplastic columnar mucosa. However, the so-called specialized intestinal metaplasia is diagnostic.
- Barrett's esophagus is a complication of a motility disorder causing severe gastroesophageal reflux disease (GERD). These patients have severe LES hypotension, esophageal contraction abnormality, and a hiatal hernia.
- Diagnosis of Barrett's esophagus requires endoscopic demonstration of upward displacement of the squamocolumnar mucosal junction in relation to the junction of the esophagus and the stomach and biopsies demonstrating intestinal metaplasia.
- Long segment (>3 cm) and short segment (<3 cm) BE is found in 5% and 15% of patients, respectively, undergoing endoscopy for GERD.
- Development of intestinal metaplasia may involve Cdx gene products.
- 0.5% of BE may develop adenocarcinoma per year.
- Progression of BE to adenocarcinoma occurs through increasingly worse grades of histologic dysplasia.
- There is considerable intra- and interobserver variability in the diagnosis of the grade of dysplasia.
- High-grade dysplasia may be confused with early adenocarcinoma and poses a very high risk of progression at carcinoma.
- No single gene has been found to predict progression to cancer; however, p53, cyclin D, and aneuploidy may be useful in this regard.
- Chromoendoscopy, optical coherence tomography, spectroscopy, and other emerging imaging techniques may aid the diagnosis of intestinal metaplasia, dysplasia, and carcinoma.
Introduction
In Barrett's esophagus, an abnormal columnar epithelium that is predisposed to malignancy replaces esophageal squamous epithelium that has been damaged by gastroesophageal reflux disease (GERD) (Figure 1).1 Gastroesophageal reflux disease and Barrett's esophagus are the major risk factors for esophageal adenocarcinoma, a tumor whose frequency has increased more than sixfold over the past several decades in the United States.2, 3 Barrett's esophagus is named for Norman Rupert Barrett, a surgeon who drew attention to the "short esophagus" in a report that he published in 1950.4 The eponym "Barrett's esophagus" has been retained even though Norman Barrett was not the first to describe the columnar-lined esophagus and even though his early speculations regarding the nature and pathogenesis of the condition were incorrect.5
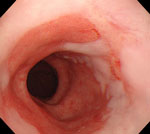
Figure 1: Endoscopic photograph of Barrett's esophagus.
Note the reddish columnar mucosa lining the distal esophagus. Erythema and erosions caused by gastroesophageal reflux disease (GERD) (reflux esophagitis) can be seen in the adjacent squamous epithelium.
Early controversies about Barrett's esophagus focused on whether the condition was congenital or acquired, and on whether the abnormal columnar epithelium in the esophagus was gastric or intestinal in type.5 Today, it is widely accepted that the condition is acquired as a consequence of chronic GERD. Although gastric fundic- and gastric cardiac-type epithelia can be found in Barrett's esophagus, specialized intestinal metaplasia is the most distinctive and important type of Barrett's epithelium, and modern authorities usually require the demonstration of specialized intestinal metaplasia to establish the diagnosis. For decades, Barrett's esophagus was not recognized unless columnar epithelium lined at least several centimeters of the distal esophagus. Since the demonstration by Spechler et al.6 in 1994 that short segments of intestinal metaplasia are present frequently in the distal esophagus, Barrett's esophagus has been categorized as "long-segment" when columnar epithelium lines more than 3 cm of the distal esophagus, and as "short-segment" when there are less than 3 cm of columnar lining.7 The cutoff value of 3 cm is arbitrary, however, and the long/short classification presently has no clear implications regarding either the pathogenesis of the condition or the clinical management of affected patients.
Epidemiology
Barrett's esophagus can be found in children, although it is extremely uncommon to find intestinal metaplasia in the esophagus of children under the age of 5 years.8 There appears to be an association between mental retardation and Barrett's esophagus in childhood.9 In adults, the average age at the time of diagnosis of Barrett's esophagus is approximately 55 years, and white men predominate in most series.10, 11 For unknown reasons, Barrett's esophagus is uncommon in blacks and Asians. In most cases, Barrett's esophagus is discovered during endoscopic examinations performed for the evaluation of GERD symptoms such as heartburn, regurgitation, and dysphagia. Long-segment Barrett's esophagus is found in fewer than 5% of such patients, whereas 10% to 15% have short-segment Barrett's esophagus.1
The prevalence of Barrett's esophagus in the general population is not known, but a recent study suggests that the condition is quite common.12 Among 961 patients scheduled for elective colonoscopy who agreed to have an upper gastrointestinal endoscopy performed for research purposes, Barrett's esophagus (predominantly short-segment) was found in 6.8%.12 Among the 556 patients who had no heartburn, Barrett's esophagus was found in 5.6%, whereas 8.3% of 384 patients who complained of heartburn had Barrett's esophagus. The difference in the frequency of short-segment Barrett's esophagus between the patients with and without heartburn was not significant. Another recent report suggests that the frequency of Barrett's esophagus in the general population is increasing.13 In this large cohort study from the Netherlands, the incidence of Barrett's esophagus increased from 14.3 per 100,000 persons in 1997 to 23.1 per 100,000 in 2002 despite a slight decline in the frequency of performing endoscopy in the Netherlands over the same time period.
The endoscopist who finds a Barrett's esophagus has no way to determine precisely when the condition developed, but one study has shed some light on this issue.14 In this study, the investigators reviewed the records of 51,311 patients who had endoscopic examinations at the Mayo Clinic between 1976 and 1989, and found 377 cases of long-segment Barrett's esophagus. The prevalence of the disorder was found to increase from 0% in patients 0 to 9 years of age to a maximum of 0.928% in patients 80 to 89 years of age. Unlike the age-related rise in prevalence, however, the length of esophagus lined by Barrett's epithelium did not appear to increase significantly with age. Twenty-year-old patients had a segment of columnar-lined esophagus similar in length to that of the octogenarians. Furthermore, no progression of Barrett's esophagus was found among 101 patients who had follow-up endoscopic examinations performed after a mean interval of 3.2 years. The prevalence data suggested that the mean age for developing Barrett's esophagus was 40 years, whereas the mean age of patients at the time of diagnosis was 63 years, an observation implying that Barrett's epithelium often develops more than 20 years before it is discovered. This report also suggests that Barrett's esophagus usually develops to its full extent all at once, many years before it is discovered, and does not progress substantially with time.
