Abstract
Randomized clinical trials (RCTs) of lifestyle modification have reported beneficial effects of interventions, compared to control. Whether participation in the control group has benefits is unknown. To determine whether control group participants experience weight loss during the course of RCTs. After prospective registration (PROSPERO CRD42021233070), we conducted searches in Medline, Scopus, Web of Science, Cochrane library and Clinicaltrials.gov databases from inception to May 2021 without language restriction to capture RCTs on dietary advice or physical activity interventions in adults with overweight, obesity or metabolic syndrome. Data extraction and study quality assessment was performed by two independent reviewers. Weight loss in the control group, i.e., the difference between baseline and post-intervention, was pooled using random effects model generating mean difference and 95% confidence interval (CI). Heterogeneity was assessed using the I2 statistical test. Subgroup meta-analysis was performed stratifying by follow-up period, type of control group protocols and high-quality studies. Among the 22 included studies (4032 participants), the risk of bias was low in 9 (40%) studies. Overall, the controls groups experienced weight loss of − 0.41 kg (95% CI − 0.53 to − 0.28; I2 = 73.5% p < 0.001). To identify a result that is an outlier, we inspected the forest plot for spread of the point estimates and the confidence intervals. The magnitude of the benefit was related to the duration of follow-up (− 0.51 kg, 95% CI − 0.68, − 0.3, for 1–4 months follow-up; − 0.32 kg, 95% CI − 0.58, − 0.07, 5–12 months; − 0.20 kg, 95% CI − 0.49, 0.10, ≥ 12 months). In high-quality studies we found an overall weight loss mean difference of − 0.16 (95% CI − 0.39, 0.09) with a considerable heterogeneity (I2 = 74%; p < 0.000). Among studies including control group in waiting lists and combining standard care, advice and material, no heterogeneity was found (I2 = 0%, p = 0.589) and (I2 = 0%, p = 0.438); and the mean difference was − 0.84 kg (95% CI − 2.47, 0.80) and − 0.65 kg (95% CI − 1.03, − 0.27) respectively. Participation in control groups of RCTs of lifestyle interventions had a benefit in terms of weight loss in meta-analysis with heterogeneity. These results should be used to interpret the benefits observed with respect to intervention effect in trials. That control groups accrue benefits should be included in patient information sheets to encourage participation in future trials among patients with overweight and obesity.
Similar content being viewed by others
Introduction
Obesity, a major cause of morbidity and mortality worldwide with over 650 million affected adults1,2, has attracted interest in preventive research of various study designs in light of its impact on the healthcare system and the economy3,4. However, it is challenging to encourage patients to take part in randomized trials, in part because of the perception that participation in control group may not be valuable5. With a median dropout rate of 24%, difficulties in recruiting, retaining and obtaining outcome data from participants are common in lifestyle randomised controlled trials (RCTs)6,7,8 and they contribute to trials being underpowered or invalid. There is a need to generate information about benefits of participation in trials to enthuse participants to engage in obesity research in a manner that robust and timely results can be produced to inform future practice and policy9.
A literature search demonstrated that participants of RCTs, on average, experienced better outcomes compared with those outside trials10,11,12,13,14,15. There is a scarcity of reviews concerning participation in lifestyle modification research16, and none is focused in overweight or obese participants being at risk of a chronic disease to assess benefits of clinical trials based in diet in the last decade. Descriptions of treatment and outcomes of control groups participants have received limited attention17,18. In obesity research it would be important to know if control groups experience any benefits inside RCTs, not only to encourage participation, but also to interpret findings of trials on effect of participation, with respect to intragroup differences in control and intervention groups. In this systematic review and meta-analysis, we aimed to determine whether participants with overweight, obesity or metabolic syndrome, allocated to control groups in lifestyle modification research experienced benefits in terms of weight loss during the course of the RCTs.
