Abstract
Delayed graft function after kidney transplantation is common and increases morbidity and health care costs. There is evidence that endotrophin, a specific fragment of pro-collagen type VI, promotes the inflammatory response in kidney diseases. We tested the hypothesis that pretransplant endotrophin in kidney transplant recipients may be associated with the risk of delayed graft function. Pretransplant plasma endotrophin was assessed using an enzyme-linked immunosorbent assay in three independent cohorts with 806 kidney transplant recipients. The primary outcome was delayed graft function, i.e., the necessity of at least one dialysis session within one-week posttransplant. In the discovery cohort median pretransplant plasma endotrophin was higher in 32 recipients (12%) who showed delayed graft function when compared to 225 recipients without delayed graft function (58.4 ng/mL [IQR 33.4–69.0]; N = 32; vs. 39.5 ng/mL [IQR 30.6–54.5]; N = 225; P = 0.009). Multivariable logistic regression, fully adjusted for confounders showed, that pretransplant plasma endotrophin as a continuous variable was independently associated with delayed graft function in both validation cohorts, odds ratio 2.09 [95% CI 1.30–3.36] and 2.06 [95% CI 1.43–2.97]. Pretransplant plasma endotrophin, a potentially modifiable factor, was independently associated with increased risk of delayed graft function and may be a new avenue for therapeutic interventions.
Similar content being viewed by others
Introduction
Delayed graft function (DGF) after kidney transplantation describes the failure of the allograft to operate properly due to ischemia–reperfusion injury and a subsequent inflammatory response1. This complication can be observed in up to 40 percent of deceased donor kidney transplant recipients, prolongs patient hospitalization, increases morbidity and health care costs2,3. DGF is defined by the requirement for dialysis within the first week after transplantation4. A systematic review showed that risk prediction models for DGF commonly include recipient age and comorbidities5. Although several causes and mechanisms leading to DGF have been described in the last years, therapeutic or preventive options are still limited6,7.
Inflammatory mechanisms during ischemia and reperfusion together with immigration of cells, e.g., neutrophils, macrophages, and T lymphocytes, are hallmarks in the pathogenesis of DGF7,8,9. Factors linked to influx of cells into the graft after ischemia reperfusion injury could therefore be new targets for therapy and prevention. One factor that may promote this immigration of cells is endotrophin, which is a Pro-collagen type VI fragment10. Endotrophin is part of the inflammatory response. Endotrophin acts as a chemoattractant on macrophages, aggravates reperfusion injury, and increases inflammation11,12,13,14,15,16. Endotrophin levels were positively correlated with C-reactive protein in patients with chronic kidney disease17. Using a doxycycline-inducible mouse model, overexpression of endotrophin increased the inflammatory response in macrophages13. In vitro, endotrophin stimulated upregulation of proinflammatory genes including Toll-like receptor13.
Recent studies indicated that endotrophin was present in fibrotic kidneys but not in histologically normal kidneys14. A prospective study in patients with type 2 diabetes and microalbuminuria showed that doubling of plasma endotrophin levels significantly increased the risk for progression of kidney disease and deterioration of glomerular filtration rate15. Furthermore, studies in collagen type VI knockout mice showed limited ischemia-induced injury16. Since reperfusion injury is the main contributor to DGF, it is biologically plausible that pretransplant endotrophin may aggravate DGF. Endotrophin, as a potentially modifiable factor, may serve as a new avenue for therapeutic intervention and prevention of DGF.
We investigated whether pretransplant plasma endotrophin in incident kidney transplant recipients was associated with DGF after kidney transplantation.
Materials and methods
Study population and data source
Details about clinical cohorts, study design, and participants, detailed immunosuppression in discovery cohort and validation cohorts, sample collection and measurements of pretransplant plasma endotrophin as well as statistical analyses are given in Supplemental Methods.
In brief, this cohort study included incident kidney transplant recipients in three European transplant centers (Odense University Hospital, Odense, Denmark; University Medical Center Groningen, Groningen, The Netherlands; Aarhus University Hospital, Aarhus, Denmark). Written informed consent was obtained from all patients before entry into the studies. Exclusion criteria were age below 18 years or missing consent.