Published estimates on the annual risk of cancer in patients with Barrett's esophagus have ranged from 0.2% to 2.9%.15, 16 However, there is compelling evidence that the cancer risk in Barrett's esophagus had been overestimated for years because of publication bias and the selective reporting of studies that have positive or extreme results.16 Modern studies suggest that patients with Barrett's esophagus develop esophageal cancer at the rate of approximately 0.5% per year (i.e., 1 cancer per 200 patients per year). Endoscopic surveillance is proposed to identify these neoplasms when they are in an early, curable stage. Although it is not clear that long- and short-segment Barrett's esophagus have the same risk for malignancy, the two conditions presently are managed similarly.
In addition to GERD and Barrett's esophagus, obesity is a strong risk factor for esophageal adenocarcinoma. Although cigarette smoking and alcohol consumption are very strong risk factors for squamous cell carcinoma of the esophagus, cigarette smoking only modestly increases the risk of esophageal adenocarcinoma, and alcohol does not appear to affect the risk at all. The use of aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs) has been found to protect against esophageal cancer, as does a diet high in fruits and vegetables.
Pathogenesis
Barrett's esophagus develops through the process of metaplasia, in which one adult cell type replaces another.17 Little is known about the molecular events that effect the transformation from esophageal squamous epithelium into specialized intestinal metaplasia, but recent studies suggest a pathogenetic role for the Cdx genes. These genes are members of the homeobox gene family of transcription factors, and they are known to mediate the differentiation of intestinal epithelial cells. The Cdx2 gene is expressed by epithelial cells in the small and large intestine, but not in the normal esophagus and stomach. Silberg et al.18 found that they could induce intestinal metaplasia in the stomachs of mice by forcing the gastric epithelial cells to express Cdx2. Immunostaining for Cdx2 has been found in 100% of biopsy specimens of Barrett's specialized intestinal metaplasia.19 One group of investigators recently detected Cdx2 messenger RNA (mRNA) expression in esophageal squamous epithelium in six of 19 specimens from patients with Barrett's esophagus.20 This finding of Cdx2 mRNA in normal-appearing squamous epithelium above specialized intestinal metaplasia suggests that Cdx2 expression by squamous cells might precede the development of Barrett's esophagus.
Esophageal metaplasia appears to be a consequence of chronic reflux esophagitis, and a number of physiologic abnormalities that predispose to severe GERD have been described in patients with long-segment Barrett's esophagus. For example, some patients have gastric acid hypersecretion and duodenogastric reflux.21, 22, 23 In those patients, reflux episodes may be exceptionally damaging to the esophagus because the gastric juice contains such high concentrations of acid and bile. Manometric studies often reveal extreme hypotension of the lower esophageal sphincter (an important barrier to gastroesophageal reflux), and patients with this abnormality are exceptionally predisposed to reflux.24 Some have poor esophageal contractility, an abnormality that may delay the clearance of noxious material from the esophagus.25 Diminished esophageal pain sensitivity has been demonstrated, and so the reflux of caustic material may not cause heartburn.26 Such patients may be unlikely to seek medical attention for GERD or to take medications that could prevent esophageal injury. Decreased salivary secretion of epidermal growth factor, a peptide that enhances the healing of peptic ulceration, has been reported in some patients.27 This abnormality might delay healing of the reflux-damaged esophagus. Individual patients may exhibit all or none of these abnormalities, and their frequency in Barrett's esophagus is disputed. For example, Hirschowitz28 did not find gastric acid hypersecretion in a large series of patients with Barrett's esophagus.
The frequency of physiologic abnormalities that predispose to GERD is especially unclear in patients with short-segment Barrett's esophagus. Many such patients have no GERD symptoms and no endoscopic signs of esophagitis.29 Some studies suggest that the length of metaplastic mucosa in Barrett's esophagus is related to the duration of esophageal acid exposure.30 Thus, patients with long-segment disease may have protracted esophageal acid exposure, whereas patients with short-segment Barrett's esophagus may have esophageal acid exposure values that are normal or only minimally increased.
Even in patients who have no signs or symptoms of GERD, recent studies have shown that the gastroesophageal junction (GEJ) region is exposed frequently to concentrated acid and other noxious materials that might induce inflammation and metaplasia. After meals, there is a pocket of acid at the GEJ that escapes the buffering effects of ingested food.31 The investigators who first described this postprandial acid pocket estimated that it had a mean length of 2 cm, beginning in the most proximal stomach and extending more than 1 cm above the squamocolumnar junction into the distal esophagus. Other investigators, who have confirmed the existence of the postprandial acid pocket, contend that it does not extend above the gastric cardia into the esophagus.32 This controversy is as yet unresolved. One recent study has shown that the very distal esophagus (5 mm above the squamocolumnar junction) of healthy volunteers is exposed to acid for more than 10% of the day.33 Potential consequences of such persistent acid exposure at the GEJ include not only acid-peptic injury, but also exposure to high concentrations of nitric oxide (NO) generated from dietary nitrate (NO3-) in green, leafy vegetables. Most ingested nitrate is absorbed by the small intestine and excreted unchanged in the urine, but approximately 25% is concentrated by the salivary glands and secreted into the mouth where bacteria on the tongue reduce the recycled nitrate to nitrite (NO2-). When swallowed nitrite encounters acidic gastric juice, the nitrite is converted rapidly to NO. After nitrate ingestion, high levels of NO have been demonstrated at the GEJ.34 Nitric oxide in these concentrations can damage DNA and, potentially, predispose to metaplasia and malignancy. Thus, the GEJ is exposed repeatedly to acid, pepsin, NO, and other noxious agents in gastric juice. Chronic exposure to these agents may induce the injury and inflammation that results in the intestinal metaplasia of Barrett's esophagus.35
Diagnosis
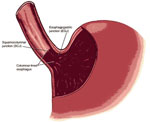
Endoscopic examination is required to recognize a columnar-lined esophagus, and the endoscopic impression of Barrett's esophagus must be confirmed by histologic evaluation of esophageal biopsy specimens. Two diagnostic criteria must be fulfilled to establish a diagnosis of Barrett's esophagus unequivocally: (1) columnar epithelium must line the distal esophagus, and (2) biopsy specimens of the columnar-lined esophagus must show specialized intestinal metaplasia. To document that columnar epithelium lines the esophagus, the endoscopist must identify both the squamocolumnar junction (SCJ) and the GEJ (Figure 2). Columnar epithelium has a reddish color and coarse texture on endoscopic examination, whereas squamous epithelium has a pale, glossy appearance. The juxtaposition of these epithelia at the SCJ forms a visible line called the Z-line. The GEJ is defined endoscopically as the level of the most proximal extent of the gastric folds.36 When the SCJ and GEJ coincide (Figure 3), then there is no columnar-lined esophagus. When the SCJ is located proximal to the GEJ (Figure 2), then there is a columnar-lined segment of esophagus. If the endoscopist takes biopsy specimens from that columnar lined segment and histologic evaluation shows specialized intestinal metaplasia, then the patient has Barrett's esophagus. Such a patient has long-segment Barrett's esophagus if the distance between the Z-line and the GEJ is 3 cm or more, and short-segment Barrett's esophagus if that distance is less than 3 cm.
