Abstract
The Indian Scad, Decapterus russelli is an important pelagic carangid widely distributed throughout the Indian Ocean and the Indo-West Pacific. Despite being widely distributed in the Indian Ocean, the information regarding genetic structuring and diversity has been lacking compared to its Indo Malay Archipelago counterparts. The present study was conducted to investigate the genetic stock structure of D. russelli based on mitochondrial (Cyt b) and nuclear (DrAldoB1) markers along Indian waters. The results indicated the presence of a single panmictic stock across the Indian Ocean region. High haplotype diversity associated with low nucleotide diversity suggested a population bottleneck followed by rapid population growth. Phylogenetic analysis revealed the absence of geographical clustering of lineages with the most common haplotype distributed globally. The pelagic life style, migratory capabilities, and larval dispersal may be the contributing factors to the observed spatial homogeneity of D. russelli. However, significant genetic differentiation was observed between the populations from Indian Ocean and Indo-Malay Archipelago. Hierarchical molecular variance analysis (AMOVA), pairwise FST comparisons and SAMOVA showed existence of two distinct genetic stocks of D. russelli in the Indian Ocean and IMA. The observed interpopulation genetic variation was high. A plausible explanation for the genetic differentiation observed between the Indo-Malay Archipelago and the Indian Ocean regions suggest the influence of historic isolation, ocean surface currents and biotic and abiotic features of the ocean. Also, there was a significant relationship between genetic distance and geographical distance between population pairs in a manner consistent with isolation-by-distance. These resulted in the evolution of a phylogeographic break for this species between these regions. The findings of these results suggest that D. russelli from the Indian Ocean shall be managed in its entire area of distribution as a single stock. Further, the Indian Ocean and Indo-Malayan stocks can be managed separately.
Similar content being viewed by others
Introduction
Understanding the population structure of any fish is of paramount importance as conservation strategies focus solely on whether the population under consideration is a single or discrete evolutionary unit. Unraveling the underlying mechanisms that lead to intra- and interspecific diversity1, as well as historical and recent demographic processes that collectively shape the spatial pattern of genetic diversity is key to creating conservation planning2. The interplay between organisms and their environment in response to ecological and anthropogenic pressures3 viz., overexploitation4,5,6, habitat loss and fragmentation7,8,9, climate change10,11, biotic exchange12,13, etc. have counterintuitive impact on the current evolutionary process, making it difficult to decipher and quantify their potential roles. In recent years, much research has focused on the genetic patterning and environmental adaptation of marine fish, finding populations that are genetically diverse14, spatially structured15,16 and locally adapted17,18. For spatially structured populations characterized by high levels of site fidelity, it is unlikely that the effects of overexploitation or habitat fragmentation at one site would have deleterious effects on neighboring populations17. On the contrary, if the populations are migratory and are highly interbreeding, one could easily manifest the effects on one population in nearby areas19.
Many marine organisms are characterized by a large effective population size and high dispersal capacity, both as larvae and adults20. These traits could result in high gene flow between populations over time and space unless separated by geographic or hydrological barriers that reduce migration between areas. Interspecific differences in population biology are exhibited by several migratory species. Several studies spanning a wide range of taxa from the Indian Ocean have identified many species with high levels of genetic structure14,21,22,23,24. However, some other species interbreed and spread extensively, resulting in low population structure25,26,27,28,29. In Alosa pseudoharengus, variations in patterns of genetic structure between landlocked and anadromous populations have been observed30. Several species of migratory fish are reported to have experienced staggering population size declines worldwide31,32,33. Pelagic sharks (Alopias spp., Carcharodon carcharias and Sphyrna spp.) have declined by over 75% in the Northwest Atlantic since 198634. Sporadic reports of similar declines have also been recorded for other predatory marine fishes35,36,37. Failure to halt this decline will ultimately result in the loss of these species from their current habitats and has evolutionary consequences. In addition, small populations often experience high levels of genetic drift, fixation of harmful alleles and loss of genetic diversity, eventually leading to loss of genetic integrity and negatively impacting their adaptive potential38. Therefore, a better understanding of a species' population structure and movement patterns is required for effective conservation and equitable management of fishery resources.
The objective of the present study was to investigate the population structure of a highly mobile pelagic fish, Decapterus russelli. It is widespread in the Indo-Pacific from the Red Sea and East Africa to Japan and Australia39,40. The Indian scad, D. russelli represents an important fishery resource in India and Southeast Asia40. It represents an important pelagic resource of India, ranking next to the Indian oil sardine41, is an inexpensive source of animal protein42 and is widely used as a live fish bait39. Indian scad mackerel inhabits at depths of 40–275 m and are most commonly found on the coast and open shores of the Indian Ocean39. The species has a prolonged spawning season, with different studies reporting different spawning period overlapping along different coasts of India: February–November43, November–December44, March–December with peak in February and March45, December and September46, December–March and June–October with a peak in January and September47 on the southwest coast; December and August on the north-west coast46; April–August46 and December–July, sometimes extending to August48, November–May49 on the east coast of India, suggesting that this species is a continuous breeder preferring to spawn only when conditions are favorable, which can vary from place to place. Ovarian development is asynchronous and the eggs are pelagic and spherical in shape with size ranging from 0.02 to 0.97 mm50. The exact spawning grounds have not yet been located. The species plays an important role in the proper functioning of the ecosystem, as it avoids direct competition by increasing the number and diversity of prey items as they grow. Planktonic crustaceans (Acetes spp., copepods and other crustaceans) formed the main diet in smaller groups45 and were replaced by fish (Lactarius spp., Myctophid spp.) and molluscs49,51,52 in larger groups. As a primary carnivore, they inevitably help keep the ecosystem in balance as they are a voracious feeder of planktonic crustaceans45 and keep their population at optimal levels. They also play a crucial role in maintaining trophic ecology and the integrity of the food web. The large-scale consumption of high-energy planktonic crustaceans45,51 makes this highly vagile species a potential link in the food chain to temporally and spatially transfer energy to subsequent trophic levels.
D. russelli has been characterized primarily for its biology and population dynamics44,48,49, length–weight relationship53, diet and feeding habits51, reproductive biology54,55,56, and population status46,57. Extensive studies of its geographic structure have also been carried out from the Indo-Malay Archipelago (IMA). Previous studies have reported that two populations of scad mackerel enter the Java Sea seasonally, one during the east monsoon from the Flores Sea and Makassar Straits and another during the west monsoon from the Indian Ocean and Sunda Straits58. In addition, it also suggested the possibility that a third population could enter the Java Sea from the South China Sea during the west monsoon. The migration and distribution of Indian scad mackerel can be ascribed to the variation in salinity associated with the monsoon cycle in the IMA58. Two putative lineages have been distinguished from IMA in D. russelli based on the sequence polymorphism of the mitochondrial cytochrome b (Cyt b) gene. The two lineages reportedly showed spatial heterogeneity in their distribution. Later, Rohfritsch and Borsa40 discovered three mitochondrial lineages of D. russelli from IMA based on the combined result of mitochondrial and nuclear markers. One of the Perrin and Borsa59 haplogroups appeared to consist of two separate lineages in Rohfritsch and Borsa40. They could also observe the co-occurrence of some mitochondrial lineages, indicating recent secondary contact between once isolated populations or admixture in samples of cryptic species60.
Genetic information on this species from the Indian Ocean is scarce apart from a study of its complete mitochondrial genome42. Furthermore, with the exception of one study using truss morphometric analysis24, D. russelli from the Indian Ocean are less studied (particularly using molecular markers) than their counterparts in the Indo-Malay Archipelago. Sen et al.24 used 23 variables to distinguish between populations and identified separate stocks on the east and west coasts of India. The genetic basis for this observed phenotypic discreteness needs to be further elucidated in order to derive effective management recommendations for the species in different geographic areas in the Indian Ocean. Therefore, we examined the genetic population structure of Indian scad mackerel collected from five locations using mitochondrial and nuclear markers. In addition, to detect any genetic breaks between the populations of D. russelli, we performed a comparative study by expanding the geographic area to IMA. Since the species has high economic value and is widely harvested, the genetic data of such a species will provide adequate information to implement effective management strategies for the sustainable use of fishery resources.