Details from the Molecular Monitoring after kidney transplantation (MoMoTx) study, the TransplantLines (TxL) Biobank and Cohort study, as well as “CONTEXT” study had been published previously18,19,20,21. The study protocols were in accordance with the ethical standards of the Declarations of Helsinki and Istanbul. Registration identifiers at ClinicalTrials.gov were NCT01515605, NCT03272841, and NCT01395719, respectively. The studies were approved by the local ethics committees (Den Videnskabsetiske Komite for Region Syddanmark, Projekt-ID: 20100098; METc 2014/077; and “CONTEXT” study21, respectively).
The primary outcome variable was DGF which was defined by United Network for Organ Sharing as dialysis within the first week after transplantation22,23. Need for dialysis was considered by the treating physicians according to local guidelines and best medical care after transplantation. Treating physicians were unaware of the pretransplant plasma endotrophin levels. Need for dialysis within the first week after transplantation was confirmed with chart review.
Statistical analysis
Data were analyzed using non-parametric Mann–Whitney test, Fisher’s exact test or chi-square tests as appropriate. We performed receiver operating characteristic (ROC) analysis and determined a cutoff value using Youden index. We also used logistic regression to characterize the association between pretransplant plasma endotrophin, which was included as a continuous variable per increase in standard deviation and DGF. We adjusted for covariates using five models, with cumulative adjustment. All statistical tests were two-sided. Two-sided P-values less than 0.05 were considered to indicate statistical significance.
Results
Baseline characteristics of patients in the “MoMoTx” discovery cohort and determinants of DGF
In the “MoMoTx” discovery cohort we included 257 incident kidney transplant recipients before transplantation, 36 recipients (14%) had ABO-blood-type-incompatible living donors, 106 recipients (41%) had ABO-blood-type-compatible living donors, and 115 recipients (45%) had donation after brain death (DBD).
Table 1 summarizes the demographic and clinical characteristics of renal transplant recipients without and with DGF. In the “MoMoTx” discovery cohort DGF occurred in 32 out of 257 recipients (12%) for the entire group, 4 out of 36 (11%) for ABO-blood-type-incompatible living donor transplants, 11 out of 106 (10%) for ABO-blood-type-compatible living donor transplants, and 17 out of 115 (15%) for DBD. Recipient age, recipient gender, cause of kidney disease, as well as donor age, number of HLA mismatches, and pretransplant plasma creatinine were similar between the groups. Recipients with longer dialysis vintage (months) were more likely to show DGF (Table 1).
Association of pretransplant plasma endotrophin levels and DGF in the “MoMoTx” discovery cohort
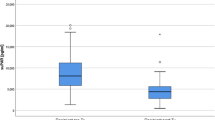
Pretransplant plasma endotrophin levels in kidney transplant recipients are provided in Table 2. The median [interquartile range, IQR] plasma endotrophin in 257 kidney transplant recipients from the “MoMoTx” discovery cohort before transplantation was 40.6 ng/mL [30.8–58.3]. Recipients showing DGF had higher median [IQR] pretransplant plasma endotrophin when compared to recipients without DGF (58.4 ng/mL [33.4–69.0]; N = 32; vs. 39.5 ng/mL [30.6–54.5]; N = 225; P = 0.009 by non-parametric Mann–Whitney test). As shown in Table 2, recipients with ABO-compatible living donor transplantation showing DGF had higher median pretransplant plasma endotrophin when compared to recipients without DGF (68.1 ng/mL [63.4–75.2]; N = 11; vs. 42.4 ng/mL [31.8–60.0]; N = 95; P = 0.0004 by non-parametric Mann–Whitney test).
For the entire “MoMoTx” discovery cohort the Receiver operating characteristic (ROC) analysis indicated that pretransplant plasma endotrophin predict DGF (Area under curve, 0.64 [95% CI 0.53–0.76]; P = 0.010). The cut-off level determined using Youden index was 61.65 ng/mL. 16 out of 51 renal transplant recipients (31%) with endotrophin higher than 61.65 ng/mL had DGF whereas only 16 out of 206 (8%) recipients with endotrophin lower than 61.65 ng/mL had DGF, which yielded an unadjusted odds ratio of 5.43 [95% CI 2.49–11.86]. Sensitivity, specificity, odds ratio, positive predictive value, negative predictive value as well as likelihood ratio are summarized in Table 3. For the entire “MoMoTx” discovery cohort the pretransplant plasma endotrophin cutoff level of 61.65 ng/mL had a negative predictive value of 0.92 [95% CI 0.88–0.95].