Figure 2: Endoscopic landmarks, part 1.
The squamocolumnar junction (SCJ or Z-line) is the visible line formed by the juxtaposition of squamous and columnar epithelia. The gastroesophageal junction (GEJ) is the imaginary line at which the esophagus ends and the stomach begins. The most proximal extent of the gastric folds has been proposed as a marker for the GEJ. When the SCJ is located proximal to the GEJ, there is a columnar-lined segment of esophagus. (Source: Spechler,37 with permission from American Gastroenterological Association.)
Intestinal Metaplasia at the Gastroesophageal Junction
Intestinal metaplasia can develop as a result of chronic inflammation in the stomach, the esophagus, or both.37 Histologically, intestinal metaplasia in the stomach can appear to be identical to intestinal metaplasia in the esophagus. During endoscopic examination, the GEJ is a dynamic structure whose position can vary by centimeters with respiration and with the extent of air insufflation. Whereas it can be difficult to localize the GEJ with great precision, it can be difficult to determine whether short segments of intestinal metaplasia found in the GEJ region are lining the distal esophagus (short-segment Barrett's esophagus) or the proximal stomach (intestinal metaplasia of the gastric cardia).17 The term intestinal metaplasia at the GEJ has been used to describe the condition in which intestinal metaplasia is found at a Z-line that appears to coincide precisely with the GEJ. Rather than constituting an independent condition, however, intestinal metaplasia at the GEJ almost certainly represents either short-segment Barrett's esophagus or intestinal metaplasia of the cardia.
Intestinal metaplasia in the stomach often results from the chronic gastritis caused by infection with Helicobacter pylori,38 whereas chronic reflux esophagitis commonly causes intestinal metaplasia in the esophagus. For some patients, therefore, intestinal metaplasia at the GEJ results from H. pylori gastritis, whereas for others the condition results from GERD that causes intestinal metaplasia in segments of esophagus so short that they cannot be distinguished from the serrations of a normal Z-line. Chronic H. pylori infection may even protect against the development of Barrett's esophagus and other complications of GERD, because the organisms can cause a diffuse gastritis that decreases gastric acid secretion.39 H. pylori gastritis is not rare in patients with Barrett's esophagus, however, and testing for H. pylori is not a reliable way to distinguish short-segment Barrett's esophagus from intestinal metaplasia of the gastric cardia.40
For patients with intestinal metaplasia at the GEJ, the distinction between short-segment Barrett's esophagus and intestinal metaplasia of the gastric cardia may have clinical importance because circumstantial evidence suggests that short-segment Barrett's esophagus has a substantially higher cancer risk. For example, Sharma et al.41 found dysplasia (the precursor of malignancy) in 20 of 177 patients (11.3%) with short-segment Barrett's esophagus, but in only one of 76 patients (1.3%) with intestinal metaplasia in the gastric cardia (41). Medical societies recommend endoscopic cancer surveillance routinely for patients with Barrett's esophagus, but not for patients with intestinal metaplasia in the stomach.42, 43 Therefore, the distinction between these two conditions has important implications for patient management. A number of histochemical and immunologic biomarkers have been proposed to differentiate intestinal metaplasia of the cardia from short-segment Barrett's esophagus including cytokeratin staining patterns,44, 45, 46, 47 immunoreactivity for mAb Das-1 (a monoclonal antibody raised against colonic epithelial cells),48 and mucosal expression of colonic-type sulfomucins.46 A recent review has concluded that the utility of biomarkers in distinguishing short-segment Barrett's esophagus from intestinal metaplasia of the gastric cardia has not been established, however, and that clinical decisions should not be based on the presence of these biomarkers.49
Dysplasia in Barrett's Esophagus
Cancers in Barrett's esophagus evolve through a series of genetic (DNA) mutations that favor cell growth. Dysplasia is the early histologic expression of that DNA damage.50 The histologic features of dysplasia include nuclear changes such as enlargement, pleomorphism, hyperchromatism, stratification, and atypical mitoses, and architectural abnormalities such as crowding of tubules and villiform surfaces.51 The finding of these histologic abnormalities suggests that the tissue has sustained DNA damage, resulting in clonal proliferations of cells with abnormal differentiation and a predisposition to malignancy. Dysplasia is categorized as low-grade or high-grade depending on the degree of histologic abnormalities, with more pronounced abnormalities reflecting more severe genetic damage and greater potential for carcinogenesis.
A nonneoplastic esophageal epithelium that is regenerating in response to GERD-induced injury can exhibit histologic changes similar to those of low-grade dysplasia. Consequently, it can be difficult for pathologists to distinguish low-grade dysplasia in Barrett's esophagus from reactive changes caused by reflux esophagitis. Interobserver agreement among experienced pathologists for the diagnosis of low-grade dysplasia may be less than 50%, whereas interobserver agreement for distinguishing high-grade dysplasia from lesser lesions is approximately 85%.52, 53, 54 Furthermore, there can be substantial interobserver disagreement when distinguishing high-grade dysplasia from intramucosal carcinoma, a lesion that has potential for lymphatic dissemination.55
Dysplasia in Barrett's esophagus often is flat, inconspicuous, and patchy both in extent and severity.56, 57 Whereas endoscopists rely on random biopsy sampling techniques to identify this elusive lesion, dysplasia can be missed because of biopsy sampling error. For patients found to have dysplasia, furthermore, foci of higher grade lesions (including invasive cancer) can be missed. In series of patients who had esophagectomies because endoscopic examination revealed high grade dysplasia in Barrett's esophagus with no apparent tumor mass, invasive cancer (missed because of biopsy sampling error) has been found in 30% to 40% of the resected esophagi.58 Extensive biopsy sampling of the Barrett esophagus can reduce biopsy sampling error, but cannot eliminate the problem entirely.59, 60
A number of molecular markers for cancer risk and endoscopic techniques for recognizing early esophageal neoplasia have been proposed as alternatives to random biopsy sampling for dysplasia in Barrett's esophagus.61, 62 Promising molecular markers include abnormalities in p53 and cyclin D1 expression, and abnormal cellular DNA content demonstrable by flow cytometry, all of which have been associated with carcinogenesis in Barrett's esophagus.63, 64, 65 Techniques to identify neoplastic areas for biopsy sampling during endoscopy include mucosal staining with dyes (chromoendoscopy), endosonography, optical coherence tomography, and spectroscopy using reflectance, absorption, light-scattering, fluorescence, and Raman detection methods.66, 67, 68, 69, 70, 71 Although preliminary study results are promising, none of these tests and techniques yet has been shown to provide sufficient clinical information to justify its routine application for surveillance purposes.