Results
Genetic diversity analysis
Mitochondrial DNA (Cyt b) analysis
The Cyt b gene sequences of a total of 125 individuals sampled from five locations along the Indian coast were sequenced. After alignment and editing of ambiguous sequences, a final sequence length of 933 bp was obtained. The aligned dataset revealed an overall of 58/933 (6.21%) variable sites where 14/993 sites (1.50%) were parsimony-informative with 38 haplotypes. All haplotypes were deposited in GenBank under accession numbers OR188721 to OR188758. Furthermore, 34 (89.47%) haplotypes were singletons and 4 (10.52%) were shared between populations. The average base composition of the sequences was 23.2% A, 27.5% T, 15.4% G and 33.9% C with a higher A/T content compared to G/C. The transition to transversion ratio was 3:1. A detailed evaluation revealed 34 nonsynonymous mutations with 33 amino acid substitutions. The overall haplotype and nucleotide diversity was 0.6723 and 0.00167, respectively. The descriptive statistics of each population from the Indian Ocean are presented in Supplementary Table S1.
The previously reported IMA sequences were also included in the study40 bringing the total number of sequences to 583. We trimmed our sequences to match those retrieved from GenBank resulting in a final length of 307 bp. These data were used for further analysis. Altogether, the dataset revealed 30 variable sites, of which 14 were parsimony informative and 16 singleton variable sites with 27 haplotypes (Table 1) with an overall haplotype diversity (h) of 0.49684 and a nucleotide diversity (π) of 0.00593. Twenty out of the 27 haplotypes were found exclusively in the IMA, while the remaining 7 were discovered from the Indian Ocean. Out of a total of 27 unique haplotypes, only 8 (30%) were found in more than one region, with the rest being region-specific. In comparison, haplotype and nucleotide diversity in the IMA population (h = 0.25029, π = 0.00364) were higher than that in the Indian Ocean population (h = 0.10916, π = 0.00078). Furthermore, haplotype and nucleotide diversity in IMA ranged from 0.00 (Arafura) to 0.48 (Makassar) and from 0.00 (Arafura) to 0.00951 (Makassar), respectively (Table 2). The haplotype and nucleotide diversity in the Indian Ocean population ranged from 0.00 (Cochin) to 0.15667 (Mangalore and Veraval) and from 0.00 (Cochin) to 0.00261 (Mangalore), respectively. The IMA populations consisted mostly of singletons (13/20–65%) (Table 1). The average base composition of the entire sequences was 21.8% A, 31.2% T, 15.6% G, and 31.3% C. The ratio of transition to transversion was 4:1. The entire dataset revealed seven non-synonymous mutations that resulted in seven amino acid changes. It has been observed that the haplotypes shared by the IMA population are not detected in the Indian Ocean population and vice versa.
The genetic diversity or distance values were converted to percentages and most of the genetic diversity within the population was 0%, except for Toli-Toli and Makassar which have a genetic distance of 1% (Supplementary Table S2). When populations with little genetic distance were excluded, genetic diversity between populations ranged from 1 to 2%. Makassar and Toli-Toli from the IMA differed the most of all, with at least 1% genetic distance from the remaining populations. The genetic distance of 2% was mainly observed between Indian Ocean and IMA populations (Mangalore and Toli-Toli; Mangalore and Makassar; Puri and Toli-Toli). Furthermore, within the IMA, a 2% divergence was observed between the populations of Toli-Toli and Tambalen (western region of the IMA). In addition, all other Indian Ocean populations are genetically separated from the IMA by 1%. However, subtle genetic differences were observed between the Indian Ocean populations and also between the populations from the western region of the IMA. The majority of FST values observed in the IMA were significantly different (p < 0.05) (Supplementary Table S2). In addition, significant differences in FST values (p < 0.05) were also observed between all IMA and Indian Ocean populations. However, non-significant values (p > 0.05) were found between populations from the Indian Ocean.
Nuclear DNA (DrAldoB1) analysis
A total of 125 individuals (250 alleles) of D. russelli from the Indian Ocean were assayed for 212 bp of intron 1 of the Aldolase b (DrAldoB1) gene. 36 unique alleles were identified of which 33 alleles were exclusively from the Indian Ocean (Table 3) and the remaining three were from IMA (IMA sequences were retrieved from NCBI-GenBank). One allele was common to all five geographical locations in the Indian Ocean (Table 3). The sequences were deposited in GenBank under accession numbers OR546246 to OR546278. Overall, the haplotype diversity was 0.3786 and nucleotide diversity was 0.01. For each population, the haplotype diversity and nucleotide diversity ranged from 0.153 (Veraval) to 0.628 (Chennai) and 0.001(Veraval) to 0.022 (Chennai) respectively (Supplementary Table S3). We observed 72 polymorphic sites out of 212 bp sequence of DrAldoB1 of which 11 sites were the sites with more than two variants. The observed intrapopulation genetic diversity varied from 0.1% (Veraval) to 1% (Puri and Mangalore). The inter population genetic distance was negligible between the Indian Ocean populations (Supplementary Table S4). Whereas, all of the Indian Ocean population displayed a higher genetic distance between IMA, ranging from 1.3% (Veraval) to 1.9% (Puri) (Supplementary Table S4. The observed global FST value was low (0.052). Pairwise FST analysis revealed a lack of genetic structure in the areas studied from the Indian Ocean and was not significant (p > 0.05) (Supplementary Table S4). However, significant FST values (p < 0.05) were obtained between IMA and Indian Ocean populations.
Population structure
Within Indian Ocean analysis
AMOVA analysis was performed on the 5 populations from the Indian Ocean subdivided into 1-4 gene pools. In all analyses, the overall levels of genetic differentiation between coasts were non-significant (p > 0.05) even for the highest variance (FCT = 0.01090, p > 0.05) with a global FST of 0.01030. The genetic differentiation among population within groups/coast was − 0.06% (FSC = − 0.00061, p > 0.05). 98.97% percentage of the total variation of D. russelli from the Indian Ocean is contributed by the genetic differences among the total populations. Furthermore, AMOVA analysis undertaken with nuclear markers also demonstrated the lack of significant genetic structure (p > 0.05) in D. russelli (Supplementary Table S5). This result indicated no subdivision or structuring within the tested populations. Additional genetic differentiation analysis revealed that estimates based on HST (0.006), NST (0.002) and KST* (0.001) were all very low and none of them were significant (p > 0.05). This also indicates a high level of gene flow among the sampled D. russelli populations from Indian Ocean (Nm = 207.34 for haplotype-based statistics and Nm = 83.97 for sequenced based statistics). On the other hand, the IMA population, divided into two geographical groups, revealed significant genetic structuring at all levels (Table 4). The mantel test showed no significant correlation between genetic distance (pairwise FST values) and geographical distance (r = 0.063, p > 0.05) among the tested population (Supplementary Fig. S1). Overall, 4 and 3 populations out of 5 were significant with negative values of Tajimas’ D and Fu’s Fs (Supplementary Table S1) suggesting historical population expansion at these sites associated with an excess of recent mutations or rare alleles61. The Harpending raggedness index (Hri = 0.46, SSD = 0.03, P = 0.02) corroborated these results. Mismatch analysis revealed a unimodal distribution providing strong evidence for a sudden population expansion (Supplementary Fig. S2). The minimum spanning network (MSN) produced a reticulation of 38 haplotypes (Supplementary Fig. S3). One haplotype was common to all geographical locations. The common haplotypes within the network are inferred to be ancestral, whereas the tip haplotypes may be derived or descended from ancestral (internal) haplotypes62. Thus, the occurrence of star like pattern radiating from these major haplotypes suggests that the D. russelli population has undergone a recent demographic expansion. Phylogenetic analysis based on mitochondrial and nuclear markers revealed no obvious phylogeographic pattern separating the 5 samples of D. russelli (Supplementary Fig. S4; Supplementary Fig. S5).