Furthermore, we also used pretransplant plasma endotrophin as a continuous variable per increase in standard deviation for logistic regression analyses which yielded an unadjusted odds ratio for DGF of 1.56 [95% CI 1.13–2.17]). We determined the additive impact of pretransplant plasma endotrophin to a model comprising pretransplant age, sex, body-mass index, and dialysis vintage. For the entire group adding pretransplant plasma endotrophin to this clinical model significantly increased net reclassification improvement (0.46 [95% CI 0.10–0.82]; P = 0.01). Spearman correlation of pretransplant plasma endotrophin was -0.18, 0.39, 0.06, and 0.54 for age, dialysis vintage (months), blood pressure, and pretransplant plasma creatinine, respectively. The variance of inflation was 1.22, 1.11, 1.13, and 1.36 for recipient age, dialysis vintage (months), blood pressure, and pretransplant plasma creatinine, respectively, thus precluding multicollinearity.
Baseline characteristics of patients in the “TxL” validation and determinants of DGF
In the “TxL” validation cohort we included 341 incident kidney transplant recipients before transplantation, 25 recipients (7%) had ABO-blood-type-incompatible living donors, 242 recipients (71%) had ABO-blood-type-compatible living donors, 26 recipients (8%) had DBD, and 48 recipients (14%) had donation after circulatory death (DCD).
Table 1 also summarizes the demographic and clinical characteristics of renal transplant recipients without and with DGF.
In the “TxL” validation cohort DGF occurred in 30 out of 341 recipients (9%) for the entire group, 1 out of 25 (4%) for ABO-blood-type-incompatible living donor transplants, 7 out of 242 (3%) for ABO-blood-type-compatible living donor transplants, 4 out of 26 (15%) for DBD, and 18 out of 48 (38%) for DCD. Recipient age, recipient gender, cause of kidney disease, as well as donor age, number of HLA mismatches, and pretransplant plasma creatinine were similar between the groups. Recipients with longer dialysis vintage (months) were more likely to show DGF (Table 1).
Confirmation of findings of pretransplant plasma endotrophin levels and DGF in the “TxL” validation cohort
The median [IQR] plasma endotrophin in 341 kidney transplant recipients from the “TxL” validation cohort before transplantation was 41.3 ng/mL [29.7–60.1] (Table 2). Recipients showing DGF had higher median [IQR] pretransplant plasma endotrophin when compared to recipients without DGF (60.0 ng/mL [41.9–100.9]; N = 30; vs. 40.0 ng/mL [29.0–58.6]; N = 311; P = 0.001 by non-parametric Mann–Whitney test).
As shown in Table 2, recipients with DBD showing DGF had higher median pretransplant plasma endotrophin when compared to recipients without DGF (96.1 ng/mL [81.3–123.6]; N = 4; vs. 55.2 ng/mL [37.8–64.0]; N = 22; P = 0.007 by non-parametric Mann–Whitney test).
We observed a positive correlation between pretransplant plasma endotrophin and C reactive protein in kidney transplant recipients (r = 0.13, p = 0.02).
Now we tested the predictive performance of the cut-off level of 61.65 ng/mL in the “TxL” validation cohort. 14 out of 80 renal transplant recipients (18%) with endotrophin higher than 61.65 ng/mL had DGF whereas only 16 out of 261 (6%) recipients with endotrophin lower than 61.65 ng/mL had DGF, which yielded an unadjusted odds ratio of 3.25 [95% CI 1.51–7.00] (Table 3). For the entire “TxL” validation cohort the pretransplant plasma endotrophin cutoff level of 61.65 ng/mL had a negative predictive value of 0.94 [95% CI 0.92–0.96].