Material and methods
We performed the systematic review after prospective registration (PROSPERO number: CRD42021233070) and reported it in accordance with relevant guidelines19.
Search and selection
We conducted a comprehensive literature search without language restrictions in electronic databases (Medline via ProQuest, Scopus, Web of Science, Cochrane library and Clinicaltrials.gov) from inception to May 2021. In addition, we hand-searched reference lists of previous reviews and included articles. The search term combination was based on MeSH terms, free-text words and word variants. The inclusion criteria lifestyle intervention RCTs based on diet, with or without physical activity, and with or without behavioural support, among adults with overweight, obesity or metabolic syndrome. In crossover RCTs, control group participants were on a waiting list with standard care to receive further intervention after a wash-up period. The combination of keywords and terms included: metabolic syndrome, obesity, overweight, diet, hypocaloric diet, Mediterranean diet, physical activity, educational intervention, preventive program, diabetes mellitus, cancer, cardiovascular disease, weight loss, mortality, randomized controlled trial, lifestyle intervention, lifestyle modification, lifestyle risk reduction (Appendix 1). All citations found were exported to Endnote where duplicates were removed.
Two reviewers (ABH and PMG) carried out a search strategy independently using electronic databases and manual searches. Both of them screened all abstracts and titles. Exclusion criteria were studies conducted on children, adolescents and pregnant women; participants with established cardiovascular disease, cancer, diabetes or eating disorders; sample selection based on special conditions like familiar hypercholesterolemia o bariatric surgery, polycystic ovary syndrome, kidney disease or chronic obstructive pulmonary disease. We also excluded studies with no control group or those which did not provide outcome data for the control group. Study designs other than RCT and types of interventions other than lifestyle modification (like drug treatments or diet supplements) were excluded. Any disagreement regarding the articles’ inclusion was resolved by taking the opinion of a third researcher (NCI). We contacted authors to achieve not available full text articles. Finally, the selection of articles was based on independent review of full texts to ensure the inclusion and exclusion criteria have been fulfilled.
Data extraction and risk of bias
The key characteristics of selected studies were extracted independently by both reviewers (ABH and PMG) after reading the full text. We used a predefined form for data extraction and, when necessary, we contacted directly the authors through ResearchGate for relevant data that were not provided in the manuscripts. Jadad scale (score range 0–5)20 was used to assess the methodological quality of randomization, blinding and patient withdrawals or dropouts. RCTs with a score of ≥ 3 was considered to be of high quality. We used this scale because the features assessed apply to control group, and also it has allowed us to verify the overall quality of the trials included. Given the type of lifestyle interventions used in these RCTs, double-blind was not possible. Disagreement was resolved by discussion between both reviewers or consultation with the third reviewer.
Data synthesis and statistical analysis
We used the outcomes of the control groups reported by the authors as the mean difference in kg of body weight lost from baseline to post-participation and its standard deviations (SD). In three reviews21,22,23, which is the 13.6% of the studies, did not provide explicitly the mean difference. We calculated the weight change from the mean values reported by the authors for control group at basal and post-participation time in the RCT. We calculated the standard deviation (SD) using the confidence interval (CI) with this formula: SD = \(\overline{x}\) ± tc (s/√n). Meta-analysis was deployed to comply with the recommended statistical approach, ensuring that the same metric unity (kg) was used to estimate mean difference and that the effect of the advice to control group was comparable across trials24, constructing forest plots with Stata v.15 software (Stata Corp., 2015, College Station, TX, USA). A random effects model was performed since each study provides information about a different effect size. We attempted to ensure that all these effect sizes are represented in the summary, and did not remove a small study by giving it a very small weight, as it would be done in a fixed-effect analysis. Heterogeneity among studies was assessed using Q test and I-squared (I2) statistics. We assumed that an I2 > 50% indicates substantial heterogeneity and I2 > 75% considerable heterogeneity25,26. In order to find out whether control group counselling was sufficiently similar across trials, we followed the criteria established by the Cochrane Handbook for Systematic Reviews of Interventions27. Subgroup meta-analysis was performed stratifying by follow-up period, type of control group protocols, and high-quality studies.
