Abstract
We investigated the clinical relevance of urinary cytokines/chemokines reflecting intrarenal immunologic micromilieu as prognostic markers and the optimal measurement timing after living donor kidney transplantation (LDKT). This prospective cohort study included 77 LDKT patients who were followed for ≥ 5 years. Patients were divided into control (n = 42) or acute rejection (AR, n = 35) group. Early AR was defined as AR occurring within 3 months. Serum and urine cytokines/chemokines were measured serially as follows: intraoperative, 8/24/72 h, 1 week, 3 months, and 1 year after LDKT. Intrarenal total leukocytes, T cells, and B cells were analyzed with immunohistochemistry followed by tissueFAXS. Urinary MCP-1 and fractalkine were also analyzed in a validation cohort. Urinary MCP-1 after one week was higher in the AR group. Urinary MCP-1, fractalkine, TNF-α, RANTES, and IL-6 after one week were significantly higher in the early AR group. Intrarenal total leukocytes and T cells were elevated in the AR group compared with the control group. Urinary fractalkine, MCP-1, and IL-10 showed positive correlation with intrarenal leukocyte infiltration. Post-KT 1 week urinary MCP-1 showed predictive value in the validation cohort. One-week post-KT urinary MCP-1 may be used as a noninvasive diagnostic marker for predicting AR after LDKT.
Similar content being viewed by others
Introduction
Kidney transplantation (KT) is the final and definitive treatment option for end-stage kidney disease (ESKD) patients. Early prediction of acute rejection (AR) and overall renal allograft function is crucial for appropriate management considering long-term outcome of KT. Although kidney biopsy is the gold standard to diagnose rejection in KT patients1,2,3, it is not convenient or feasible as a follow-up diagnostic tool because of its invasiveness, especially during the immediate postoperative period after KT. Therefore, non-invasive surrogate markers for early diagnosis and treatment of AR are required.
With the advance of molecular biology, a number of biomarkers have been evaluated for prediction, diagnosis, and risk stratification in KT patients. However, there is no consensus regarding specific types of noninvasive biomarkers and timing of sample acquisition in KT patients4. Urinary cytokines and chemokines have been reported as promising biomarkers substituting kidney biopsy because urine was expected to contain inflammatory mediators involved in alloimmune responses leading to AR or interstitial fibrosis/tubular atrophy after KT5,6,7,8. Since the association of pretransplant serum levels of CXCL9 and AR was reported9, urinary CXCL9 at 6 months post-KT was suggested as a promising biomarker in KT patients10. If specific urine cytokines/chemokines can be used as surrogate markers substituting kidney biopsy, especially during the early postoperative period, it would be very helpful for both KT patients and physicians.
In this study, we aimed to evaluate the diagnostic and prognostic potential of urinary cytokines/chemokines at different time points after KT and to identify specific cytokines/chemokines reflecting intrarenal immunologic micromilieu as well as the optimal sample acquisition time for early diagnosis of AR.
Results
Baseline characteristics
Table 1 shows patient baseline characteristics according to the group. Patients’ age ranged from 18 to 69 years (mean: 42.4 years) and 52 patients were male. Three patients received a second KT. A total of 35 patients experienced AR after KT. There was no difference in age or sex between the control and AR groups. Hypertension was more prevalent in the control group while lupus nephritis and autosomal dominant polycystic kidney disease were more prevalent as the underlying disease of ESKD in the AR group. There were no differences in proportions of induction therapy agents and ABO-incompatible KT. The proportions of patients with ABO incompatibility, PRA positivity, and DSA positivity were comparable between the groups.
Changes in renal function for 5 years after KT
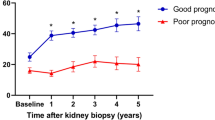
Changes in renal function were assessed as serial changes in eGFR over 5 years. Renal function of the AR group was comparable with the control group until 3 months after KT. The mean eGFR from 2 years after KT was significantly lower in the AR group compared with the control group. (P < 0.05) (Fig. 1).
Serial changes in renal function. The eGFR from 2 years after KT was significantly lower in the AR group compared with the control group. *P < 0.05 compared with the control group. AR acute rejection, eGFR estimated glomerular filtration rate, KT kidney transplantation. Data are shown as mean ± standard error of mean.