Furthermore, we also used pretransplant plasma endotrophin levels as a continuous variable per increase in standard deviation for logistic regression analyses which yielded an unadjusted odds ratio for DGF of 1.89 [95% CI 1.39–2.56]). The association between pretransplant plasma endotrophin and DGF remained independent of adjustment for age, sex, race, and dialysis vintage (model 2; adjusted odds ratio 1.65; 95% CI 1.16–2.35), further cumulative adjustment for blood pressure and transplant type (model 3; adjusted odds ratio 1.84; 95% CI 1.21–2.80), further cumulative adjustment for pretransplant plasma creatinine (model 4; adjusted odds ratio 2.12; 95% CI 1.33–3.40), and further cumulative adjustment for cold ischemic time (model 5; adjusted odds ratio 2.09; 95% CI 1.30–3.36; Fig. 1). Further cumulative adjustment for diabetes mellitus showed (model 6) an adjusted odds ratio, 2.11; 95% CI 1.31–3.42. In the subgroup with recipients with ABO-compatible living donor transplantation pretransplant plasma endotrophin yielded an odds ratio for DGF of 1.77 [95% CI 1.06–2.96]. In the subgroup with recipients with deceased donors pretransplant plasma endotrophin yielded an odds ratio for DGF of 2.01 [95% CI 1.15–3.52].
Risk of delayed graft function in the two independent validation cohorts, “TxL” and “CONTEXT”. The graphs show the odds ratios and 95% confidence intervals using logistic regression to characterize the association between continuous pretransplant plasma endotrophin per increase in standard deviation and delayed graft function. Model 1 was unadjusted. Model 2 was adjusted for age, sex, race, and dialysis vintage (months). Model 3 was further cumulative adjusted for blood pressure and transplant type. Model 4 was further cumulative adjusted for pretransplant plasma creatinine. Model 5 was further cumulative adjusted for cold ischemic time. Adjustment for “CONTEXT” validation cohort did not include race, dialysis vintage (months), and blood pressure.
Baseline characteristics of patients in the “CONTEXT” validation cohort and determinants of DGF
In the “CONTEXT” validation cohort we included 208 incident kidney transplant recipients before transplantation, 192 recipients (92%) had DBD, and 16 recipients (8%) had DCD.
In the “CONTEXT” validation cohort DGF occurred in 63 out of 208 recipients (30%) for the entire group, 51 out of 192 (27%) for DBD, and 12 out of 16 (75%) for DCD.
Table 1 also summarizes the demographic and clinical characteristics of renal transplant recipients without and with DGF. Recipient age, recipient gender, as well as donor age, number of HLA mismatches, and pretransplant plasma creatinine were similar between the groups (Table 1).
Confirmation of findings of pretransplant plasma endotrophin levels and DGF in the “CONTEXT” validation cohort
The median [IQR] plasma endotrophin in 208 kidney transplant recipients with deceased donors from the “CONTEXT” validation cohort before transplantation was 50.6 ng/mL [36.0–65.5] (Table 2). Recipients showing DGF had higher median [IQR] pretransplant plasma endotrophin when compared to recipients without DGF (56.6 ng/mL [48.4–79.2]; N = 63; vs. 45.3 ng/mL [32.2–60.9]; N = 145; P = 0.0001 by non-parametric Mann–Whitney test).
As shown in Table 2, recipients with DBD showing DGF had higher median pretransplant plasma endotrophin when compared to recipients without DGF (56.1 ng/mL [50.1–77.5]; N = 51; vs. 45.3 ng/mL [32.3–60.7]; N = 141; P = 0.0001 by non-parametric Mann–Whitney test).
Next, we tested the predictive performance of the cut-off level of 61.65 ng/mL in the “CONTEXT” validation cohort. 26 out of 60 renal transplant recipients (43%) with endotrophin higher than 61.65 ng/mL had DGF whereas only 37 out of 148 (25%) recipients with endotrophin lower than 61.65 ng/mL had DGF, which yielded an unadjusted odds ratio of 2.29 [95% CI 1.22–4.32] (Table 3). For the entire “CONTEXT” validation cohort the pretransplant plasma endotrophin cutoff level of 61.65 ng/mL had a negative predictive value of 0.75 [95% CI 0.71–0.79].
Furthermore, we also used pretransplant plasma endotrophin levels as a continuous variable per increase in standard deviation for logistic regression analyses which yielded an unadjusted odds ratio for DGF of 1.88 [95% CI 1.37–2.58]). The association between pretransplant plasma endotrophin and DGF remained independent of adjustment for age and sex (model 2; adjusted odds ratio 1.57; 95% CI 1.11–2.22), further cumulative adjustment for transplant type (model 3; adjusted odds ratio 1.62; 95% CI 1.14–2.31), further cumulative adjustment for pretransplant plasma creatinine (model 4; adjusted odds ratio 1.69; 95% CI 1.14–2.52), and further cumulative adjustment for cold ischemic time (model 5; adjusted odds ratio 2.06; 95% CI 1.43–2.97; Fig. 1).