Results
Study selection and quality assessment
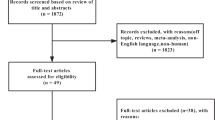
A total of 846 records were identified initially. In total, 22 studies with 4032 participants were finally included (Fig. 1). The main characteristics of the studies included are summarized in Table 1. In all RCTs a lifestyle intervention was performed. The studies were conducted in several countries United Kingdom (3), United States (3), Spain (3), Japan (2), Australia (2), China (1), South Korea (1), Netherlands (1), Denmark (1), Thailand (1), Finland (1), Germany (1), Italy (1) and Saudi Arabia (1). In total, only 2 studies were published before 2010. Of the total, 9 studies (40%) were considered of high quality and 13 studies (60%) were classified as low quality (Fig. 2). Cohen's Kappa coefficient (κ) was 0.80 indicating a high inter-rater reliability between the two reviewers concerning study quality assessment.
Characteristics of control groups
In our selected studies the sample size ranged from 32 to 626 participants, aged between 18 and 70 years old (mean age 53.92 years). Each study applied different inclusion criteria concerning the BMI. The mean of BMI was 31.93 kg/m2 in control group at baseline, ranging from 25.6 kg/m2 to 39,8 kg/m2,21,28. Four studies included only women22,23,29,30 whereas three studies enrolled only men28,31,32. One article21 set obstructive sleep apnoea hypopnoea syndrome as other inclusion criteria, while another study33 included prediabetic patients that have overweight or obesity. In one of our studies34, having overweight or obesity was not an inclusion criterion, but all participants had overweight with a BMI > 30.2 in the intervention and > 29.9 in the control group, that why we decided to include it in the review.
In six studies30,31,34,35,36,37 control group received only standard care, while in three studies29,32,38 they were given extra nutritional advice. In nine studies21,22,28,33,39,40,41,42,43 they received extra material, like written information, educational booklets or leaflets. Finally, in four studies23,44,45,46 control group participants were waitlisted to receive the programme after data extraction. The length of the follow-up ranged from 1 to 24 months. We considered as time points the end-point of the intervention provided by the authors. If these data were not available, post-intervention follow-up value was considered, like in one study47 where outcomes were measured at 6 months, although the follow-up lasted up to 12 months. The same criterion was applied to another study29, where the effects of only the first three months of intervention were reported, whilst the intervention lasted up to 12 months. In two studies32,35 the intervention was carried out during 12 months and, afterwards, the participants were followed up for other 12 months (post-intervention). Finally, in a RCT21 the intervention lasted 3 months, although the follow-up was extended to 6,5 months. The lifestyle interventions were carried out by dietitians or nutritionists in three studies33,42,47, and in collaboration with other health professionals (e.g., nurses, physicians, psychologists, sport coaches or trainers) in the rest of the studies. All of the RCT included physical activity (n = 22) as part of the intervention. Control groups received the standard or usual care, or were wait-listed to receive the lifestyle program after data collection in the RCT.