Postoperative serial follow-ups of urinary cytokines/chemokines
Urinary level of MCP-1 at 1 week after KT was higher in the AR group (Fig. 2a). To compare the nearest previous value of urinary cytokines/chemokines from when AR was diagnosed, urinary samples at 1 week after KT (1–9 weeks prior to biopsy) from 13 patients who experienced early AR within 3 months and the control group were compared. Urinary fractalkine, MCP-1, RANTES, TNF-α, and IL-6 at 1 week after KT were significantly higher in patients who were diagnosed with early AR within 3 months compared with the control group (Fig. 2b).
Urinary cytokines/chemokines of the control and AR groups. (a) Serial changes in urinary cytokines/chemokines of the control and AR groups. Urinary MCP-1 at 1 week post-KT was significantly higher in the AR group. (b) Post-KT 1-week urinary cytokines/chemokines of the control and early AR (AR within 3 months) groups. The early AR group showed higher urinary fractalkine, MCP-1, RANTES, TNF-α, and IL-6 compared with the control group. *P < 0.05 compared with the control group. AR acute rejection, Cr creatinine, IL interleukin, KT kidney transplantation, MCP-1 monocyte chemoattractant protein-1, RANTES regulated on activation, normal T cell expressed and secreted, TNF-α tumor necrosis factor-α, VEGF vascular endothelial growth factor.
Postoperative serial follow-ups of serum cytokines/chemokines
Serial changes in serum cytokines/chemokines according to AR are shown in Fig. 3. Serum cytokines/chemokines at all time points were comparable between the control and AR groups. Serum cytokines/chemokines at each time point were not correlated with urinary cytokines/chemokines.
Serial changes in serum cytokines/chemokines of the control and AR groups. Serum cytokines/chemokines were comparable in the control and AR groups at each time point for the first year after KT. AR acute rejection, MCP-1 monocyte chemoattractant protein-1, IL interleukin, KT kidney transplantation, RANTES regulated on activation, normal T cell expressed and secreted, TNF-α tumor necrosis factor-α, VEGF vascular endothelial growth factor.
Correlations between urinary cytokines/chemokines and intrarenal leukocyte infiltration
Intrarenal infiltration of total leukocytes, T cells, and B cells was analyzed in renal allograft biopsy tissues using immunohistochemistry with anti-CD45, anti-CD3, and anti-CD20. Total leukocytes, T cells, and B cells were significantly elevated in the AR group (Fig. 4).
Semiquantitative analysis of intrarenal leukocytes infiltration. (a) Intrarenal infiltration of total leukocytes expressing CD45, T cells expressing CD3, and B cells expressing CD20 was compared with immunohistochemistry followed by semiquantitative analysis using tissueFAXS. Arrows indicate CD45-positive leukocytes, CD3-positive T cells, or CD20-positive B cells (×200). (b) The AR group displayed a significantly higher infiltration of total leukocytes, T cells, and B cells compared with the control group. *P < 0.05 compared with the control group. AR acute rejection, CD clusters of differentiation.
Urinary fractalkine, MCP-1, and IL-10 showed positive correlation with total leukocyte infiltration. Urinary excretion of fractalkine, MCP-1, and IL-10 was positively correlated with intrarenal T cells and B cells (Fig. 5).
Correlation between intrarenal leukocytes infiltration and urinary cytokines/chemokines. (a) Urinary fractalkine, MCP-1, and IL-10 showed positive correlation with infiltration of total leukocytes expressing CD45. (b) Urinary fractalkine, MCP-1, and IL-10 showed positive correlation with intrarenal T cells expressing CD3. (c) Urinary fractalkine, MCP-1, and IL-10 showed positive correlation with intrarenal B cells expressing CD20. CD clusters of differentiation, Cr creatinine, IL interleukin, MCP-1 monocyte chemoattractant protein-1.
Potential value of urinary MCP-1 and fractalkine for predicting AR and early AR
Potential predictive value of urinary MCP-1 and fractalkine was further analyzed using sensitivity analysis. The best thresholds for predicting early AR and AR were 1100 pg/mg for urinary MCP-1/creatinine and 300 pg/mg for urinary fractalkine/creatinine. The results of the sensitivity analysis are summarized in Table 2.
Validation of urinary MCP-1 and fractalkine
Internal validation was performed using bootstrapping (bootstrap B = 1000). The area under the receiver operating characteristic (AUROC) of urinary MCP-1 predicting early AR and AR was 0.795 (95% confidence interval (CI) 0.659–0.931) and 0.691 (95% CI 0.563–0.818), respectively (Fig. 6a). AUROC of urinary fractalkine predicting early AR and AR was 0.670 (95% CI 0.485–0.854) and 0.643 (95% CI 0.511–0.776), respectively (Fig. 6b).