The “CONTEXT” study indicated that the intervention, i.e., the repetitive inflation and deflation of a cuff around the thigh of the recipient, did not produce any effect on early kidney transplant functions21. In line with these results the association between pretransplant plasma endotrophin and DGF was observed in 107 recipients who had the sham procedure (1.97 [95% CI 1.22–3.17]) as well in 101 recipients who had the intervention (1.83 [95% CI 1.21–2.76]).
Figure 2 shows odds ratios and 95% CI for DGF using logistic regression for several subgroups from the “TxL” and “CONTEXT” validation cohorts. These subgroup analyses showed that pretransplant plasma endotrophin was associated with DGF in male as well as female recipients, and in recipients younger as well as older than 60 years.
The graphs show the odds ratios and 95% confidence intervals (95% CI) for delayed raft function in the validation cohorts “TxL” and “CONTEXT”, using logistic regression to characterize the association between continuous pretransplant plasma endotrophin per increase in standard deviation and delayed graft function according to subgroup in the unadjusted analysis (Model 1). Subgroups included: Age < 60 years; Age ≥ 60 years; Male recipient; Female recipient.
Discussion
We investigated the association between pretransplant endotrophin and DGF after incident kidney transplantation with recipients who obtained living as well as deceased donors. We found that higher pretransplant plasma endotrophin was associated with subsequent DGF in three independent prospective cohorts. Determination of pretransplant plasma endotrophin in recipients may help to reduce delayed graft function after kidney transplant, since higher pretransplant plasma endotrophin levels may be attributed to worse outcome. There is an urgent need to reduce DGF because it is associated with longer hospitalizations and kidney rejection23,24. A systematic review and meta-analysis indicated that compared to patients without DGF, patients with DGF had a 41% increased risk of graft loss at 3.2 years of follow-up24. Tapiawala et al. reported that 23% out of 50,246 kidney transplant recipients showed DGF24. Compared with recipients without DGF, recipients with DGF were significantly more likely to die with a functioning graft25.
Some markers including urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and calprotectin, have been investigated posttransplant to indicate DGF. They are early markers whose levels rise only after kidney injury has occurred26,27,28. Endotrophin can clearly be distinguished from these markers which allow early detection, but not pretransplant association with DGF. Notably, the association between pretransplant plasma endotrophin and DGF persisted independent of adjustment for clinically important covariates, including age, sex, dialysis vintage (months) pretransplant plasma creatinine, and cold ischemic time.
We observed the association of pretransplant plasma endotrophin with DGF in the discovery cohort. It should be noted that recipients’ factors including diabetes as well as donor age were similar in recipients who had DGF and no-DGF. We performed Receiver operator characteristics (ROC) analyses to define a cut-off in the discovery cohort and tested that cut-off value in the validation cohorts. We found consistent results in all cohorts with a broad spectrum of incident kidney transplant recipients. The size of the “TxL” validation cohort was larger, and the number of deceased donor transplants was higher in the “CONTEXT” validation cohort compared with the discovery cohort. Furthermore, using pretransplant plasma endotrophin levels as a continuous variable per increase in standard deviation for logistic regression analyses yielded to fully adjusted odds ratio for DGF of 2.06 and 2.09 in the validation cohorts, respectively. These findings indicate that pretransplant plasma endotrophin is associated with the development of DGF in real-world transplant settings, and that this finding is independently replicable. Notably, our fully adjusted models showed that pretransplant plasma endotrophin levels were associated with DGF, whereas characteristics including age, pretransplant plasma creatinine, and cold ischemic time were not associated with DGF. We observed the association of pretransplant plasma endotrophin with DGF when looking at a clinically relevant cut-off level as well as when using pretransplant plasma endotrophin as a continuous variable per increase in standard deviation.
The primary outcome, DGF, was defined according to well-established criteria of the United Network for Organ Sharing22,23. Notably, the incidence of DGF in the cohorts was between approximately 3% in living donor transplant recipients and approximately 30% in deceased donor transplant recipients, respectively, which is in line with previous reports22 indicating that our cohorts are representative for kidney transplant recipients in general.