Between Indian Ocean and IMA analysis
The AMOVA analysis was also performed on the 15 populations (10 from the IMA and 5 from the Indian Ocean) that were subdivided into two geographical groups (Group 1: Indian Ocean; Group 2: IMA) to determine whether there is any genetic structuring over the geographical area. AMOVA analysis revealed that genetic differentiation was significant at all levels (Table 4), with 72.09% of the total variation attributed to variation among regions/groups, implying significant differentiation across geographical areas. The analysis was also repeated with the IMA populations divided into three geographical locations. The test is to discover whether the genetic differentiation was visible at the finer spatial scales across the IMA. Thus, the sample was divided into 4 groups (Group 1: Indian Ocean; Group 2: Makassar strait/Sulawesi Sea; Group 3: Arafura Sea; and Group 4: western region of the Indo-Malay Archipelago). The analysis generated a non-significant albeit low FSC value, whereas significant differentiation among groups (FCT = 0.8267, p < 0.05) was observed accounting for the highest percentage (82.77) of the total variation. The analysis was repeated splitting the Indian Ocean population into two groups based on coast, bringing the total group to five. The groups were, Group 1: Western Indian Ocean; Group 2: Eastern Indian Ocean; Group 3: Makassar strait/Sulawesi Sea; Group 4: Arafura Sea; Group 5: western region of the IMA. It produced similar outcomes as the previous group of four. In addition, genetic differentiation estimates were analyzed by dividing the whole population into two: Indian Ocean and IMA. All values generated (HST = 0.60, NST = 0.79 and KST* = 0.54) were high and significant. DrAldoB1 also revealed significant genetic structuring (p < 0.05) between IMA and Indian Ocean populations (Supplementary Table S5).
The result from the SAMOVA was consistent with other analyses, with k = 2 showing the highest FCT value (FCT = 0.8362, p < 0.05), clearly demonstrating the existence of two genetically distinct D. russelli stocks among the populations under study, namely the IMA and Indian Ocean populations. The mantel test showed a significant correlation between genetic differentiation (pairwise FST) and geographical distance (r = 0.11, p < 0.05) among the tested population.
Phylogenetic relationship
The Bayesian phylogenetic tree revealed three mitochondrial lineages (Fig. 1). The two lineages were haplogroups M and A formerly identified by Perrin and Borsa59. Two distinct mitochondrial lineages with heterogeneous geographic distribution were found for Toli-Toli, Pekalogan, and Labuan. The third lineage consisted of populations from the Indian Ocean. In addition, the Perrin and Borsa’s59 M haplogroups formed a sister lineage with the populations from the Indian Ocean. The two major lineages were observed to be separated by an average nucleotide divergence of 2%. Haplogroup M and the Indian Ocean population were separated from haplogroup A by an average nucleotide difference of 2.2% and 1.8% respectively, when the three lineages were considered. In addition, the haplogroup M and the Indian Ocean population are separated by a genetic divergence of 2.2.%.
The Bayesian phylogenetic tree produced from DrAldoB1 formed 2 lineages representing an Indian Ocean lineage and an IMA lineage (Fig. 2). These two lineages were observed to be separated by an average nucleotide divergence of 1.6%.
Haplotype network diagram
The haplotype network diagram (Fig. 3) derived from partial sequences of the mitochondrial Cyt b gene, delineated three major clusters similar to the phylogenetic tree: haplogroup A, haplogroup M and Indian Ocean populations. The rarer haplotypes from IMA, h1, h2 and i comprising the M haplogroup formed a distinct cluster separated from haplotype M, the most frequent haplotype in the haplogroup M by 4 genetic mutations, supporting the finding of Perrin and Borsa59. The haplogroup M has been separated from haplogroup A by 6 mutational steps. The cluster formed by the Indian Ocean population was separated from haplogroups A and M by 8 and 4 mutational steps, respectively.
Demographic history
Overall, the 11 and 10 populations out of the total populations tested were significant with negative Tajimas D and Fu’s Fs (Table 2) values respectively, indicating a recent historical directional selection (selective sweep) or population expansion63 at these sites. In addition, the Harpending Raggedness index, Hri = 0.5, SSD = 0.04 (p = 0.2), corroborated this finding with the recent population growth hypothesis64. Furthermore, based on the mismatch distribution of the combined population, past population demography of D. russelli revealed three highly divergent peaks, representing the three lineages (Supplementary Fig. S2). A bimodal mismatch distribution was found in the IMA lineage 2 after additional independent analysis of the three lineages. However, further comprehensive studies from the IMA are needed to confirm this preliminary finding. The IMA lineage 1 and the Indian Ocean (IO) lineage on the other hand, has a unimodal distribution indicating a rapid expansion of its population.
The time of demographic and spatial expansion for the major lineages 1 (IMA lineage 1) and 2 (IMA lineage 2 + IO lineage) using the formula τ = 2 μt are estimated to occur 8,58,934–4,46,254 years ago and 4,57,680–4,46,254 years ago respectively. Therefore, the spatial expansion coincided with the demographic expansion. Furthermore, the spatial and demographic expansion of IO lineage occurred between 6,11,285–3,17,589 years ago. In addition, the demographic expansion of lineage 2 (IMA lineage 2) occurred between 7,93,1596–3,17,589 years ago, whereas the spatial expansion of its population was between 3,66,771–1,90,553 years ago. Therefore, this lineage experienced demographic expansion following spatial expansion.
Discussion
Genetic data based on mitochondrial and nuclear markers revealed little divergence among the five populations analyzed, suggesting the existence of panmictic populations of Indian Scad, D. russelli from Indian waters. The lack of significant genetic differentiation between populations as indicated by AMOVA analysis (FST > 0.05) suggests that D. russelli populations can be managed as a single stock in Indian waters. These findings contradicted the previous study which suggested the presence of two distinct stocks of D. russelli along the east and west coasts of India24. The previous study was conducted based on truss morphometry over a small geographical area, with opportunistically collected individuals over a short period of time probably resulting in a potential artifact of population subdivision. Morphometric and meristic characters are particularly vulnerable to environmental influences and are considerably impacted once the species is exposed to environmental stressors. These environmental stressors act as selection pressures affecting the structure and function of organisms, reducing their Neo-Darwinian fitness65. Environmental conditions such as currents or water masses and various stressors, including biotic and abiotic factors such as food availability, salinity and temperature are subject to temporal and spatial variability and affect the morphometry of the species at each site at any given time. Although it is widely acknowledged that the environment plays a role in shaping the phenotype, there are additional important factors influencing phenotypic variation that are being investigated at the molecular level66. Thus, the variations reported by Sen et al.24 in the specimens from the Arabian Sea and Bay of Bengal can be proposed as a consequence of phenotypic plasticity in response to temporal variations in environmental and oceanographic parameters.
In this study, the high haplotype diversity, Hd (values ranging from 0.43 to 0.90) coupled with low nucleotide diversity, π (ranging from 0.0007 to 0.002) observed in all populations was concordant with previous reports of several pelagic marine species from the Indian Ocean. Previous studies from the Indian Ocean include Euthynnus affinis (Hd = 0.9, π = 0.01)67, Xiphias gladius (Hd = 0.88 π = 0.002)68, Rastrelliger kanagurta (Hd = 0.95 π = 0.008)69 and Thunnus obesus (Hd = 0.99 π = 0.04)27. High haplotypic diversity within populations can be maintained for a variety of reasons, including large population sizes, environmental variation, and life-history factors that favor rapid population growth70,71. The high-level haplotype diversity observed in this study may be due to the large effective population size and wide distribution as suggested for other pelagic fishes72,73. D. russelli is also the most abundant carangid in Indian waters41.
It has been proposed that population history of marine species can be classified into four categories based on different values of haplotype diversity (Hd) and nucleotide diversity (π) of mt DNA gene sequences74. High Hd values and low π values observed in the present study indicate a recent population expansion from a small effective population size, i.e. a rapid population increase is seen in D. russelli population after a bottleneck event with mutation accumulation (second category; Grant and Bowen74). The unimodal mismatch analysis, the significant negative Fu's Fs and Tajima’s D values, non-significant deviation from the sum of squared deviations (SSD) and the Harpending’s raggedness index (Hri) were also consistent in supporting the occurrence of population expansion of D. russelli in Indian waters.