Internal validation of post-KT 1 week urinary MCP-1 and fractalkine. (a) AUROC of urinary MCP-1 predicting early AR and AR was 0.795 and 0.691, respectively. (b) AUROC of urinary fractalkine predicting early AR and AR was 0.670 and 0.643, respectively. AR acute rejection, AUROC area under the receiver operating characteristic, CI confidence interval, KT kidney transplantation, MCP-1 monocyte chemoattractant protein-1.
Urinary MCP-1 and fractalkine were further validated using ELISA in an independent cohort (Table 3). Time separate independent cohort included a total of 79 patients with 38 patients in the control group and 41 patients in the AR group. In the AR group, 28 patients had early AR. A logistic regression of AR and early AR on a continuous, normally distributed variable (X) with a sample size of 79 observations achieves 80% power at a 0.05 significance level to detect an odds ratio of 1.96 and 2.03, respectively, when the prevalence of AR and early AR in the population is 0.52 and 0.35, respectively 11. In this independent cohort, post-KT 1 week urinary MCP-1 was significantly higher in the early AR and AR groups than in the control group (Fig. 7a). However, post-KT 1 week urinary fractalkine was comparable in all groups (Fig. 7b).
Post-KT 1 week urinary MCP-1 and fractalkine in a validation cohort. (a) In the validation cohort including 79 patients, both early AR and AR groups showed significantly higher urinary MCP-1 at 1 week after KT than the control group. (b) Post-KT 1 week urinary fractalkine was comparable in both control and AR groups. AR acute rejection, KT kidney transplantation, MCP-1 monocyte chemoattractant protein-1.
Reliability of measurements for triplicates of each sample was analyzed with both variability and absolute agreement using coefficient of variation (CV) and intraclass correlation coefficient (ICC), respectively. MCP-1 and fractalkine showed low variability (CV = 0.181 and 0.091), and high agreement (ICC = 0.972 [0.959, 0.981] and 0.956 [0.932, 0.972]) 12,13, showing good reliability of measurements for both MCP-1 and fractalkine.
Discussion
In this study, the diagnostic and prognostic potentials of several serum and urinary cytokines/chemokines were analyzed with serially collected urine samples after LDKT. Urinary MCP-1 at 1 week after KT was identified as an important predictive factor of AR development. Urinary fractalkine, TNF-α, RANTES, and IL-6 at 1 week after KT showed predictive potential for early AR within 3 months post-KT. Urinary fractalkine, MCP-1, and IL-10 exhibited positive correlation with intrarenal total leukocytes, T cells, and B cells. These results suggest clinical usefulness of urinary cytokines/chemokines for early diagnosis of AR in LDKT patients and support the potential role of urinary cytokines/chemokines as surrogate markers of intrarenal immunologic micromilieu.
AR is a serious problem after KT and exerts significant impacts on both allograft and patient survival. Early diagnosis and treatment of AR are crucial for long-term allograft survival. Although kidney biopsy is the gold standard for assessing kidney injury, timely detection of early alterations in allograft immunologic micromilieu has not been feasible with current techniques14. Therefore, new biomarkers for predicting changes in intrarenal immunologic micromilieu after KT are required15. Ideal biomarkers should be measured reproducibly in noninvasively collected samples. Urine biomarkers are ideal for immune monitoring after KT because they are directly produced by the allograft and can be collected noninvasively and repeatedly. Cytokines and chemokines have been reported to play crucial roles in controlling immune responses within renal allografts16. After KT, proinflammatory cytokines are released from allografts and subsequently induce local chemokine secretion17,18. Based on urinary cytokines/chemokines can reflect overall changes in intrarenal cytokines/chemokines, our study aimed to find the most appropriate timing and types of urinary cytokines/chemokines during the early postoperative period for predicting AR.
In our study, urinary fractalkine, MCP-1, RANTES, TNF-α, and IL-6 at 1 week after KT were significantly higher in patients who developed early AR within 3 months post-KT. Urinary MCP-1 at 1 week post-KT showed the most prominent increment in early AR patients compared with the control group. Elevation of serum proinflammatory cytokines and chemokines depending on allograft status was previously reported in KT patients19,20. MCP-1 and RANTES, important members of the C–C chemokine family, are known to substantially contribute to glomerular and tubulointerstitial damage in diabetic nephropathy through a positive feedback loop of inflammation21. Blocking of the MCP-1/CCR2 chemokine pathway was reported to improve survival of islet allografts22. Intrarenal tissue expression of RANTES and MCP-1 was elevated in KT patients with chronic allograft dysfunction23. Urine MCP-1 was also reported to be elevated in patients with AR24,25 or polyomavirus nephropathy26. However, most previous studies investigated these cytokines/chemokines using a cross-sectional study design. Our results support these previous findings and further reveal the diagnostic potential of urinary MCP-1 as a surrogate biomarker for predicting AR via serial measurement.