Natural History of Dysplasia
Cancers in Barrett's esophagus evolve through a sequence of genetic alterations that may be heralded by dysplastic changes of progressive severity. Thus, low-grade dysplasia appears to progress to high-grade dysplasia, which, in turn, progresses to cancer. Few studies document the natural history of dysplasia, however, and the rate with which low-grade dysplasia progresses to high-grade dysplasia and cancer is not clear. Furthermore, the fact that low-grade dysplasia in Barrett's esophagus is not diagnosed reliably is a major problem that confounds the interpretation of all studies on this condition. Diagnostic difficulties undoubtedly underlie some of the disparities among published series regarding the prevalence and incidence of low-grade dysplasia in Barrett's esophagus. For example, low-grade dysplasia was described in approximately 70% of patients in one large series from a Veterans Administration hospital.72 Another recent study that reviewed the pathology archives of three large university hospitals using strict diagnostic criteria for dysplasia identified low-grade dysplasia in only 37 (4.7%) of 790 cases of Barrett's esophagus.73
One study of 48 patients with low-grade dysplasia followed for a mean duration of 41 months found that four progressed to high-grade dysplasia (8%) and one progressed to adenocarcinoma (2%).74 Another group described a 12% cumulative incidence of adenocarcinoma at 5 years for 43 patients with low-grade dysplasia on baseline endoscopy.65 Skacel et al.53 followed 25 patients with low-grade dysplasia for a mean duration of 26 months, during which seven (28%) progressed to high-grade dysplasia (five patients) or adenocarcinoma (two patients). In this series, agreement among pathologists in the diagnosis of low-grade dysplasia was associated with neoplastic progression. Seven of the 17 patients (41%) in whom at least two of the three study pathologists agreed on the diagnosis of low-grade dysplasia exhibited progression, whereas progression was seen in four of the five patients (80%) for whom there was unanimous agreement among the study pathologists.
There are substantial variations among reported estimates on the rate at which high-grade dysplasia in Barrett's esophagus progresses to cancer. One report described eight patients with high-grade dysplasia in Barrett's esophagus, five (63%) of whom were found to have adenocarcinomas on repeat endoscopic examinations performed within 1 year.74 Reid et al.65 reported a 59% five-year cumulative cancer incidence in 76 patients with high-grade dysplasia in Barrett's esophagus. In Buttar et al.'s75 series of 100 consecutive patients with high-grade dysplasia, cancers were detected in 32% during a follow-up period of up to 8 years. In contrast, Schnell et al.72 reported that only 12 (16%) of his 75 Veteran patients with high-grade dysplasia in Barrett's esophagus developed adenocarcinoma during a mean follow-up period of 7.3 years. The reasons underlying the large disparities in the results of these studies are not clear, but probably involve differences among pathologists in their interpretation of dysplastic changes and differences regarding whether patients found to have cancer within 1 year of the initial diagnosis of high-grade dysplasia were included or excluded from the outcome analyses. Buttar et al.75 found that the extent of high-grade dysplasia in Barrett's esophagus correlated with the risk for adenocarcinoma, but other investigators have not confirmed this observation.76, 77 Spechler50 recently reviewed these data and concluded that 10% to 30% of patients with high-grade dysplasia in Barrett's esophagus will develop a demonstrable malignancy within 5 years of the initial diagnosis.
Screening and Surveillance for Barrett's Esophagus
Two major unresolved clinical issues in Barrett's esophagus are whether patients with GERD symptoms should be screened endoscopically for the condition, and whether patients found to have Barrett's esophagus should have regular endoscopic surveillance for dysplasia.78, 79 The rationale for these practices includes the following assumptions: (1) screening will reliably identify those individuals at highest risk for developing esophageal adenocarcinoma; (2) without intervention, patients with Barrett's esophagus will have decreased survival because of deaths from esophageal adenocarcinoma; (3) surveillance will reliably detect dysplasia in Barrett's esophagus; and (4) treatment of the dysplasia found by surveillance will prolong survival and improve quality of life by preventing death and morbidity from esophageal cancer. All of these assumptions are unproved and questionable.
It has not been established that screening patients with GERD symptoms reliably identifies those individuals at high risk for esophageal adenocarcinoma. Indeed, studies suggest that approximately 40% of patients with esophageal adenocarcinomas have no history of GERD symptoms (3). Therefore, screening programs that target only patients with heartburn can have only limited impact on cancer mortality rates, and there is little evidence that these programs have prevented deaths from esophageal adenocarcinomas. In published series of patients found to have these tumors, fewer than 5% were known to have had Barrett's esophagus before they sought medical attention for symptoms of esophageal cancer.80
A number of studies have suggested that survival for patients with Barrett's esophagus does not differ significantly from that for control subjects in the general population.81, 82, 83, 84 Those studies have comprised predominantly older patients, although these were patients who often succumbed to common diseases of the elderly (e.g., coronary artery disease) rather than to esophageal adenocarcinoma. Proponents of surveillance argue that the results of those studies may not be applicable to younger, healthier patients with Barrett's esophagus.
Observational studies have shown that endoscopic surveillance can detect curable neoplasms in Barrett's esophagus, and that asymptomatic cancers discovered during surveillance are less advanced than those found in patients who present with cancer symptoms like dysphagia and weight loss.85, 86, 87, 88 However, those studies are highly susceptible to a number of biases such as healthy volunteer bias, lead-time bias, and length-time bias. It is not appropriate to conclude that surveillance is beneficial based solely on the observation that patients with asymptomatic neoplasms survive longer than patients who have symptoms owing to esophageal cancer.89 No study has established the reliability of surveillance in detecting curable dysplasia, and a number of reports have documented the development of incurable malignancies in some patients despite adherence to endoscopic surveillance programs.85, 86 Furthermore, hazardous invasive therapies for dysplasia like esophagectomy ultimately might do more harm than good. Nevertheless, no observational study has documented an overall survival disadvantage for patients in surveillance programs for Barrett's esophagus.