Marine fishes are reported to have strong intraspecific gene flow than freshwater fishes and hence reduced genetic structure75. This is probably because the marine environment imposes fewer barriers to species dispersal. In particular, there are reports of low levels of genetic differentiation in species with a pelagic life history both within27,67,68,69 and between the oceans25,68,76, largely due to the existence of a continuous circumglobal pelagic environment and the presence of a wide range of suitable spawning grounds. One of the possible explanations for the observed lack of population subdivision in D. russelli samples includes seasonal fluctuations in water circulation caused by the monsoon currents in the Indian Ocean. During the northeast monsoon, the flow of the upper ocean is directed westward towards the Arabian sea. The flow direction changes during the southwest monsoon, flowing eastward from Somalia towards the Bay of Bengal77. This seasonal reversal of monsoon currents may homogenize D. russelli populations in Indian waters. In addition, the Indian Ocean is divided into two hemispheres by the equator, resulting in the well-known marine bioecological impacts determined by latitudes68. Furthermore, this ocean is characterized by the westward South Equatorial Current (SEC)78 and hydrochemical south Tropical Front which separate the two large oligotrophic areas—Indian Monsoon Gyre Province (MONS) in the north and the Indian South Subtropical Gyre Province (ISSG)79. However, these physical and ecological divisions have little impact on the genetic structure of D. russelli in contrast to the Indo Malay Archipelago counterparts. In addition, the life history traits of D. russelli like long distance migration58, fast growth rate53 and high fecundity55 contribute to a high level of gene flow, which in turn led to the low level of genetic subdivision in this species. Furthermore, the phylogenetic groups that were evident from the Bayesian analysis of both Cyt b and DrAldoB1 genes data of D. russelli revealed the absence of geographical clustering of lineages among the Indian Ocean populations. Moreover, the haplotypes from all samples were interspersed throughout the tree.
Analysis of mitochondrial Cyt b and DrAldoB1 sequences indicated strong isolation between the Indian Ocean and IMA with significant level of genetic differentiation. The Indian Ocean is impacted by climatic variability on a larger scale77. It displays a variety of climate variability modalities, most of which are associated with the robust seasonal cycle ranging from intra-seasonal to inter-annual and even longer time scales. It differs from the other oceans in a number of climatically important ways78—first, the Asian landmass blocks the northern entrance to the Indian Ocean, preventing heat export to the north. Second, the strongest monsoon on earth originates from the Asian continent. These monsoonal winds cause significant seasonal variations in ocean currents including annual reversals. In addition, unlike other oceans, the Indian Ocean does not experience consistent equatorial easterlies, which prevents it from experiencing climatological equatorial upwelling. Finally, the Indian Ocean has the Indonesian Throughflow (ITF), a low-latitude exchange route with the Pacific. The central Indo-West Pacific region is geographically and hydrologically complex and possesses the world’s largest marine diversity hotspot80. The region experienced major changes to its shorelines during the Pleistocene81,82. The shallow seas of the Sunda shelf and Sahul shelf (Arafura Sea) were above the current sea level during the Pleistocene. Land barriers are also being built to separate the South China Sea from the Indian Ocean at the southernmost point and from the Sulu Sea to the east. The repeated fluctuation in sea level associated with the ice ages may be the reason for the geographic isolation of several inland seas, leading to the genetic differentiation of the species they harbored. This is believed to be the reason for the geographic discontinuity observed in many marine species between the Indian and Pacific Oceans and also for the greater species diversity observed in the Indo-Malay region81. These environmental factors together with the emergence of land barriers to movement between ocean basins appear to be the strongest factor for the observed genetic heterogeneity of D. russelli populations between the Indian Ocean and the IMA. This may also be responsible for shaping the modern genetic structure of many species that inhabit this region as we see it today81,82,83,84,85.
Three phylogenetic groups were evident in the Cyt b data for D. russelli. The tree showed evidence for the existence of distinct geographical lineages present among IMA and between IMA and Indian Ocean populations. All IMA sites grouped in Clades 1 and 2 correspond to the haplotypes A and M recognized by Perrin and Borsa59 and Rohfritsch and Borsa40 with an obvious pattern of geographic structuring. In clade 2, one lineage is consists entirely of Makassar and Toli-Toli individuals (haplotype M) and the next lineage encompasses haplotypes h1, h2 and i as in Perrin and Borsa59 and Rohfritsch and Borsa40. Clade 3 consisted entirely of Indian Ocean individuals. The previous reports of the co-occurrence of separate mitochondrial lineages in some samples and their heterogeneous geographical distribution (western populations and easternmost populations)40,59 were confirmed in this study. Due to the high connectivity of the seas in the Indo-Malay Archipelago, one might anticipate a high level of gene flow between populations. However, seasonal variations in the surface currents in the western part of the archipelago in response to the typical monsoon cycle may cause changes in water circulation and salinity, which can affect the migration patterns of adult pelagic species and the dispersal of their larvae58. However, the divergence of these clades may have occurred in the past during the Pleistocene lowering of sea level.
The average rate of nucleotide changes in the Cyt b gene observed in this study (1.8–2.2%) between various lineages lies in the range described for marine fishes (between the extremes of sharks and mammals (2.5% and 1.0% per million years respectively)). This indicates that the split between the lineages dates back to the mid-Pleistocene. In addition, the co-occurrence of the Haplogroup M of Rohfritsch and Borsa40 and the Indian Ocean population to form a major lineage and their heterogeneous geographical distribution suggests that differences may be due to vicariance. The average genetic divergence between the haplogroup M and the Indian Ocean population corroborates the above findings (while considering the whole population (2.2%) vs only these two (haplogroup M and IO population) (1.8%)).
Past geological and climatic events have undoubtedly played a major role in this inferred population expansion. The paleogeography of IMA changed dramatically during the Quaternary period10. During the last interglacial period, there were marine connections between the Indian Ocean and the south China Sea via the Strait of Singapore85. These connections may have allowed D. russelli, an essentially pelagic species to freely migrate and expand its population across this region at that time. Estimation of the time since expansion of various lineages of D. russelli suggests the timing of these events is consistent with the cyclic sea level rises that occurred during the late Pleistocene between 1,600,000 to 10,000 years ago86. The land bridges, which were later exposed as sea level fell, impeded the spread of D. russelli. Furthermore, the last glacial maximum (LGM), 30,000 to 19,000 years ago led to the final decline in sea level up to the present86,87. A substantial portion of Sunda and Sahul shelves was exposed as the sea level declined with glaciation up to 200 m below their present level82. This process would have also impeded the dispersal of many marine taxa. By this time the adjacent South China Sea considerably shrank in size and transitioned into a semi closed marginal sea exposing a massive low gradient on the Sundaland carton86. In addition, around this time the modern Malayan Penisula, Borneo and Sumatra formed highlands to the south88 adding yet another potential barrier to population migration. The co-occurrence of an IMA lineage and an IO lineage (clade 2 and clade 3) as well as their distinct geographic distributions, as seen in this study, can be attributed to the remnants of these earlier marine links and the emergence of geographic barriers brought about by ice ages associated with the Pleistocene events respectively. Such isolated marine populations experienced extensive inbreeding leading to the accumulation of genetic variation and the structuring of their population at the genetic level. Similar scenarios have been reported for the genetic heterogeneity of many marine species between oceans68,69. MSN analysis also supported this structuring of D. russelli across the IMA and between the Indian Ocean and the IMA.
Marine species with high dispersal potential usually display high level of gene flow resulting in low level of genetic structuring89. This would limit or reduce adaptive divergence among local populations90. The low and non-significant FST values observed here among the five D. russelli populations from Indian waters are consistent with the pattern of Cyt b homogeneity and high gene flow (Nm = 208.34). Various evolutionary processes including migration, mutation and drift may play a role in determining genetic differentiation between populations. Thus, a highly migratory species with a large population has a tendency to show low level of population differentiation in contrast to species with small populations and reduced migration rates91. Several other investigations have also reported relative panmixia in marine species across Indian waters. For example, an apparent lack of genetic structure was observed in Bigeye tuna (Thunnus obesus)27 and Euthynnus affinis67 based on the analysis of mitochondrial control region. Furthermore, mitochondrial (ND2) and nuclear DNA (microsatellites) analyzes of Xiphias gladius have shown its homogenous distribution throughout the Indian Ocean68.
The present study also identified a significant population differentiation between the IMA and the Indian Ocean (Nm = 0.13). Although it is true that gene flow between populations can often prevent subpopulation isolation92,93, this relationship is not as simple as it first appears. The hierarchical AMOVA results revealed significant FST and FCT values indicative of the genetic structure among populations of D. russelli. This was confirmed by spatial genetic heterogeneity (k = 2 in SAMOVA) between groups comprising the populations in the IMA vs Indian Ocean. In addition, the geographical distance also contributed to the divergence among populations. Overall genetic distance increased with geographical distance consistent with isolation by distance. Thus, a more plausible explanation for the observed population differentiation of two distinct groups of D. russelli is the combined effects of several factors including historical physical isolation during the Pleistocene, larval dispersal factors, biotic and abiotic features of the ocean basins, ocean currents and geographical distance.