In our validation cohorts we showed that higher pretransplant plasma endotrophin was associated with DGF in recipients with DBD grafts, but not with DCD grafts. These findings are in line with results from a recent meta-analysis confirming that DCD grafts per se are much more susceptible to DGF than DBD grafts which is mainly related to the donation process29. That implies that properties of the kidney transplant recipients have less impact in DCD grafts. Furthermore, our study gave strong evidence that properties of the kidney transplant recipients are of major importance with grafts of high quality and best donor processing, i.e., ABO-compatible living donor transplantation.
The present study showed that pretransplant plasma endotrophin was associated with acute allograft function. Furthermore, there is evidence that in patients with type 2 diabetes and microalbuminuria doubling of plasma endotrophin levels increased the risk for progression of kidney disease and deterioration of glomerular filtration rate15. Furthermore, plasma endotrophin was positively correlated with Banff interstitial fibrosis/tubular atrophy (IF/TA) scoring in transplant biopsies30. That may indicate that endotrophin may also be important for long term allograft outcome.
Standard induction therapy in our cohorts included different substances, e.g., basiliximab, anti-thymocyte globulin, and rituximab. Several previous studies indicated that different induction therapies showed similar rates of DGF31,32. Currently it is unknown whether plasma exchange or other therapies which are used for ABO-incompatible living donor transplantation may affect endotrophin levels. However, the present study had overall very small number of events in ABO-incompatible living donor transplantation, therefore future studies are necessary to evaluate further therapeutic opportunities.
Recent publications gave evidence that endotrophin is a potentially modifiable factor. Plasma endotrophin was determined in samples which had been obtained in a multicentre, open-label, randomised trial, which compared the 52-week treatment with the glucagon-like peptide-1 receptor agonist, dulaglutide, to insulin glargine in 329 patients with type 2 diabetes and moderate-to-severe chronic kidney disease33. Compared with insulin glargine, treatment with dulaglutide significantly attenuated the rise of plasma endotrophin33. In an animal model, the administration of the thiazolidinedione, rosiglitazone, to FP365PyMT mice reduced endotrophin transcripts as well as endotrophin proteins in tissue34.
Data availability
Access to data will be granted, on condition that researchers have appropriate ethical permission and sign the appropriate Material Transfer Agreement form.
Abbreviations
- DGF:
-
Delayed graft function
- DBD:
-
Donation after brain death
- DCD:
-
Donation after circulatory death
- Endotrophin:
-
Pro-collagen type VI fragment
- OR:
-
Odds ratio
- 95% CI:
-
95 Percent confidence interval
References
Cooper, J. E. & Wiseman, A. C. Acute kidney injury in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 22, 698–703. https://doi.org/10.1097/MNH.0b013e328365b388 (2013).
Johnston, O. et al. Reduced graft function (with or without dialysis) vs immediate graft function—A comparison of long-term renal allograft survival. Nephrol. Dial. Transplant. 21, 2270–2274. https://doi.org/10.1093/ndt/gfl103 (2006).
Yarlagadda, S. G. et al. Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol. Dial. Transplant. 23, 2995–3003. https://doi.org/10.1093/ndt/gfn158 (2013).
Mallon, D. H., Summers, D. M., Bradley, J. A. & Pettigrew, G. J. Defining delayed graft function after renal transplantation: Simplest is best. Transplantation 96, 885–889. https://doi.org/10.1097/TP.0b013e3182a19348 (2013).
Kabore, R., Haller, M., Harambat, J., Heinze, G. & Leffondre, K. Risk prediction models for graft failure in kidney transplantation: A systematic review. Nephrol. Dial. Transplant. 32(suppl_2), 68–76. https://doi.org/10.1093/ndt/gfw405 (2017).
Wijermars, L. G. M., Schaapherder, A. F., George, T., Sinharoy, P. & Gross, E. R. Association of impaired reactive aldehyde metabolism with delayed graft function in human kidney transplantation. Oxid. Med. Cell Longev. 3704129, 2018. https://doi.org/10.1155/2018/3704129.eCollection (2018).
Siedlecki, A., Irish, W. & Brennan, D. C. Delayed graft function in the kidney transplant. Am. J. Transplant. 11, 2279–2296. https://doi.org/10.1111/j.1600-6143.2011.03754.x (2011).