Data synthesis
The results of the meta-analysis showed an overall weight loss control group mean difference of − 0.41 (95% CI − 0.53, − 0.28). These results show statistical significance with a substantial heterogeneity (I2 = 73.5%; p < 0.001) (Fig. 3). For studies with a follow-up period of 1–4 months, the heterogeneity was substantial (I2 = 72.3%; p = < 0.003) and the mean difference was − 0.51 kg (95% CI − 0.68, − 0.34), studies with 5–12 months had a considerable heterogeneity (I2 = 76.8%; p = < 0.001) and mean difference − 0.32 kg (95% CI − 0.58, − 0.07), whereas when the follow-up was > 12 months, there was a substantial heterogeneity (I2 = 70.3%; p = < 0.018) and mean difference − 0.20 kg (95% CI − 0.49, 0.10) (Fig. 4). We performed a meta-analysis of high-quality studies with an overall weight loss control group mean difference of − 0.16 (95% CI − 0.39, 0.09) and a considerable heterogeneity (I2 = 74%; p < 0.000) (Fig. 5). As the exploration of heterogeneity leads to more meaningful, high-value conclusions, we also performed a meta-analysis comparing subgroups by type of care protocols in control group. Among studies including control group in waiting lists and combining standard care, advice and material, no heterogeneity was found (I2 = 0%, p = 0.589) and (I2 = 0%, p = 0.438), and the mean difference was − 0.84 kg (95% CI − 2.47, 0.80) and − 0.65 kg (95% CI − 1.03, − 0.27), respectively. In studies with standard care and material, the heterogeneity was substantial (I2 = 68,2%, p = 0.004) and the mean difference was − 0.47 kg (95% CI − 0.65, − 0.28). Finally, in the studies where control group participants received standard care, or standard care and advice, we found a considerable heterogeneity (I2 = 85.4%, p = 0.000) and (I2 = 85.8%, p = 0.001) with a mean difference of − 0.48 kg (95% CI − 0.76, − 0.20) and 0.00 kg (-0.30, 0.30) (Fig. 6).
Discussion
Our meta-analysis of over four thousand participants combined showed that control groups in obesity research lost weight overall, confirming that it is safe and beneficial to participate in trials even if the allocation is not to the intervention arm.
To our knowledge, this is the first systematic review and meta-analysis focusing on control group outcomes in lifestyle intervention studies. Our findings confirm the hypothesis of health improvement of control participants, in contrast to the results on overall weight changes in a meta-regression study on behavioural weight loss interventions16. Our search was unrestricted, without limitations regarding language or dataset inception, to capture the highest possible number of relevant studies. There was reviewer agreement in the search, selection and quality assessment of studies adding to reliability of our work. However, our main finding was within the limitations placed by heterogeneity. This is an expected, possibly unavoidable, limitation when addressing lifestyle interventions48. In our review there are various possible sources of heterogeneity. Standard healthcare in control groups may vary between participants depending on the health systems in the countries where trials are carried out. We also found a diversity of approaches in handling control group engagement, e.g., providing health educational contents with a variable frequency, within the trials included, which may have different effect on weight loss16. With a considerable sample size, we could precisely estimate the control group weight loss. The reporting of some of the studies did not facilitate the analysis of the control group, as findings were mainly reported for intergroup differences. However, in the three mentioned articles not providing required parameters for meta-analysis, we were able to estimate them from the available data. Despite the issues arising from data reporting quality, our overall result was statistically significant.
How did the control group come to benefit? The observed benefits may be due to a trial effect, which increases adherence to care protocols12 and encourages interaction between patients and professionals49. Additionally, Hawthorne effect could improve control group outcome through modification of the behaviour of research participants just because they are observed in the course of a trial50. The observed fact that the control groups benefit is generally in line with the view that participating in RCTs is good for participants10,11,16. This finding is particularly important as the prevalent overweight and obesity rates are high. For example, in Spanish population aged 55–64 years the prevalence of overweight and obesity reaches 44% and 22% respectively51. As the mean age of the control group participants in Spain35,43,46 was 60 years, trial participation could be thought of as a strategy for weight control. The same theme is repeated for the USA, where north American studies33,40,42 showed a mean age of 51 years and the prevalence of overweight and obesity in the over 50 s is 70%52. Despite the magnitude of the effect in control group participants is not large, the fact that they experienced a weight loss inverses population trends of progressive gain during adult life53. According to the preventive paradox of Rose et al.54,55, beyond the individual benefit, this weight loss may have a high impact of the health outcomes when extended to general population, in terms of improvement of health status and reduction of burden for health systems. Health services should also consider implementing lifestyle intervention trials as part of programs for people with overweight and obesity56.