The movement of marine fishes, especially as larvae and adults, can greatly be influenced by Ocean surface currents. In the Indian Ocean, it is largely influenced by monsoon currents, also known as monsoon drifts, which flow between the Bay of Bengal and the Arabian Sea94. In contrast, the Kuroshio current, the largest surface current in the South China Sea affects the waters surrounding the IMA95. These two sea surface currents can contribute to, and also act as, powerful factors in genetic differentiation among D. russelli populations between the IMA and the Indian Ocean.
The significant genetic differentiation detected between Indian Ocean and IMA needs to be corroborated by advanced genomic markers like SNPs detected through genome scans. Genome scans also will provide insights regarding loci under selection in response to heterogeneous environmental conditions of the Indian ocean and IMA96,97. In addition, tagging programs and microchemical analyzes of specific structures (eg: otolith) may also be helpful to devise management measures for D. russelli in the Indian Ocean. Finally, a comprehensive understanding of reproductive strategies, population dynamics in various regions, and spawning areas within the Indian Ocean is required to fully understand the nature and behavior of its population98.
Conclusion
The results of this study demonstrated that the Indian Ocean D. russelli constituted a single panmictic population and can be managed as a single stock. This study revealed two discrete populations of D. russelli, Indian Ocean populations and IMA populations. The inferred demographic history indicates that D. russelli populations may have experienced a potential population expansion during the Pleistocene. The subsequent appearance of a barrier to gene flow may have limited their ability to migrate between the Indian Ocean and the IMA as seen in the extant populations.
Materials and methods
Sample collection
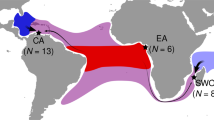
A total of 125 D. russelli individuals were collected from the east (Odisha (Puri) and Tamil Nadu (Chennai)) and west (Gujarat (Veraval), Mangalore (Karnataka) and Kerala (Cochin)) coasts of India between 2019–2020 (Fig. 4) A piece of caudal fin basal tissue was collected from each individual and preserved in 95% ethanol until DNA isolation. The fish sample used in this study was treated in accordance with the recommendations made by De Tolla et al.99 for the handling and use of fish in research. The protocols were approved by the ethical committee of the ICAR—Central Marine Research Institute, Kochi. These methods are also reported following ARRIVE guidelines (http://arriveguidelines.org).
Map of sampling sites, site used for comparative analysis and targeted species. (a) Samples were collected from respective landing sites (red pin) from 5 localities of the Indian Ocean. (b) A comparative analysis of D. russelli populations in the Indian Ocean and the Indo-Malay Archipelago. (c) Specimen of Indian Scad, D. russelli. The map was obtained from Google Earth and the sampling sites were marked using the placemark tool in Google Earth available at http://earth.google.com. The images were then combined and edited manually in Adobe Photoshop CS6.0.
DNA isolation and PCR amplification
Genomic DNA was isolated from tissue using the standard phenol–chloroform protocol100. The isolated DNA was then eluted in T.E. buffer (1 ×) and the DNA samples were stored at 4 °C prior to amplification by PCR. The quality and quantity (concentration) of the extracted DNA was checked using gel electrophoresis or NanoDrop One Spectrophotometer (Thermoscientific, USA). To facilitate comparative analysis, a new primer set was designed that targets a 933 bp fragment of mitochondrial Cyt b gene sequences spanning the regions analyzed by Rohfritsch and Borsa40. The primer sequences were as follows: 5′-AATCTCCGTAAAACCCACCC-3′ (forward) and 5′-AAAAACTAGGAATAGGAGGAAGT-3′ (reverse). The primers were designed based on the aligned full-length Cyt b gene sequences of carangids using Geneious R7 (Biomatters Ltd., New Zealand). The PCR reaction mixture consisted of 15.6 μl of sterile deionized water, 2 μl of 10 × buffer, 10 mM of each dNTP, 10 μM of each oligonucleotide, 1 unit of Taq DNA polymerase, and ~ 50 ng of genomic DNA as template. Amplification conditions were: initial denaturation at 94 °C (4 min); 32 cycles of 94 °C (30 s), 55.4 °C (30 s), 72 °C (1 min 10 s) and a final extension at 72 °C (10 min) before the reaction termination at 4 °C. Amplification of this 20 μl reaction volume was performed in a Biorad T100 thermal cycler (Bio-Rad, USA). Intron 1 of the aldolase b (DrAldoB1) gene was targeted with the following primers; 5′-GCTCCAGGAAAGGGAATCCTGGC-3′ (forward) and 5′-CTCGTGGAAGAAGATGATCCCGCC-3′ (reverse)101. PCR amplification was performed in a Biorad T100 thermal cycler (Bio-Rad, USA) with a final volume of 25 μl, consisting of 12.5 μl of 2 × EmeraldAmp® GT PCR Master Mix (TaKaRa, Shiga, Japan), 0.3 μl of each primer (10 mM each), 2.0 μl of genomic DNA (50 ng/ml) followed by nuclease-free water to achieve final reaction volume. Thermal cycling conditions were as follows: initial denaturation at 94 °C for 4 min, followed by 35 cycles of 30 s denaturation at 94 °C, 12 s annealing at 52 °C, 20 s extension at 72 °C followed by final extension of 3 min at 72 °C. DNA amplification products were then separated in 1.5% agarose gels at 90 V in 1× Tris–borate–EDTA (TBE) buffer, stained with ethidium bromide and visualized using a gel documentation system (Bio-Rad, USA). All PCR products were then sequenced using Sanger sequencing in both the forward and reverse directions. The nucleotide sequences were checked visually to ensure that the sequence information was consistent in both directions. The sequences were also verified by comparison with known sequences from GenBank.
Data analysis
Both forward and reverse raw sequences were edited and assembled after removing the ambiguous bases in MEGA X102. The edited sequences of each locus were aligned with Clustal W implemented in the same software. The coding sequences of Cyt b were translated into amino acids, checked for stop codons and then back translated into the corresponding nucleotide sequences. A total of 933 bp sequences of Cyt b and 212 bp sequences of DrAldoB1 were obtained for the subsequent analysis. Further, Cyt b (accession numbers: AF307494–AF307510 and AF515757–AF515759) and DrAldoB1 (accession numbers: AF500103–AF500105) gene sequences deposited from IMA were also retrieved for comparative analysis. Prior to data analysis, the PHASE103 program implemented in DnaSP6104 was used to resolve heterogeneous sites in DrAldoB1 sequences and to reconstruct haplotypes.
Genetic diversity analysis
Genetic distance within and between populations was estimated using K2P model, selected based on the lowest Bayesian information criterion (BIC) score using MEGA X102. These values were also used to assess the possibility of occurrence of subspecies or cryptic species (if intra-specific variation exceeds the threshold of 2% in marine species)105,106,107,108. The complete aligned dataset was analyzed for nucleotide variable sites, parsimony informative sites, haplotype number, haplotype frequencies and nucleotide frequencies in DnaSP6104. The ratio of transition to transversion was analyzed using MEGAX102.
Population genetic structure analysis
Population pairwise FST values were calculated using ARLEQUIN v3.5109. Hierarchical analysis of molecular variance (AMOVA) was executed to estimate population subdivision and structuring at different hierarchical levels using ARLEQUIN v3.5109. The AMOVA analysis was performed at three levels: among the Indian Ocean population (1), among the IMA population (2) and between the Indian Ocean and the IMA populations (3). For the Indian Ocean population, a hierarchical AMOVA analysis was performed by partitioning the variance among and within populations. (i) one gene pool comparison considering the whole population as a single gene pool, (ii) two gene pools [West coast (Cochin, Mangalore and Veraval); East coast (Chennai and Puri)] (iii) three gene pools [North West (Veraval); South West (Mangalore and Cochin); East (Chennai and Puri), and (iv) four gene pools [North West (Veraval); South West (Mangalore and Cochin); South East (Chennai) and North East (Puri)]. Further the variation was assessed among groups, within groups and within populations. The IMA population was divided into two geographically distinct gene pools based on the previous findings on their distribution40: Makassar Strait and Sulawesi Sea populations vs the populations from the entire western region of IMA. For the comparative analysis, the whole data set was divided into two groups: one comprising the populations from the Indian Ocean and the other comprising the entire population from the IMA. The variance among populations and the relative contribution of variance were estimated at three different levels; FST (variation among individuals within populations), FSC (variation among populations within region) and FCT (variation among regions)110. In addition, the spatial structure was examined using spatial analysis of molecular variance (SAMOVA) v2.0111,112 to identify groups of populations that were geographically homogenous and maximally differentiated from each other. Gene flow (Nm) between populations based on both haplotype and sequence statistics was estimated according to Nei113 and Hudson et al.114 using DnaSP6104. Genetic differentiation estimates based on HST, NST and KST* were determined in DnaSP6104. The isolation by distance or Mantel test has been conducted to confirm the relationship between genetic distance and geographical distance115.