Nguyen, M. T. J. P. et al. Pretransplant recipient circulating CD4+CD127lo/- tumor necrosis factor receptor 2+ regulatory T cells: A surrogate of regulatory T cell-suppressive function and predictor of delayed and slow graft function after kidney transplantation. Transplantation 100, 314–324. https://doi.org/10.1097/TP.0000000000000942 (2016).
Menon, M. C., Murphy, B. & Heeger, P. S. Moving biomarkers toward clinical implementation in kidney transplantation. J. Am. Soc. Nephrol. 28, 35–747. https://doi.org/10.1681/ASN.2016080858 (2017).
Aigner, T., Hambach, L., Söder, S., Schlötzer-Schrehardt, U. & Pöschl, E. The C5 domain of Col6A3 is cleaved off from the Col6 fibrils immediately after secretion. Biochem. Biophys. Res. Commun. 748, 743–748. https://doi.org/10.1006/bbrc.2001.6227 (2002).
Park, J. & Scherer, P. E. Adipocyte-derived endotrophin promotes malignant tumor progression. J. Clin. Investig. 122, 4243–4256. https://doi.org/10.1172/JCI63930 (2012).
Sun, K. et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 5, 3485. https://doi.org/10.1038/ncomms4485 (2014).
Zhao, Y. et al. Divergent functions of endotrophin on different cell populations in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 311, E952–E963. https://doi.org/10.1152/ajpendo.00314.2016 (2013).
Rasmussen, D. G. K. et al. Urinary endotrophin predicts disease progression in patients with chronic kidney disease. Sci. Rep. 7, 17328. https://doi.org/10.1038/s41598-017-17470-3 (2017).
Rasmussen, D. G. K. et al. Higher collagen VI formation is associated with all-cause mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care 41, 1493–1500. https://doi.org/10.2337/dc17-2392 (2018).
Luther, D. J. et al. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ. Res. 110, 851–856. https://doi.org/10.1161/CIRCRESAHA.111.252734 (2012).
Fenton, A. et al. Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease. PLoS ONE 12, e0175200. https://doi.org/10.1371/journal.pone.0175200 (2017) (eCollection 2017).
Tepel, M., Beck, H. C., Tan, Q., Borst, C. & Rasmussen, L. M. The 82-plex plasma protein signature that predicts increasing inflammation. Sci. Rep. 5, 14882. https://doi.org/10.1038/srep14882 (2015).
Nagarajah, S., Rasmussen, M., Hoegh, S. V. & Tepel, M. Prospective study of long non-coding RNA, MGAT3-AS1, and viremia of BK polyomavirus and cytomegalovirus in living-donor renal transplant recipients. Kidney Int. Rep. 5, 2218–2227. https://doi.org/10.1016/j.ekir.2020.09.005 (2020) (eCollection 2020 Dec).
Eisenga, M. F. et al. Rationale and design of TransplantLines: A prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 8, e024502. https://doi.org/10.1136/bmjopen-2018-024502 (2018).
Krogstrup, N. V. et al. Remote ischemic conditioning on recipients of deceased renal transplants does not improve early graft function: A multicenter randomized, controlled clinical trial. Am. J. Transplant. 17, 1042–1049. https://doi.org/10.1111/ajt.14075 (2017).
Wu, W. K., Famure, O., Li, Y. & Kim, S. J. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 88, 851–858. https://doi.org/10.1038/ki.2015.190 (2015).
Mogulla, M. R., Bhattacharjya, S. & Clayton, P. A. Risk factors for and outcomes of delayed graft function in live donor kidney transplantation—A retrospective study. Transpl. Int. 32, 1151–1160. https://doi.org/10.1111/tri.13472 (2019).
Yarlagadda, S. G., Coca, S. G., Formica, R. N., Poggio, E. D. & Parikh, C. R. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 24, 1039–1047. https://doi.org/10.1093/ndt/gfn667 (2009).
Tapiawala, S. N. et al. Delayed graft function and the risk for death with a functioning graft. J. Am. Soc. Nephrol. 21, 153–161. https://doi.org/10.1681/ASN.2009040412 (2010).
van Timmeren, M. M. et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 84, 1625–1630. https://doi.org/10.1097/01.tp.0000295982.78039.ef (2007).