Lifestyle research has shown health benefits of intervention compared to control in terms of adiposity and cardiovascular risk decrease57,58. Our findings also show a benefit in the outcome of the control groups. Future research should examine if the benefits gained by participation in the control groups can be maintained over time as a healthy weight loss has a tendency to be gradually regained48,59. These benefits should be used to encourage participation in future obesity research to generate the timely evidence for practice and policy.
Conclusions
Our systematic review showed that participation in control groups of RCTs of lifestyle interventions had a benefit in terms of weight loss in meta-analysis with heterogeneity. These results should be used to interpret the benefits observed with respect to intervention effect in trials. That control groups accrue benefits should be included in patient information sheets to encourage participation in future trials among patients with overweight or obesity.
Change history
24 August 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-18828-y
Abbreviations
- RCTs:
-
Randomized controlled trials
- BMI:
-
Body Mass Index
References
Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. https://doi.org/10.1038/s41574-019-0176-8 (2019).
The Lancet, G., & Hepatology. Obesity: another ongoing pandemic. Lancet Gastroenterol. Hepatol. 6, 411. https://doi.org/10.1016/S2468-1253(21)00143-6 (2021).
Zhao, N., Tao, K., Wang, G. & Xia, Z. Global obesity research trends during 1999 to 2017: A bibliometric analysis. Medicine 98, e14132. https://doi.org/10.1097/md.0000000000014132 (2019).
Tremmel, M., Gerdtham, U. G., Nilsson, P. M., & Saha, S. Economic burden of obesity: A systematic literature review. Int. J. Environ. Res. Public Health. https://doi.org/10.3390/ijerph14040435 (2017).
Lindström, D., Sundberg-Petersson, I., Adami, J. & Tönnesen, H. Disappointment and drop-out rate after being allocated to control group in a smoking cessation trial. Contemp. Clin. Trials 31, 22–26. https://doi.org/10.1016/j.cct.2009.09.003 (2010).
Groeneveld, I. F., Proper, K. I., van der Beek, A. J., Hildebrandt, V. H. & van Mechelen, W. Factors associated with non-participation and drop-out in a lifestyle intervention for workers with an elevated risk of cardiovascular disease. Int. J. Behav. Nutr. Phys. Act 6, 80. https://doi.org/10.1186/1479-5868-6-80 (2009).
Lemstra, M., Bird, Y., Nwankwo, C., Rogers, M. & Moraros, J. Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Prefer. Adherence 10, 1547–1559. https://doi.org/10.2147/ppa.S103649 (2016).
Mutsaerts, M. A., Kuchenbecker, W. K., Mol, B. W., Land, J. A. & Hoek, A. Dropout is a problem in lifestyle intervention programs for overweight and obese infertile women: A systematic review. Hum. Reprod. 28, 979–986. https://doi.org/10.1093/humrep/det026 (2013).
Eaglehouse, Y. L. et al. Impact of a community-based lifestyle intervention program on health-related quality of life. Qual. Life Res. 25, 1903–1912. https://doi.org/10.1007/s11136-016-1240-7 (2016).
Vist, G. E., Bryant, D., Somerville, L., Birminghem, T. & Oxman, A. D. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database Syst. Rev. 2008, Mr000009. https://doi.org/10.1002/14651858.MR000009.pub4 (2008).
Nijjar, S. K. et al. Participation in clinical trials improves outcomes in women’s health: A systematic review and meta-analysis. BJOG 124, 863–871. https://doi.org/10.1111/1471-0528.14528 (2017).
Clarke, M. & Loudon, K. Effects on patients of their healthcare practitioner’s or institution’s participation in clinical trials: A systematic review. Trials 12, 16. https://doi.org/10.1186/1745-6215-12-16 (2011).