Phylogenetic analysis
The phylogenetic relationship among haplotypes was examined by constructing a phylogenetic tree based on Bayesian inference (BI) in MrBayes with 1,000,000 Markov Chain Monte Carlo (MCMC) generations. Posterior distributions were sampled for every 10,000 generations. The sequence of D. macarellus (NCBI, GenBank Accession No. NC_026718) was employed as an outgroup for phylogenetic tree construction based on Cyt b sequences and the sequences of D. macarellus (Accession No. AF500103) and D. macrosoma (Accession No. AF500104, AF500105) were used as outgroups for phylogenetic tree construction based on DrAldoB1 sequences. A network diagram of all haplotypes was constructed based on median joining calculations as implemented in PopARTv1.7116,117.
Demographic history
The historical demographic expansion of each population was tested using Tajima’s D63 and Fu’s F61 statistics in ARELIQUIN v3.5109. In addition, Harpending’s raggedness index, Hri64 implemented in ARLEQUIN v3.5109 and the mismatch distribution118,119 implemented in DnaSp6104 were applied to find out whether the sampled populations were demographically stable or expanding or decreasing over time. Both tests were applied in order to detect whether the populations deviate from what would be expected from a sudden population expansion model. A significant Hri value (p < 0.05) rejects a model of sudden population expansion112.
The precise time for expansion was calculated using the equation τ = 2ut118; where t is the expansion time. 2u was calculated using the equation 2u = μk120, where μ is the mutation rate which is 1–2%/site/year for the mitochondrial Cyt b gene in fishes (Carangidae)121,122,123 and k is the number of nucleotides covered in the data (307 bp).
Data availability
All data generated or analyzed during this study is included in this published article (and its supplementary information files). In addition, the sequence data generated in this study is available in the NCBI-GenBank under the accession numbers: OR188721 to OR188758 and OR546246 to OR546278 (https://www.ncbi.nlm.nih.gov/genbank/).
References
Correia, A. M. & Lopes, L. F. Revisiting biodiversity and ecosystem functioning through the lens of complex adaptive systems. Diversity 15, 895 (2023).
Lawton-Rauh, A. Demographic processes shaping genetic variation. Curr. Opin. Plant Biol. 11, 103–109 (2008).
Santamaría, L. & Méndez, P. F. Evolution in biodiversity policy—Current gaps and future needs. Evol. Appl. 5, 202–218 (2012).
Dunlop, E. S., Baskett, M. L., Heino, M. & Dieckmann, U. Propensity of marine reserves to reduce the evolutionary effects of fishing in a migratory species. Evol. Appl. 2, 371–393 (2009).
Hutchings, J. A. Life history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 62, 824–832 (2005).
Taboun, Z. S., Walter, R. P., Ovenden, J. R. & Heath, D. D. Spatial and temporal genetic variation in an exploited reef fish: The effects of exploitation on cohort genetic structure. Evol. Appl. 14, 1286–1300 (2021).
Esguícero, A. L. H. & Arcifa, M. S. Fragmentation of a Neotropical migratory fish population by a century-old dam. Hydrobiologia 638, 41–53 (2010).
Junge, C., Museth, J., Hindar, K., Kraabøl, M. & Vøllestad, L. A. Assessing the consequences of habitat fragmentation for two migratory salmonid fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 297–311 (2014).
Sterling, K. A., Reed, D. H., Noonan, B. P. & Warren, M. L. Genetic effects of habitat fragmentation and population isolation on Etheostoma raneyi (Percidae). Conserv. Genet. 13, 859–872 (2012).
Nelson, J. S., Hoddell, R. J., Chou, L. M., Chan, W. K. & Phang, V. P. E. Phylogeographic structure of false clownfish, Amphiprion ocellaris, explained by sea level changes on the Sunda shelf. Mar. Biol. 137, 727–736 (2000).
Stewart, J. R. The evolutionary consequence of the individualistic response to climate change. J. Evol. Biol. 22, 2363–2375 (2009).
Bagley, J. C. & Johnson, J. B. Phylogeography and biogeography of the lower Central American Neotropics: Diversification between two continents and between two seas. Biol. Rev. 89, 767–790 (2014).
Unmack, P. J., Barriga, J. P., Battini, M. A., Habit, E. M. & Johnson, J. B. Phylogeography of the catfish Hatcheria macraei reveals a negligible role of drainage divides in structuring populations. Mol. Ecol. 21, 942–959 (2012).
Sebastian, W., Sukumaran, S., Zacharia, P. U. & Gopalakrishnan, A. Genetic population structure of Indian oil sardine, Sardinella longiceps assessed using microsatellite markers. Conserv. Genet. 18, 951–964 (2017).
Coleman, R. A. et al. Artificial barriers prevent genetic recovery of small isolated populations of a low-mobility freshwater fish. Heredity 120, 515–532 (2018).
Planes, S. & Fauvelot, C. Isolation by distance and vicariance drive genetic structure of a coral reef fish in the Pacific Ocean. Evolution 56, 378–399 (2002).
Fraser, D. J., Weir, L. K., Bernatchez, L., Hansen, M. M. & Taylor, E. B. Extent and scale of local adaptation in salmonid fishes: Review and meta-analysis. Heredity 106, 404–420 (2011).
Sebastian, W. et al. Signals of selection in the mitogenome provide insights into adaptation mechanisms in heterogeneous habitats in a widely distributed pelagic fish. Sci. Rep. 10, 9081 (2020).
Kell, L. T. et al. Lumpers or splitters? Evaluating recovery and management plans for metapopulations of herring. ICES J. Mar. Sci. 66, 1776–1783 (2009).
Hellberg, M. E. Gene flow and isolation among populations of marine animals. Annu. Rev. Ecol. Evol. Syst. 40, 291–310 (2009).
Divya, P. R. et al. Mitochondrial ATPase 6/8 genes to infer the population genetic structure of silver pomfret fish Pampus argenteus along the Indian waters. Mitochondrial DNA 26, 189–194 (2015).
Habib, K. A. et al. Population structure, phylogeography and demographic history of Tenualosa ilisha populations in the Indian Ocean region inferred from mitochondrial DNA sequence variation. Reg. Stud. Mar. Sci. 54, 102478 (2022).
Lu, C. P., Chen, C. A., Hui, C. F., Tzeng, T. D. & Yeh, S. Y. Population genetic structure of the swordfish, Xiphias gladius (Linnaeus, 1758), in the Indian Ocean and West Pacific inferred from the complete DNA sequence of the mitochondrial control region. Zool. Stud. 45, 269–279 (2006).
Sen, S. et al. Stock structure analysis of Decapterus russelli (Ruppell, 1830) from east and west coast of India using truss network analysis. Fish. Res. 112, 38–43 (2011).
Appleyard, S. A., Williams, R. & Ward, R. D. Population genetic structure of patagonian toothfish in the west Indian Ocean sector of the Southern Ocean. CCAML Sci. 11, 21–32 (2004).
Castro, A. L. F. et al. Population genetic structure of Earth’s largest fish, the whale shark (Rhincodon typus). Mol. Ecol. 16, 5183–5192 (2007).
Chiang, H. C., Hsu, C. C., Wu, G. C. C., Chang, S. K. & Yang, H. Y. Population structure of bigeye tuna (Thunnus obesus) in the Indian Ocean inferred from mitochondrial DNA. Fish. Res. 90, 305–312 (2008).
Muths, D., Grewe, P., Jean, C. & Bourjea, J. Genetic population structure of the Swordfish (Xiphias gladius) in the southwest Indian Ocean: Sex-biased differentiation, congruency between markers and its incidence in a way of stock assessment. Fish. Res. 97, 263–269 (2009).
Sukumaran, S., Sebastian, W. & Gopalakrishnan, A. Population genetic structure of Indian oil sardine, Sardinella longiceps along Indian coast. Gene 576, 372–378 (2016).