Hall, I. E. et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J. Am. Soc. Nephrol. 21, 189–197. https://doi.org/10.1681/ASN.2009030264 (2010).
Tepel, M. et al. Urinary calprotectin and posttransplant renal allograft injury. PLoS One 9, e113006. https://doi.org/10.1371/journal.pone.0113006 (2014).
Gavriilidis, P. & Inston, N. G. Recipient and allograft survival following donation after circulatory death versus donation after brain death for renal transplantation: A systematic review and meta-analysis. Transplant. Rev. (Orlando). 34, 100563. https://doi.org/10.1016/j.trre.2020.100563 (2020).
Yepes-Calderón, M. et al. Biopsy-controlled non-invasive quantification of collagen type VI in kidney transplant recipients: A post-hoc analysis of the MECANO trial. J. Clin. Med. 9, 3216. https://doi.org/10.3390/jcm9103216 (2020).
Brennan, D. C., Daller, J. A., Lake, K. D., Cibrik, D. & Del Castillo, D. Thymoglobulin Induction Study Group: Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N. Engl. J. Med. 355, 1967–1977. https://doi.org/10.1056/NEJMoa060068 (2006).
Butler, T. & Hayde, N. Impact of induction therapy on delayed graft function following kidney transplantation in mated kidneys. Transplant. Proc. 49, 1739–1742. https://doi.org/10.1016/j.transproceed.2017.06.032 (2017).
Tuttle, K. R. et al. Dulaglutide improves kidney fibrosis biomarker levels in patients with type 2 diabetes and moderate-to-severe chronic kidney disease. Diabetes 69(Suppl.1), 949-P. https://doi.org/10.2337/db20-949-P (2020).
Park, J., Morley, T. S. & Scherer, P. E. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol. Med. 5, 935–948. https://doi.org/10.1002/emmm.201202006 (2013).
Acknowledgements
Supported by a grant from the European Union, Eurostars (Project E!12850 PRO-C6-Rec), the Innovation Fund Denmark, and the Danish Research Foundation. We thank Mihai Oltean, Sahlgrenska University Hospital, Gothenburg, Sweden, Gertrude J. Nieuwenhuijs-Moeke, University Medical Center Groningen, The Netherlands, and Frank JMF Dor, Erasmus University Medical Center, Rotterdam, The Netherlands, for their contribution to the CONTEXT-study group.
Funding
The funder had no role in the writing of the manuscript or the decision to submit it for publication. The funder had no role in data collection, analysis, or interpretation; trial design; patient recruitment; or any aspect pertinent to the study. There was no payment for writing this article by a pharmaceutical company or other agency.
Author information
Authors and Affiliations
Contributions
M.T., S.J.L.B., O.T., and D.G.K.R. conceived and designed the study. M.T., F.F.A., D.K., S.N., Q.S., N.V.K., N.S. retrieved and validated data. N.S., F.G., M.A.K., and D.G.K.R. planed, supervised, and interpreted endotrophin measurements. M.T., F.F.A., D.K., S.J.L.B., Q.S., R.N., N.S., and D.G.K.R. did the statistical analysis and interpreted the data. M.T., S.J.L.B., and D.G.K.R. wrote the draft of the report. M.T., F.F.A., D.K., S.J.L.B., O.T., S.N., Q.S., S.P.B., J.B., N.V.K., M.B.N., R.N., B.J., N.S., F.G., M.A.K., and D.G.K.R. revised the report for important intellectual content. M.T., S.J.L.B., B.J., and D.G.K.R. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M.T. and F.F.A. contributed equally.
Corresponding author
Ethics declarations
Competing interests
NS, FG, MAK, and DGKR are full-time employees at Nordic Bioscience and FG MAK and DGKR hold stocks. Nordic Bioscience is a privately-owned, small–medium-sized enterprise partly focused on the development of biomarkers and owns the patent for the ELISA used to measure endotrophin levels. No authors received fees, bonuses, or other benefits for the work described in the manuscript, and Nordic Bioscience did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tepel, M., Alkaff, F.F., Kremer, D. et al. Pretransplant endotrophin predicts delayed graft function after kidney transplantation. Sci Rep 12, 4079 (2022). https://doi.org/10.1038/s41598-022-07645-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07645-y
This article is cited by
-
The effects of apoptosis inhibitor of macrophage in kidney diseases
European Journal of Medical Research (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.