Urinary fractalkine at 1 week after KT showed not only diagnostic potential for predicting early AR, but also a positive correlation with intrarenal T cells and B cells in protocol biopsy. Fractalkine and its interaction with receptors on leukocytes mediate chemoattraction and play an important role in recruiting NK cells into cardiac allografts27. Fractalkine expression was significantly higher in the tubular epithelium and endothelium of the allografts in AR patients28. Increased excretion of urinary TNF-α and IL-6 was reported in patients with AR29,30,31. Although our findings were consistent with previous reports and further revealed that excretion of these proinflammatory cytokines/chemokines increased early 1 week after KT in patients at risk for AR, external validation of urinary fractalkine using an independent cohort did not show reproducible results. Further studies in a larger cohort may be required to evaluate the clinical value of urinary fractalkine as a biomarker in LDKT patients.
The activity and subsets of intrarenal leukocytes are known to change actively within renal allografts and create a specific intrarenal immune environment such as renal tissue memory T cells32,33. Increased infiltrating leukocytes into renal allografts and activated intrarenal T cells were reported in AR34. In our study, the AR group showed more enhanced infiltration of total leukocytes, T cells, and B cells into renal allografts. Urinary fractalkine, MCP-1, and IL-10 showed significant correlation with intrarenal infiltration of total leukocytes, T cells, and B cells. A previous study analyzing the mechanisms of AR in a baboon KT model reported a significant correlation between urinary excretion and intrarenal expression of interferon-γ inducible protein-10 and monokine35. Our study supports the clinical usefulness of urinary cytokines/chemokines, especially urinary fractalkine and MCP-1, as surrogate biomarkers that reflect the intrarenal immunologic micromilieu.
A few limitations of this study require consideration. First, there were some time differences in the collection of urine samples and kidney biopsies, ranging from 5 days to 1 month, which was inevitable given the prospective study design and serial collection of urine samples. Second, dividing the raw concentration of urinary cytokines/chemokines by urinary creatinine concentration might not be ideal for correcting for differences in urine concentration. Diverse ranges of proteinuria have been reported in KT patients36,37,38,39, and dividing the raw concentration of urinary cytokines/chemokines by the urinary protein-to-creatinine ratio might be a more appropriate method40. However, correction for urinary concentration differences using urinary creatinine concentration has been widely used in previous studies investigating urine biomarkers41,42, and using the urinary protein-to-creatinine ratio as a denominator can cause other confounding effects because the total amount of urinary protein excretion per se already includes urinary cytokines/chemokines. We also protocolized the procedures for urine collection, storage, and analysis since standardized management of urine samples is critical for consistent results. Third, some discrepancies in the types of cytokines/chemokines showing correlations with AR or intrarenal leukocytes were found. Further studies including a large cohort of KT patients are required to confirm the types of urinary cytokines/chemokines that more precisely reflect both cellular and humoral factors of intrarenal immunologic micromilieu. Fourth, a power study estimation could not be performed in our original cohort because of the prospective design and serial measurements of multiple serum and urine samples. The validation cohort was designed with a power study estimation to overcome this limitation.
In conclusion, urinary MCP-1 at 1 week after KT can be used as a noninvasive surrogate marker for predicting early AR in LDKT patients. Moreover, positive correlation with intrarenal leukocytes infiltration and urinary fractalkine, MCP-1, and IL-10 suggests the clinical usefulness of these urinary cytokines/chemokines as biomarkers that reflect the intrarenal immunologic micromilieu.
Methods
Study design and participants
This prospective cohort study recruited 77 adult patients who underwent living donor KT (LDKT) from April 2011 to August 2013 at Samsung Medical Center and were followed-up for more than 5 years. Adult (≥ 18 years) LDKT patients with no history of cancer whose urine samples were collected at each time point were included in the study. Exclusion criteria were (1) serum samples missing at more than 3 time points, (2) patients with autosomal-dominant polycystic kidney disease who received simultaneous bilateral nephrectomy of native kidneys with LDKT, and (3) significant bleeding or infection during the immediate postoperative period.