Palkovacs, E. P., Dion, K. B., Post, D. M. & Caccone, A. Independent evolutionary origins of landlocked alewife populations and rapid parallel evolution of phenotypic traits. Mol. Ecol. 17, 582–597 (2008).
Haro, A. et al. Population decline of the American eel: Implications for research and management. Fisheries 25, 7–16 (2000).
Hutchings, J. A. Collapse and recovery of marine fishes. Nature 406, 882–885 (2000).
Schoen, E. Insights into drivers of population declines offer a ray of hope for migratory fishes in the temperate rainforest. Glob. Change Biol. 28, 4–5 (2021).
Baum, J. K. et al. Collapse and conservation of shark populations in the Northwest Atlantic. Science 299, 389–392 (2003).
Christensen, V. et al. Hundred-year decline of North Atlantic predatory fishes. Fish Fish. 4, 1–24 (2003).
Ferretti, F., Myers, R. A., Serena, F. & Lotze, H. K. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 22, 952–964 (2008).
O’Connell, M. T., Shepherd, T. D., O’Connell, A. M. U. & Myers, R. A. Long-term declines in two apex predators, bull sharks (Carcharhinus leucas) and alligator gar (Atractosteus spatula), in Lake Pontchartrain, an oligohaline estuary in southeastern Louisiana. Estuaries Coasts 30, 567–574 (2007).
Bourret, V., O’Reilly, P. T., Carr, J. W., Berg, P. R. & Bernatchez, L. Temporal change in genetic integrity suggests loss of local adaptation in a wild Atlantic salmon (Salmo salar) population following introgression by farmed escapees. Heredity 106, 500–510 (2011).
Froese, R. & Pauly, D. FishBase. World Wide Web electronic publication. http://www.fishbase.org (2021).
Rohfritsch, A. & Borsa, P. Genetic structure of Indian scad mackerel Decapterus russelli: Pleistocene vicariance and secondary contact in the Central Indo-West Pacific Seas. Heredity 95, 315–326 (2005).
CMFRI. CMFRI Annual Report 2019 http://eprints.cmfri.org.in/14753/ (2019).
Jose, A. et al. Comparative mitogenomics and phylogenetics of the family Carangidae with special emphasis on the mitogenome of the Indian Scad Decapterus russelli. Sci. Rep. 12, 5642 (2022).
Sreenivasan, P. V. Length-weight relationship in Decapterus dayi Wakiya. Indian J. Fish. 28, 283–286 (1981).
Balasubrahmanian, N. K. & Natarajan, P. Studies on the biology of the scads, Decapterus russelli and Decapterus macrosoma at Vizhinjam, southwest coast of India. Indian J. Fish. 47, 291–300 (2000).
Manojkumar, P. P. Stock assessment of Indian scad, Decapterus russelli (Ruppell, 1830) off Malabar. J. Mar. Biol. Assoc. India 49, 79–80 (2007).
Reuben, S. et al. Fishery, biology and stock assessment of carangid resources from the Indian seas. Indian J. Fish. 39, 195–234 (1992).
Poojary, N., Tiwari, L. R. & Sundaram, S. Reproductive biology of the Indian scad, Decapterus russelli (Ruppell, 1830) from Maharashtra waters, Northwest coast of India. J. Mar. Biol. Assoc. India 57, 71–77 (2015).
Murty, V. S. Observations on some aspects of biology and population dynamics of the Scad Decapterus russelli (Ruppell) (Carangidae) in the trawling grounds off Kakinada. J. Mar. Biol. Assoc. India 33, 396–408 (1991).
Raje, S. G. On some aspects of biology of the mackerel scad Decapterus russelli (Ruppell). Indian J. Fish. 44, 97–99 (1997).
Poojary, N., Tiwari, L. R. & Dasari, B. Histological studies on the gonads of the Indian scad Decapterus russelli (Ruppell, 1830) from Maharashtra waters, north-west coast of India. Indian J. Fish. 62, 75–81 (2015).
Poojary, N., Tiwari, L. & Jaiswar, A. Food and feeding habits of the Indian scad, Decapterus russelli (Ruppell, 1830) from Mumbai waters, north-west coast of India. Indian J. Fish. 57, 93–99 (2010).
Sreenivasan, P. V. On two new records of carangid fishes from Indian seas. Indian J. Fish. 21, 20–28 (1974).
Jaiswar, A. K., George, J. P., Gulati, D. K. & Swamy, R. P. A study on length-weight relationship, food and feeding habits of Indian scad, Decapterus russelli (Ruppel, 1830) along the northwest coast of India. J. Indian Fish. Assoc. 23, 1–6 (1993).
Moiseyeva, Y. B. & Zhuk, N. N. Oogenesis in some species of carangids in the west Indian Ocean. J. Ichthyol. 35, 137–149 (1995).
Manojkumar, P. P. Maturation and spawning of Decapterus russelli (Ruppell, 1830) along the Malabar Coast. Indian J. Fish. 52, 171–178 (2005).
Sunil, V. & Suryanarayanan, H. The reproductive biology of Ambassis gymnocephalus and Decapterus russelli in the Neendakara Zone, Kerala. J. Anim. Morphol. Physiol. 41, 119–123 (1994).
Kalhoro, M. et al. Stock assessment of Indian scad, Decapterus Russelli in Pakistani marine waters and its impact on the national economy. Fish. Aquac. J. 8, 1–10 (2017).
Hardenberg, J. D. F. Preliminary report on a migration of fish in the java sea. Treubia 16, 293–300 (2016).
Perrin, C. & Borsa, P. Mitochondrial DNA analysis of the geographic structure of Indian scad mackerel in the Indo-Malay archipelago. J. Fish Biol. 59, 1421–1426 (2001).
Hoarau, G. & Borsa, P. Extensive gene flow within sibling species in the deep-sea fish Beryx splendens. C. R. Seances Acad Sci. Ser. 3 Sciences de la Vie 323, 315–325 (2000).
Fu, Y.-X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147, 915–925 (1997).
Posada, D. & Crandall, K. A. Intraspecific gene genealogies: Trees grafting into networks. Trends Ecol. Evol. 16, 37–45 (2001).
Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595 (1989).
Harpending, H. C. Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum. Biol. 66, 591–600 (1994).
Calow, P. Proximate and ultimate responses to stress in biological systems. Biol. J. Linn. Soc. 37, 173–181 (1989).
Burga, A. & Lehner, B. Beyond genotype to phenotype: Why the phenotype of an individual cannot always be predicted from their genome sequence and the environment that they experience. FEBS J. 279, 3765–3775 (2012).
Kumar, G., Kunal, S. P., Menezes, M. R. & Meena, R. M. Single genetic stock of kawakawa Euthynnus affinis (Cantor, 1849) along the Indian coast inferred from sequence analyses of mitochondrial DNA D-loop region. Conserv. Genet. 13, 1119–1131 (2012).
Muths, D., Couls, S. L., Evano, H., Grewe, P. & Bourjea, J. Multi-genetic marker approach and spatio-temporal analysis suggest there is a single panmictic population of swordfish Xiphias gladius in the Indian Ocean. PLoS One 8, e63558. https://doi.org/10.1371/journal.pone.0063558 (2013).
Akib, N. A. M. et al. High connectivity in Rastrelliger kanagurta: Influence of historical signatures and migratory behaviour inferred from mtDNA cytochrome b. PLoS One 10, e0119749. https://doi.org/10.1371/journal.pone.0119749 (2015).
Avise, J. C. Empirical intraspecific phylogeography. In Phylogeography: The History and Formation of Species 135–212 (Harvard University Press, 2000).
Nei, M. Molecular Evolutionary Genetics 102–143 (Columbia University Press, 1987).
Ely, B. et al. Consequences of the historical demography on the global population structure of two highly migratory cosmopolitan marine fishes: The yellowfin tuna (Thunnus albacares) and the skipjack tuna (Katsuwonus pelamis). BMC Evol. Biol. 5, 19 (2005).
Menezes, M. R., Ikeda, M. & Taniguchi, N. Genetic variation in skipjack tuna Katsuwonus pelamis (L.) using PCR-RFLP analysis of the mitochondrial DNA D-loop region. J. Fish Biol. 68, 156–161 (2006).
Grant, W. & Bowen, B. Shallow population histories in deep evolutionary lineages of marine fishes: Insights from sardines and anchovies and lessons for conservation. J. Hered. 89, 415–426 (1998).