Braunholtz, D. A., Edwards, S. J. & Lilford, R. J. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J. Clin. Epidemiol. 54, 217–224. https://doi.org/10.1016/s0895-4356(00)00305-x (2001).
Gross, C. P., Krumholz, H. M., Van Wye, G., Emanuel, E. J. & Wendler, D. Does random treatment assignment cause harm to research participants?. PLoS Med. 3, e188. https://doi.org/10.1371/journal.pmed.0030188 (2006).
Fernandes, N. et al. Outcomes for patients with the same disease treated inside and outside of randomized trials: A systematic review and meta-analysis. CMAJ 186, E596-609. https://doi.org/10.1503/cmaj.131693 (2014).
Waters, L., George, A. S., Chey, T. & Bauman, A. Weight change in control group participants in behavioural weight loss interventions: A systematic review and meta-regression study. BMC Med. Res. Methodol. https://doi.org/10.1186/1471-2288-12-120 (2012).
Byrd-Bredbenner, C. et al. Systematic review of control groups in nutrition education intervention research. Int. J. Behav. Nutr. Phys. Act 14, 91. https://doi.org/10.1186/s12966-017-0546-3 (2017).
Glanz, K., Sorensen, G. & Farmer, A. The health impact of worksite nutrition and cholesterol intervention programs. Am. J. Health Promot. 10, 453–470. https://doi.org/10.4278/0890-1171-10.6.453 (1996).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. https://doi.org/10.1001/jama.283.15.2008 (2000).
Jadad, A. R. et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Control Clin. Trials 17, 1–12. https://doi.org/10.1016/0197-2456(95)00134-4 (1996).
Moss, J. et al. Effects of a pragmatic lifestyle intervention for reducing body mass in obese adults with obstructive sleep apnoea: A randomised controlled trial. BioMed Res. Int. https://doi.org/10.1155/2014/102164 (2014).
Oh, E.-G. et al. A randomized controlled trial of therapeutic lifestyle modification in rural women with metabolic syndrome: A pilot study. Metab. Clin. Exp. 57, 255–261. https://doi.org/10.1016/j.metabol.2007.09.009 (2008).
Share, B. L. et al. Effects of a multi-disciplinary lifestyle intervention on cardiometabolic risk factors in young women with abdominal obesity: A randomised controlled trial. PLoS ONE. https://doi.org/10.1371/journal.pone.0130270 (2015).
Morris, S. B. & DeShon, R. P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 7, 105–125. https://doi.org/10.1037/1082-989x.7.1.105 (2002).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560. https://doi.org/10.1136/bmj.327.7414.557 (2003).
Deeks, J. J., Higgins, J. P., Altman, D. G. & Group, o. b. o. t. C. S. M. in Cochrane Handbook for Systematic Reviews of Interventions 241–284 (2019).
McKenzie, J. E. et al. in Cochrane Handbook for Systematic Reviews of Interventions 33–65 (2019).
Nanri, A. et al. Effect of six months lifestyle intervention in Japanese men with metabolic syndrome: Randomized controlled trial. J. Occup. Health 54, 215–222. https://doi.org/10.1539/joh.11-0238-OA (2012).
Christensen, J. R. et al. Diet, physical exercise and cognitive behavioral training as a combined workplace based intervention to reduce body weight and increase physical capacity in health care workers—A randomized controlled trial. BMC Public Health. https://doi.org/10.1186/1471-2458-11-671 (2011).
Thiabpho, C. et al. Intensive lifestyle modification program on weight loss and metabolic syndrome risk reduction among obese women in rural areas of Thailand. J. Health Res. https://doi.org/10.1108/JHR-05-2018-022 (2018).
Maruyama, C., Kimura, M., Okumura, H., Hayashi, K. & Arao, T. Effect of a worksite-based intervention program on metabolic parameters in middle-aged male white-collar workers: A randomized controlled trial. Prev. Med. 51, 11–17. https://doi.org/10.1016/j.ypmed.2010.04.008 (2010).