Some computer models have shown that endoscopic screening and surveillance for Barrett's esophagus can be beneficial provided that certain baseline assumptions are valid.90, 91, 92, 93, 94 Such models do not provide a single definitive answer, however, but rather a range of possible outcomes that vary with changes in the baseline assumptions. One group used a decision tree to explore the utility of one-time endoscopic screening for high-grade dysplasia in 60-year-old patients with GERD symptoms. With favorable baseline assumptions (e.g., relatively high prevalence of dysplasia in the group screened, low cost for endoscopy, good health-related quality of life after esophagectomy), this model estimated that screening might cost $24,700 per life-year saved.91 Another group used a Markov model to construct a computer cohort simulation of 10,000 middle-aged patients with Barrett's esophagus, and assumed that esophagectomy would be performed for those whose surveillance endoscopies showed high-grade dysplasia. For an annual cancer incidence rate of 0.4%, this analysis suggested that endoscopic surveillance performed every 5 years was the preferred strategy, costing $98,000 per quality-adjusted life year gained.90 Another, more recent cost-utility analysis contradicted the findings of the latter model, concluding that whereas screening for Barrett's esophagus might be cost-effective, surveillance is not.93 None of these computer models can be considered definitive, however, because all incorporate numerous layers of soft data and questionable assumptions.
Spechler79 has summarized the dilemma regarding screening and surveillance for Barrett's esophagus as follows: (1) Endoscopy is expensive. (2) There is no proof that endoscopic screening of patients with GERD for Barrett's esophagus has any impact on survival. (3) No "proof" in the form of a randomized controlled trial is likely to become available in the near future. (4) Available observational studies, which are subject to numerous forms of bias, suggest that screening and surveillance are beneficial. (5) Available computer models, which are based on some soft data and questionable assumptions, suggest that screening can be beneficial. (6) Although endoscopic screening clearly can be associated with risks (i.e., complications resulting both from endoscopy and from the invasive procedures used to treat conditions found by endoscopy), no study has shown an overall survival disadvantage for patients in screening and surveillance programs. In this murky situation, where the indirect evidence available suggests that screening and surveillance are beneficial and the major objection is cost, it seems better to err by performing unnecessary endoscopy rather than by missing curable esophageal neoplasms.
Treatment of Barrett's Esophagus
Treatment of GERD in Barrett's Esophagus
The primary goals of antireflux therapy, irrespective of the presence of Barrett's esophagus, are elimination of the symptoms and signs of reflux esophagitis, and prevention of GERD complications such as peptic esophageal stricture. Authorities debate whether proton pump inhibitors (PPIs) or histamine H2-receptor antagonists (H2RAs) should be used as initial therapy for patients with GERD of mild to moderate severity.95 Reliable healing of severe GERD generally requires treatment with PPIs, however, and there is a consensus that PPIs should be the initial medical therapy for patients with severe GERD.95, 96 Whereas long-segment Barrett's esophagus is associated with severe reflux esophagitis, PPIs usually are prescribed as first-line therapy for GERD in such patients.97 Patients with short-segment Barrett's esophagus often have mild GERD, however, and H2RA therapy might be sufficient to control their symptoms and signs of reflux disease. Some patients with Barrett's esophagus have no signs or symptoms of active GERD, and it is not clear that any antireflux therapy is warranted in these cases.98
Although medical antisecretory therapy reduces gastric acid secretion in most patients with Barrett's esophagus, the effect of antisecretory therapy on GERD symptoms does not accurately reflect the level of acid suppression achieved. Esophageal pH monitoring studies frequently reveal pathologic levels of acid reflux in patients with severe GERD (with and without Barrett's esophagus) who are rendered asymptomatic by PPIs administered in conventional dosages.99, 100 Approximately 80% of patients with Barrett's esophagus treated with a PPI twice daily experience the phenomenon of nocturnal gastric acid breakthrough (defined as a fall in gastric pH below 4 for more than 1 hour at night) which is often associated with episodes of acid reflux.101
It is sometimes possible to achieve almost complete achlorhydria by administering PPIs in high dosages, or by adding a bedtime dose of an H2RA to a regimen of high-dose PPI therapy.102 Authorities now debate the advisability of prescribing such aggressive antireflux therapy, designed to eliminate rather than merely reduce acid reflux, for all patients with Barrett's esophagus, irrespective of the severity of their underlying GERD. Proponents of this aggressive approach contend that gastroesophageal reflux is the major factor promoting carcinogenesis in specialized intestinal metaplasia, and that elimination of acid reflux by pharmacologic or surgical means should prevent cancer. Opponents argue that the role of acid reflux in promoting carcinogenesis in Barrett's esophagus is not clear, and available data on this issue are too weak to support the routine prescription of aggressive antireflux therapy (with its considerable expense and inconvenience) in all cases.
The notions that GERD promotes malignancy in Barrett's esophagus and that GERD treatment might prevent cancer are supported by indirect evidence only. For example, biopsy specimens of specialized intestinal metaplasia maintained in organ culture have been shown to exhibit hyperproliferation and increased expression of cyclooxygenase-2 (a mediator of proliferation) when exposed to acid for 1 hour.103, 104 Brief esophageal acid exposure also has been shown to activate the mitogen-activated protein kinase (MAPK) pathways that can increase proliferation and decrease apoptosis in Barrett's esophagus.105 These observations suggest that acid reflux in patients with Barrett's esophagus might stimulate hyperproliferation, suppress apoptosis, and thereby promote carcinogenesis in their specialized intestinal metaplasia.
One study evaluated biopsy specimens from 39 patients with Barrett's esophagus at baseline and after 6 months of therapy with PPIs given only in doses sufficient to eliminate GERD symptoms.106 The expression of proliferating cell nuclear antigen (PCNA) (a proliferation marker) decreased and the expression of villin (a differentiation marker) increased significantly in biopsy specimens from the 24 patients in whom PPIs normalized esophageal acid exposure, but not in the 15 with persistently abnormal acid reflux during PPI therapy. Another group found no significant change in the proliferative activity of Barrett's esophagus (as assessed by in vitro labeling with 5-bromo-2-deoxyuridine) in 22 patients treated with a PPI for 2 years, whereas proliferative activity increased significantly in 23 patients treated for the same time with an H2RA.107
Extrapolation of the results of these studies to the practice of prescribing aggressive antireflux therapy for patients with Barrett's esophagus requires assumptions that may not be warranted. The acute effects of acid exposure on tissue maintained in organ culture ex vivo may not reflect the chronic effects of GERD on Barrett's esophagus in vivo. Even if the ex vivo observations are valid, it is unclear to what extent acid exposure must be reduced in order to be beneficial to patients with Barrett's esophagus. It is often difficult to achieve achlorhydria even with PPIs administered in high dosages and in combination with H2RAs,108 and the ex vivo experiments suggest that a treatment that reduces acid reflux to brief episodes conceivably could stimulate cellular proliferation and promote carcinogenesis. The aforementioned clinical studies suggest that reduction of esophageal acid exposure can be beneficial,106, 107 but this conclusion is based on the dubious assumption that effects on MAPK pathways, PCNA and villin expression, and 5-bromo-2-deoxyuridine labeling reflect important changes in cancer risk.