Ward, R. D., Woodwark, M. & Skibinski, D. O. F. A comparison of genetic diversity levels in marine, freshwater, and anadromous fishes. J. Fish Biol. 44, 213–232 (1994).
Durand, J.-D., Collet, A., Chow, S., Guinand, B. & Borsa, P. Nuclear and mitochondrial DNA markers indicate unidirectional gene flow of Indo-Pacific to Atlantic bigeye tuna (Thunnus obesus) populations, and their admixture off southern Africa. Mar. Biol. 147, 313–322 (2005).
Schott, F. A. & McCreary, J. P. The monsoon circulation of the Indian Ocean. Prog. Oceanogr. 51, 1–123 (2001).
Schott, F. A., Xie, S.-P. & McCreary, J. P. Jr. Indian Ocean circulation and climate variability. Rev. Geophys. 47, RG1002 (2009).
Longhurst, A. Biogeographic partition of the Ocean. In Ecological Geography of the Sea 19–31 (Academic Press, 1998).
Mora, C., Chittaro, P. M., Sale, P. F., Kritzer, J. P. & Ludsin, S. A. Patterns and processes in reef fish diversity. Nature 421, 933–936 (2003).
Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913 (2000).
Voris, H. K. Maps of Pleistocene Sea levels in Southeast Asia: Shorelines, river systems and time durations. J. Biogeogr. 27, 1153–1167 (2000).
Avise, J. C. Genealogical concordance: Towards speciation and beyond. In Phylogeography: The History and Formation of Species 135–212 (Harvard University Press, 2000).
Bird, M. I., Taylor, D. & Hunt, C. Palaeoenvironments of insular Southeast Asia during the Last Glacial Period: A savanna corridor in Sundaland?. Quatern. Sci. Rev. 24, 2228–2242 (2005).
Bird, M. I., Pang, W. C. & Lambeck, K. The age and origin of the Straits of Singapore. Palaeogeogr. Palaeoclimatol. Palaeoecol. 241, 531–538 (2006).
Hanebuth, T., Stattegger, K. & Grootes, P. M. Rapid flooding of the Sunda Shelf: A late-glacial sea-level record. Science 288, 1033–1035 (2000).
Lambeck, K., Esat, T. M. & Potter, E.-K. Links between climate and sea levels for the past three million years. Nature 419, 199–206 (2002).
Tjia, H. D. & Liew, K. K. Changes in tectonic stress field in northern Sunda Shelf basins. Geol. Soc. Spec. Publ. 106, 291–306 (1996).
Palumbi, S. R. Population genetics, demographic connectivity, and the design of marine reserves. Ecol. Appl. 13, 146–158 (2003).
Nielsen, E. E., Nielsen, P. H., Meldrup, D. & Hansen, M. M. Genetic population structure of turbot (Scophthalmus maximus L.) supports the presence of multiple hybrid zones for marine fishes in the transition zone between the Baltic Sea and the North Sea. Mol. Ecol. 13, 585–595 (2004).
Wright, S. Evolution in Mendelian populations. Genetics 16, 97–159 (1931).
Frankel, O. & Soule, M. E. Population genetics and conservation. In Conservation and Evolution 31–75 (Cambridge University Press, 1981).
Franklin, I. R. Evolutionary change in small populations. In Conservation biology—An evolutionary–ecological perspective (eds Soule, M. E. & Wilcox, B. A.) 135–149 (Sinauer Associates, 1980).
Shankar, D., Vinayachandran, P. N. & Unnikrishnan, A. S. The monsoon currents in the north Indian Ocean. Prog. Oceanogr. 52, 63–120 (2002).
Sun, P., Shi, Z., Yin, F. & Peng, S. Population genetic structure and demographic history of Pampus argenteus in the Indo-West Pacific inferred from mitochondrial cytochrome b sequences. Biochem. Syst. Ecol. 43, 54–63 (2012).
Nielsen, E. E., Hemmer-Hansen, J., Larsen, P. F. & Bekkevold, D. Population genomics of marine fishes: Identifying adaptive variation in space and time. Mol. Ecol. 18, 3128–3150 (2009).
Russello, M. A., Kirk, S. L., Frazer, K. K. & Askey, P. J. Detection of outlier loci and their utility for fisheries management. Evol. Appl. 5, 39–52 (2012).
Waples, R. S. & Gaggiotti, O. Invited review: What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol. Ecol. 15, 1419–1439 (2006).
DeTolla, L. J. et al. Guidelines for the care and use of fish in research. ILAR J. 37, 159–173 (1995).
Sambrook, J. D. & Russell, W. Molecular Cloning: A Laboratory Manual 2028 (Cold Spring Harbor Laboratory Press, 2001).
Hassan, M., Lemaire, C., Fauvelot, C. & Bonhomme, F. Seventeen new exon-primed intron-crossing polymerase chain reaction amplifiable introns in fish. Mol. Ecol. Notes 2, 334–340 (2002).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Stephens, M., Smith, N. J. & Donnelly, P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 68, 978–989 (2001).
Rozas, J. et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 34, 3299–3302 (2017).
Chanthran, S. S. D. et al. Genetic diversity and population structure of Terapon jarbua (Forskål, 1775) (Teleostei, Terapontidae) in Malaysian waters. ZooKeys 911, 139–160 (2020).
Hebert, P. D. N., Cywinska, A., Ball, S. L. & de Waard, J. R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 270, 313–321 (2003).
Jamaludin, N.-A. et al. Phylogeography of the Japanese scad, Decapterus maruadsi (Teleostei; Carangidae) across the Central Indo-West Pacific: Evidence of strong regional structure and cryptic diversity. Mitochondrial DNA Part A 31, 298–310 (2020).
Ward, R. D. Genetics in fisheries management. Hydrobiologia 420, 191–201 (2000).
Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567 (2010).
Excoffier, L., Smouse, P. E. & Quattro, J. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 131, 479–491 (1992).
Dupanloup, I., Schneider, S. & Excoffier, L. A simulated annealing approach to define the genetic structure of populations. Mol. Ecol. 11, 2571–2581 (2002).
Schneider, S. & Excoffier, L. Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: Application to human mitochondrial DNA. Genetics 152, 1079–1089 (1999).
Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. 70, 3321–3323 (1973).
Hudson, R. R., Boos, D. D. & Kaplan, N. L. A statistical test for detecting geographic subdivision. Mol. Biol. Evol. 9, 138–151 (1992).
Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220 (1967).
Bandelt, H. J., Forster, P. & Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16, 37–48 (1999).
Leigh, J. W. & Bryant, D. popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 110–116 (2015).
Rogers, A. R. & Harpending, H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 9, 552–569 (1992).
Slatkin, M. & Hudson, R. R. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129, 555–562 (1991).
Nei, M. & Tajima, F. DNA polymorphism detectable by restriction endonucleases. Genetics 97, 145–163 (1981).
Cárdenas, L. et al. Origin, diversification, and historical biogeography of the genus Trachurus (Perciformes: Carangidae). Mol. Phylogenet. Evol. 35, 496–507 (2005).
Johns, G. C. & Avise, J. C. A comparative summary of genetic distances in the vertebrates from the mitochondrial cytochrome b gene. Mol. Biol. Evol. 15, 1481–1490 (1998).
Rousset, F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145, 1219–1228 (1997).
Acknowledgements
We would like to thank Dr. S.R. Krupesha Sharma, Head in charge, Marine Biotechnology Fish Nutrition and Health Division (MBFNHD) and Director, ICAR-Central Marine Fisheries Research Institute (CMFRI) (No. MBT/GNM/25), Kochi for providing the necessary facilities for conducting the research. Anjaly Jose is grateful to the Council of Scientific and Industrial Research (CSIR) for the financial assistance in the form of a research fellowship.
Author information
Authors and Affiliations
Contributions
A.J. performed the experiments, analyzed and interpreted the data, and prepared the main manuscript text. S.S. conceptualized and coordinated the study and reviewed and edited the manuscript. S.K.R., A.A.P. and S.J.K. participated in the sample collection. N.R. and N.K. participated in the data analysis. A.G. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jose, A., Sukumaran, S., Roul, S.K. et al. Genetic analyses reveal panmixia in Indian waters and population subdivision across Indian Ocean and Indo-Malay Archipelago for Decapterus russelli. Sci Rep 13, 22860 (2023). https://doi.org/10.1038/s41598-023-49805-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49805-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.