Puhkala, J. et al. Lifestyle counseling to reduce body weight and cardiometabolic risk factors among truck and bus drivers–a randomized controlled trial. Scand. J. Work Environ. Health 41, 54–64. https://doi.org/10.5271/sjweh.3463 (2015).
Weinhold, K. R. et al. A randomized controlled trial translating the diabetes prevention program to a university worksite, Ohio, 2012–2014. Preventing Chronic Disease. https://doi.org/10.5888/pcd12.150301 (2015).
Duijzer, G. et al. Effect and maintenance of the SLIMMER diabetes prevention lifestyle intervention in Dutch primary healthcare: A randomised controlled trial. Nutr. Diabetes. https://doi.org/10.1038/nutd.2017.21 (2017).
Fernández-Ruiz, V. E. et al. Effectiveness of the I 2 AO 2 interdisciplinary programme led by nurses on metabolic syndrome and cardiovascular risk: a randomized, controlled trial. J. Int. Med. Res. 46, 2202–2218. https://doi.org/10.1177/0300060518757604 (2018).
Bo, S. et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J. Gen. Intern. Med. 22, 1695–1703. https://doi.org/10.1007/s11606-007-0399-6 (2007).
Anderson, A. S. et al. A novel approach to increasing community capacity for weight management a volunteer-delivered programme (ActWELL) initiated within breast screening clinics: A randomised controlled trial. Int. J. Behav. Nutr. Phys. Activity. https://doi.org/10.1186/s12966-021-01099-7 (2021).
Cai, H. et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. https://doi.org/10.1186/s12876-019-1132-8 (2019).
Greaves, C. et al. Waste the waist: A pilot randomised controlled trial of a primary care based intervention to support lifestyle change in people with high cardiovascular risk. Int. J. Behav. Nutr. Phys. Activity. doi:https://doi.org/10.1186/s12966-014-0159-z (2015).
Lin, M. et al. Tailored, interactive text messages for enhancing weight loss among African American Adults: The TRIMM randomized controlled trial. Am. J. Med. 128, 896–904. https://doi.org/10.1016/j.amjmed.2015.03.013 (2015).
Alghamdi, R. Q. A randomized controlled trial of a 12-week intensive lifestyle intervention program at a primary care obesity clinic for adults in western Saudi Arabia. Saudi Med. J. 38, 837–845. https://doi.org/10.15537/smj.2017.8.20553 (2017).
Kandula, N. R. et al. Translating a heart disease lifestyle intervention into the community: the South Asian Heart Lifestyle Intervention (SAHELI) study; A randomized control trial. BMC Public Health 15, 1064. https://doi.org/10.1186/s12889-015-2401-2 (2015).
Salas-Salvadó, J. et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean Diet and exercise on weight loss and cardiovascular risk factors: One-year results of the PREDIMED-Plus Trial. Diabetes Care 42, 777–788. https://doi.org/10.2337/dc18-0836 (2019).
Blackford, K. et al. Home-based lifestyle intervention for rural adults improves metabolic syndrome parameters and cardiovascular risk factors: A randomised controlled trial. Prev. Med. 89, 15–22. https://doi.org/10.1016/j.ypmed.2016.05.012 (2016).
Röhling, M. et al. Weight reduction by the low-insulin-method—a randomized controlled trial. Nutrients 12, 1–17. https://doi.org/10.3390/nu12103004 (2020).
Pablos, A. et al. Effects of a lifestyle intervention program for treating obesity in lower socioeconomic status adults: a randomized controlled trial. Gazzetta Medica Italiana Archivio Per Le Scienze Mediche 176, 467–477. https://doi.org/10.23736/s0393-3660.17.03407-6 (2017).