Another line of evidence suggesting that aggressive antireflux therapy might prevent cancer in Barrett's esophagus is the observation that such therapy can cause partial regression of the specialized intestinal metaplasia.109 During chronic PPI therapy, most patients develop islands of squamous epithelium (evidence of partial regression) within their metaplastic columnar lining.110 It is not clear that this partial regression is beneficial, however. Biopsy specimens of the squamous islands show underlying intestinal metaplasia in approximately 40% of cases, suggesting that the islands may result from an overgrowth of squamous epithelium rather than a regression of the metaplastic mucosa.111 Furthermore, biopsy specimens from the squamous islands frequently exhibit abnormalities in Ki-67 staining (a proliferation marker) and p53 expression that might favor carcinogenesis.112 These observations suggest that the partial regression of metaplasia induced by antireflux therapy might not decrease the cancer risk in Barrett's esophagus.
A recent study of 236 veteran patients with Barrett's esophagus who were followed for a total of 1170 patient-years has found that the use of PPIs is associated with a reduced incidence of dysplasia.113 In a Kaplan-Meier survival analysis, the 10-year cumulative incidence of dysplasia was 21% for the 155 patients on PPI therapy, compared to 58% for the 81 patients treated with either a histamine H2-receptor antagonist or no antisecretory therapy.
Surgeons have proposed that fundoplication might be more effective than antisecretory therapy for preventing cancer in Barrett's esophagus.114 Several uncontrolled, observational studies have found fewer cases of dysplasia and cancer among patients with Barrett's esophagus who had antireflux surgery than among those who had received medical treatment.115, 116, 117 Some even have proposed that antisecretory therapy might predispose to malignancy,118, 119 and that the increasing use of antisecretory medications might underlie the rising frequency of esophageal adenocarcinoma in Western countries.120 However, the limited studies that have addressed this issue directly have not found a significant association between esophageal adenocarcinoma and the use of antisecretory agents per se.121, 122
The long-term outcome of a randomized trial of medical and surgical therapies for 247 veteran patients with complicated GERD (including 108 with Barrett's esophagus) does not support the contention that fundoplication prevents esophageal cancer better than antisecretory therapy.123 During 10 to 13 years of follow-up, four of 165 patients (2.4%) in the medical group and one of 82 (1.2%) in the surgical group developed an esophageal adenocarcinoma. The difference between the treatment groups in the incidence of this tumor was not statistically significant but, with such a low observed rate of cancer development, the study did not have sufficient statistical power to detect small differences in the incidence of esophageal cancer. However, any potential cancer-preventive benefit of surgery was offset by an unexplained, but significant, decrease in survival for the surgical patients owing to excess deaths from heart disease.
A report describing the results of a large, Swedish, population-based cohort study also refutes the contention that antireflux surgery prevents esophageal adenocarcinoma.124 In this study, patients with GERD were followed for up to 32 years. The relative risk for developing esophageal adenocarcinoma (compared to the general population) among 35,274 men who received medical antireflux therapy was 6.3 [95% confidence interval (CI) 4.5–8.7], whereas the relative risk for 6406 men treated with fundoplication was 14.1 (95% CI 8.0–22.8). Finally, a large, retrospective cohort study of Veteran Administration patients125 and a recent meta-analysis of published reports both found no significant differences in esophageal cancer incidence among medically and surgically treated patients with Barrett's esophagus.126 Although none of these studies is definitive, the bulk of evidence suggests that antireflux surgery should not be advised with the expectation that the procedure will prolong life by preventing esophageal cancer.
Prevention of Adenocarcinoma
The Role of Nonsteroidal Antiinflammatory Drugs
Epidemiologic data suggest that aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs), which inhibit cyclooxygenase (COX), may protect against cancer in Barrett's esophagus.127 The specialized intestinal metaplasia of Barrett's esophagus exhibits increased expression of COX-2,128 and inhibition of COX-2 has antiproliferative and proapoptotic effects in Barrett's-associated esophageal adenocarcinoma cell lines.129 Furthermore, COX-2 inhibitors have been found to prevent the development of esophageal adenocarcinoma in an animal model of Barrett's esophagus.130 Nevertheless, prospective clinical studies are needed before NSAIDs can be recommended for chemoprevention in patients with Barrett's esophagus. Even if efficacy in cancer prevention could be demonstrated, it is not clear that the high cost and potential cardiovascular risks of the COX-2 selective NSAIDs would be justified for routine clinical use in patients with Barrett's esophagus. Aspirin, an inexpensive, nonselective NSAID that can prevent cardiovascular as well as neoplastic complications, might be a useful drug if its protective effects can be shown to outweigh its risk of gastrointestinal complications. Currently, a large, controlled trial of aspirin for the chemoprophylaxis of Barrett's esophagus is underway in the United Kingdom.
Treatment of Dysplasia in Barrett's Esophagus
For patients with verified high-grade dysplasia in Barrett's esophagus, there are generally four proposed management options: (1) esophagectomy, (2) endoscopic therapies that ablate the neoplastic tissue, (3) endoscopic mucosal resection, and (4) intensive endoscopic surveillance in which invasive therapies are withheld until biopsy specimens reveal adenocarcinoma. Each of these options is associated with substantial risks and unclear benefits.
Esophagectomy
Esophagectomy is the only therapy for high-grade dysplasia that clearly removes all of the neoplastic epithelium, but this definitive therapy has the highest rates of short-term mortality and long-term morbidity of all the treatment options. Studies on esophagectomy for high-grade dysplasia in Barrett's esophagus typically have involved small numbers of patients from a single institution, and such reports are of limited value for estimating the morbidity and mortality of esophagectomy for patients with dysplasia. Much larger series are available on the results of esophagectomy for esophageal cancer, but it may not be appropriate to extrapolate the results of surgery performed on debilitated patients with esophageal malignancies to those for otherwise healthy patients with dysplasia. Nevertheless, there are some important lessons to be learned from the large series that may be applicable to patients with precancerous lesions of the esophagus.
One lesson is that there is an inverse relationship between the mortality rate for esophagectomy and the frequency with which the operation is performed at any given institution. In one study of 340 esophagectomies performed at 25 different hospitals, the mortality rate was 3.0% for patients who had the operation at institutions that did five or more esophagectomies per year, compared to 12.2% for patients treated at institutions where the operation was performed less frequently.132 In a study of data from the Dutch National Medical Registry, the mortality rates for esophagectomy were 12.1%, 7.5%, and 4.9% at centers performing one to 10, 11 to 20, and >50 esophagectomies per year, respectively.133
The average hospital stay for open esophagectomy is approximately 2 weeks, and 30% to 50% of patients develop at least one serious postoperative complication such as pneumonia, arrhythmia, myocardial infarction, heart failure, wound infection, and anastomotic leak.132, 133, 134, 135 Available data on minimally invasive techniques for esophagectomy are promising but limited, and it is not yet clear that these approaches are safer or preferable to the open procedure.136, 137
Esophagectomy frequently causes long-term morbidities such as dysphagia, weight loss, gastroesophageal reflux, and dumping syndrome. Nevertheless, the limited quantitative data available suggest that the impact of these problems on quality of life may be surprisingly small. One group performed a utility assessment of patients who had esophagectomies at least 1 year earlier, and found that they rated their median quality of life at 0.97 on a scale of 0 to 1 (where 0 = death and 1 = perfect health).90 In another study of 53 patients who had esophagectomies for high-grade dysplasia in Barrett's esophagus and who completed the Medical Outcomes Study (MOS) Short Form 36 (SF-36) Health Status Questionnaire, there appeared to be few important differences between the patients and a normal control population.138 Specialized intestinal metaplasia develops frequently in the esophageal remnant in patients who have had esophagectomy with esophagogastrostomy, presumably as the result of the reflux esophagitis that often accompanies this procedure.139, 140, 141 Presumably, those patients will be at risk for developing esophageal adenocarcinoma in the future, but I have found only one report of this occurrence.142
Endoscopic Ablative Therapies
Endoscopic ablative therapies with, for example, potassium titanyl phosphate (KTP), argon, or neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers, multipolar electrocoagulation, argon plasma coagulation, or photodynamic therapy, use thermal or photochemical energy to ablate the abnormal epithelium in Barrett's esophagus.171143, 144 After ablation, patients are given potent antireflux therapy so that the injured mucosa heals with the growth of new squamous epithelium. The relative merits of the various endoscopic ablative therapies are disputed, and there appears to be an inverse relationship between the completeness of mucosal ablation and the frequency of complications.
One major concern regarding endoscopic ablative therapies for dysplasia is that the procedures will not eradicate all of the dysplastic cells. Indeed, endoscopic ablation commonly leaves visible foci of metaplastic mucosa behind.143, 144 Partially ablated metaplastic mucosa can heal with an overlying layer of squamous epithelium that buries the abnormal tissue and hides it from the endoscopist. There are reports of adenocarcinoma developing from buried metaplastic tissue.145 Furthermore, partially ablated metaplastic epithelium can develop new abnormalities in the expression of proliferation markers and p53, raising the possibility that incomplete ablation of Barrett's esophagus might even increase the risk of carcinogenesis.146
A number of deficiencies limit the conclusions that can be drawn from reports on ablative therapies for dysplasia in Barrett's esophagus. Most studies are not randomized or controlled, involve relatively few patients, and have short durations of follow-up. Photodynamic therapy (PDT) is the most extensively studied of the ablative techniques. For PDT, patients are given a systemic dose of a light-activated chemical like a porphyrin that is taken up by the esophageal cells. The esophagus is then irradiated using a low-power laser that activates the chemical, which transfers the energy acquired from laser light to oxygen. This results in the formation of singlet oxygen, a toxic molecule that destroys the abnormal cells and their vasculature.
Overholt et al.147 performed PDT using porfimer sodium for 103 patients with early cancer or dysplasia in Barrett's esophagus who were followed for an average of 51 months (range 2–122 months). For nine patients with early-stage cancers, the malignancy appeared to be eliminated in four cases (44%). Follow-up endoscopy showed no dysplastic epithelium in 62 of 80 patients (78%) who had PDT for high-grade dysplasia, and in 13 of 14 patients (93%) who had the procedure for low-grade dysplasia. Most patients who receive PDT with porfimer sodium experience chest pain and dysphagia, most develop small pleural effusions, and some develop transient atrial fibrillation in the week after treatment.148 Patients who do not adequately shield themselves from sun exposure in the weeks following the porfimer injection experience skin injury, and one third of patients develop esophageal strictures.
The results of a large, multicenter, randomized trial of PDT using porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus recently have been presented in abstract form.149 Patients with high-grade dysplasia (n = 208) were randomized to receive either PDT with omeprazole 20 mg b.i.d., or omeprazole 20 mg b.i.d. alone (without PDT). Patients were followed for 2 to 4.5 years with endoscopic surveillance performed every 3 months. No dysplasia was seen on follow-up in 77% of the patients treated with PDT, and in 39% of the patients who received omeprazole alone (p <.0001). Thirteen percent of the PDT patients developed cancer, compared to 28% of those treated with omeprazole alone (p = .006). There was no procedure-related mortality, but esophageal strictures developed in 37% of those who received PDT. These results show that PDT clearly is superior to omeprazole alone for eradicating dysplasia and preventing cancer in Barrett's esophagus. Nevertheless, enthusiasm for PDT must be tempered by the fact that 13% of the patients still develop cancer within 5 years of treatment.
Although the reports discussed above document the feasibility of ablating neoplastic epithelium with PDT, they do not establish the benefit of the technique. Photodynamic therapy is an expensive treatment that entails substantial risk and inconvenience. Furthermore, with no histologic examination of a resected esophagus and with durations of follow-up that are generally much less than 5 years, it is not possible to verify claims that dysplasia and cancer indeed are "eliminated" by PDT. Residual foci of metaplasia remain in most patients after PDT, and some of these foci may be buried under a superficial layer of squamous epithelium where they are invisible to the endoscopist. No study yet has established that PDT decreases the long-term risk for cancer development in Barrett's esophagus.
Endoscopic Mucosal Resection
In endoscopic mucosal resection (EMR), a large segment of esophageal mucosa is removed using a diathermy snare or endoscopic knife.71, 150, 151 Endoscopic ultrasonography (EUS) often is performed first to estimate the depth of the neoplastic lesion, but the accuracy of EUS in predicting that depth in this setting may be poor.152 Consequently, authorities dispute the necessity and even the advisability of performing EUS prior to EMR. If there is no evidence of extension into the submucosa, which is a contraindication to EMR, the endoscopist elevates the mucosal target by injecting saline into the submucosa. For the snare EMR methods, the saline-elevated mucosa is removed either by a strip biopsy technique using the snare alone or by a cap-assisted technique in which the mucosa first is suctioned into a cap that fits over the tip of the endoscope, and the snare is tightened around the suctioned area. In the endoscopic knife techniques, a large segment of mucosa is dissected and removed en bloc. Unlike the endoscopic ablative techniques, EMR provides large tissue specimens that can be examined by the pathologist to determine the character and extent of the lesion, and the adequacy of resection.