Cai, R. et al. Effect of community-based lifestyle interventions on weight loss and cardiometabolic risk factors in obese elderly in China: A randomized controlled trial. Exp. Gerontol. https://doi.org/10.1016/j.exger.2019.110749 (2019).
Malakou, E. et al. The combined effect of promoting the mediterranean diet and physical activity on metabolic risk factors in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients. https://doi.org/10.3390/nu10111577 (2018).
Montesi, L. et al. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab. Syndr. Obes. 9, 37–46. https://doi.org/10.2147/dmso.S89836 (2016).
McCambridge, J., Witton, J. & Elbourne, D. R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 67, 267–277. https://doi.org/10.1016/j.jclinepi.2013.08.015 (2014).
Índice de masa corporal población adulta según sexo y grupo de edad. Población de 18 y más años, <https://www.ine.es/jaxi/Datos.htm?path=/t15/p419/a2017/p06/l0/&file=01001.px#!tabs-tabla> (2021).
Roser, H. R. & Max. Obesity. https://ourworldindata.org/obesity (2021).
Alvarez León, E. E. & Vioque, J. [Weight gain along adult life]. Med Clin (Barc) 117, 172–174. https://doi.org/10.1016/s0025-7753(01)72052-0 (2001).
Rose, G. Sick individuals and sick populations. Int. J. Epidemiol. 14, 32–38. https://doi.org/10.1093/ije/14.1.32 (1985).
Rose, G. Strategy of prevention: Lessons from cardiovascular disease. Br. Med. J. (Clin. Res. Ed.) 282, 1847–1851. https://doi.org/10.1136/bmj.282.6279.1847 (1981).
van Namen, M., Prendergast, L. & Peiris, C. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: A systematic review and meta-analysis. Metabolism 101, 153988. https://doi.org/10.1016/j.metabol.2019.153988 (2019).
Cano-Ibáñez, N. et al. Diet quality and nutrient density in subjects with metabolic syndrome: Influence of socioeconomic status and lifestyle factors. A cross-sectional assessment in the PREDIMED-Plus study. Clin. Nutr. 39, 1161–1173. https://doi.org/10.1016/j.clnu.2019.04.032 (2020).
Cano-Ibanez, N. et al. Effect of changes in adherence to Mediterranean diet on nutrient density after 1-year of follow-up: Results from the PREDIMED-Plus Study. Eur. J. Nutr. 59, 2395–2409. https://doi.org/10.1007/s00394-019-02087-1 (2020).
Christian, J. G., Tsai, A. G. & Bessesen, D. H. Interpreting weight losses from lifestyle modification trials: using categorical data. Int. J. Obes. (Lond.) 34, 207–209. https://doi.org/10.1038/ijo.2009.213 (2010).
Acknowledgements
The first author would like to acknowledge support by the CIBER Epidemiología y Salud Pública (CIBERESP/ CB06/02/1014). Professor Khan is a Distinguished Investigator at the University of Granada funded by the Beatriz Galindo (senior modality) program of the Spanish Ministry of Education.
Funding
This research has received funding from the Ministry of Science and Innovation, Instituto de Salud Carlos III, FEDER co-funding from European Union (PI20/01532 project), and the Centro de Investigación Biomédica en Red-Epidemiología y Salud Pública (CIBERESP/CB06/02/1014).
Author information
Authors and Affiliations
Contributions
All authors contributed in the conception of the research question and designed the study. A.B.H. did the literature search, study selection and data extraction, and double checked by P.M.G. N.C.I., A.B., K.S.K. and A.B.C. did the statistical analysis. The figures, tables and appendices were designed by A.B.H. All authors contributed to the drafts and final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the Funding section. Full information regarding the correction made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bouzalmate Hajjaj, A., Massó Guijarro, P., Khan, K.S. et al. A systematic review and meta-analysis of weight loss in control group participants of lifestyle randomized trials. Sci Rep 12, 12252 (2022). https://doi.org/10.1038/s41598-022-15770-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15770-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.