The esophageal mucosa has abundant lymphatic vessels, and so neoplastic cells that enter the esophageal lamina propria (as they do in patients with intramucosal adenocarcinoma) have the potential to disseminate. Nevertheless, limited data from surgical series suggest that fewer than 5% of patients with intramucosal adenocarcinoma in Barrett's esophagus have lymph node metastases.153, 154 In contrast, for esophageal squamous cell carcinomas with a similar early T stage, lymphatic spread appears to be more common.155 It has been proposed that the reflux esophagitis that often accompanies Barrett's esophagus may occlude mucosal lymphatic channels and thereby prevent lymphatic spread of early esophageal adenocarcinomas.154 Once the tumor cells breach the muscularis mucosae to enter the submucosa, however, the frequency of lymph node metastases exceeds 20%, even for patients without bulky tumors.153, 154 Consequently, EMR cannot be considered definitive therapy for neoplasms that extend into the submucosa.
Preliminary studies have established the feasibility of EMR, either alone or in combination with an ablative therapy like PDT, for treating high-grade dysplasia in Barrett's esophagus.156, 157, 158, 159, 160, 161 The small and limited studies available so far have documented surprisingly few serious complications (bleeding, perforation, stricture) and, as yet, no procedure-related mortality. The mean durations of follow-up reported (10 to 36 months) are woefully inadequate for meaningful conclusions regarding the efficacy of EMR in decreasing the risk of cancer development. Furthermore, a recent report has raised serious questions regarding the adequacy of cap-assisted EMR as a treatment for high-grade dysplasia.162 Histologic examination of EMR specimens from 88 patients with high-grade dysplasia revealed dysplasia at the margins of the specimens in 72 cases (82%). This suggests that cap-assisted EMR leaves neoplastic cells behind in the large majority of cases. An alternative approach is circumferential EMR, in which the endoscopist attempts to remove all of the metaplastic epithelium, not just a localized segment as in the cap-assisted technique. Preliminary results for circumferential EMR are promising, but as yet too limited to make meaningful conclusions regarding the safety and efficacy of the technique.163
Intensive Endoscopic Surveillance
Noting the risks of the invasive therapies, some authorities have recommended a program of expectant management with intensive endoscopic surveillance (i.e., endoscopic examinations every 3 to 6 months) for patients with high-grade dysplasia in Barrett's esophagus. With this approach, invasive treatments like esophagectomy are withheld until biopsy specimens reveal adenocarcinoma.72, 164 Although this practice has been endorsed as a management option by the American College of Gastroenterology,42 few published data directly support the safety and efficacy of intensive surveillance for high-grade dysplasia.
Schnell et al.72 described 12 patients who developed adenocarcinomas during intensive endoscopic surveillance for high-grade dysplasia in Barrett's esophagus. The cancers were deemed potentially curable at the time of detection in all 11 patients who were compliant with the surveillance program, but one patient who was lost to follow-up returned 10 years later with an unresectable tumor. In Reid et al.'s165 series of 32 patients with high-grade dysplasia who developed adenocarcinoma during intensive endoscopic surveillance, only one patient (3%) had incurable disease (metastases) when the cancer was first detected on surveillance endoscopy. Weston et al.77 performed intensive endoscopic surveillance in 15 patients with high-grade dysplasia for a mean duration of 36.8 months, during which four developed adenocarcinoma. One of those four had metastatic disease, and the authors concluded that an observational approach to the management of high-grade dysplasia should be discouraged.
Duration of Follow-Up for Determining Cure
Patients treated for epithelial malignancies traditionally have been deemed cured when they have no evidence of recurrence at 5 years, because it is assumed that any cancer cells that survived the treatment would have proliferated and become clinically manifest within that time period.50 Studies on the natural history of dysplasia in Barrett's esophagus suggest that, even without invasive therapy, only 10% to 30% of patients with high-grade dysplasia will develop a demonstrable cancer within 5 years, and early esophageal cancers can take years to become manifest clinically.50, 131 In theory, if a treatment for dysplasia leaves even one dysplastic cell behind, that cell can proliferate and eventually become malignant. Consequently, it is not appropriate to conclude that the cancer risk has been eliminated for a patient who has survived 5 years after treatment of dysplasia in Barrett's esophagus. Indeed, 5 years might be considered the absolute minimum for a meaningful follow-up of this condition, and many more years would be required before one could reasonably consider that the risk of dysplasia-associated cancer has been eradicated. Unfortunately, the follow-up duration in most studies on treatments for dysplasia in Barrett's esophagus is considerably less than 5 years.
Management Recommendations
No management strategy for patients with Barrett's esophagus has been proved to prolong life or to prevent death from esophageal adenocarcinoma. Some of the controversies regarding the management of this condition were highlighted in a recent, critical review conducted by an 18-member panel of experts (the AGA Chicago workshop).166 The management strategy that has been endorsed by the American College of Gastroenterology is arguably the most complete and widely followed of the published guidelines for the management of patients with Barrett's esophagus.42 Their guidelines, with minor modifications, are as follows:
- Patients with Barrett's esophagus should have regular surveillance endoscopy to obtain esophageal biopsy specimens. Gastroesophageal reflux disease should be treated prior to surveillance to minimize confusion caused by inflammation in the interpretation of dysplasia.
- For patients who have had two consecutive endoscopies that show no dysplasia, surveillance endoscopy is recommended at an interval of every 3 years.
- If dysplasia is noted, another endoscopy should be performed with extensive biopsy sampling (especially from areas with mucosal irregularity) to look for invasive cancer, and the histology slides should be interpreted by an expert pathologist.
- For patients with verified low-grade dysplasia after extensive biopsy sampling, yearly surveillance endoscopy is recommended.
- For patients with verified, multifocal high-grade dysplasia, intervention (e.g., esophagectomy) may be considered.
Although not specifically recommended in the practice guidelines, clinicians can consider the use of experimental endoscopic therapies such as photodynamic therapy or endoscopic mucosal resection for their patients with high-grade dysplasia in Barrett's esophagus, provided the therapy is administered as part of an established, approved research protocol. The use of endoscopic therapies outside of research protocols cannot be condoned at this time.