Validation cohort for post-KT 1 week urinary MCP-1 and fractalkine included 79 adult patients who underwent LDKT from September 2013 to December 2015. Sample size was determined to achieve 80% power at a 0.05 significance level considering the incidence of AR and early AR 11.
This study was approved by the Institutional Review Board of Samsung Medical Center in compliance with the Declaration of Helsinki (IRB number: 2011-06-117). Informed consent was obtained from all participants before enrollment.
Data collection
Clinical data including baseline characteristics, induction therapy for KT, immunologic risk factors, and serial changes in renal function were collected from the electronic medical record system. Immunologic risk factors included the presence of ABO incompatibility, panel reactive antibodies (PRA), and donor specific antibody (DSA).
Patients who did not experience AR were classified into the control group and those who developed acute cellular rejection were classified as the AR group. Renal allograft biopsy was used as the diagnostic standard and AR was scored according to the Banff criteria2. There were no cases of antibody-mediated rejection. Early AR was defined as AR within 3 months. Renal function was compared with the estimated glomerular filtrate rate (eGFR), which was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Sample collection and cytokine/chemokine assays
Both urine and serum samples were serially collected at the following time points to measure cytokines/chemokines: intraoperative (during KT) and postoperative 8 h, 24 h, 72 h, 1 week, 3 months, and 1 year. The collected urine samples were centrifuged at 2500 rpm for 15 min under − 4 °C. The supernatant of each sample was aliquoted with 1.4 mL of urine and 1.0 mL of serum. After adding 1/100 volume of protease inhibitor and 0.1 M PMSF (Sigma Aldrich, St. Louis, MO, USA) to each sample, all samples were stored at − 70 °C until analysis. A total of 8 cytokines/chemokines were measured in each sample as follows: regulated on activation, normal T cell expressed and secreted (RANTES), fractalkine, interleukin (IL)-4, IL-6, IL-10, monocyte chemoattractant protein (MCP)-1, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF). These 8 cytokines/chemokines were measured using a Milliplex MAP human cytokine/chemokine kit (Merck Millipore, Burlington, MA, USA) according to the manufacturer’s instructions. In the validation cohort, urinary MCP-1 and fractalkine were measured using Quantikine ELISA Kits (R&D Systems, Inc. Minneapolis, MN, USA) according to the manufacturer’s instructions. The concentrations of urinary cytokines/chemokines (pg/mL) were normalized by dividing the raw value by urinary creatinine concentration (mg/dL). Therefore, final values of urinary cytokines/chemokines are expressed as pg/mg.
Quantification of infiltrating immune cells into renal allografts
Renal allograft biopsy was performed between 12 and 14 days after KT as protocol biopsies in 37 patients or when AR was suspected in 9 patients. Immunohistochemical staining with CD45, CD3, and CD20 was performed on formalin-fixed kidney tissues. Sections (4 μm) were deparaffinized with xylene, rehydrated in a graded alcohol series, and then placed in citrate buffer solution (pH 6.0). Slides were placed in a pressure cooker and heated with microwaves for 10 min to enhance antigen retrieval. After cooling, sections were immersed in hydrogen peroxide solution (DAKO, Carpinteria, CA, USA) for 30 min to block endogenous peroxidase and then treated with serum-free protein block (DAKO) at 4 °C overnight. The next day, slides were incubated for 1 h at room temperature with a 1:100 dilution of monoclonal antibody to CD45, CD3, and CD20. After incubation of slides with a mixed solution containing dextran coupled with peroxidase molecules and goat secondary antibody molecules (DAKO) for 30 min at room temperature, 3,3′-diaminobenzidine tetrahydrochloride (DAKO) was applied to the slides to develop a brown color. Then, slides were counterstained with Mayer’s hematoxylin (DAKO). To calculate the percentage of CD45-, CD3-, and CD20-positive cells among total nucleated cells in renal allografts, the whole field of the slide was scanned and analyzed with TissueFAXS. (TissueGnostics, Vienna, Austria)43,44,45.
Statistical methods
All results were expressed as mean ± standard deviation (SD) or standard error of the mean (SEM) as appropriate. SPSS 24.0 statistical software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) were used for statistical analyses. Group means were compared using the Mann–Whitney test. The Chi-square test or Fisher's exact test was used for comparing baseline characteristics between the control and AR groups. The correlations between each cytokine/chemokine and intrarenal leukocytes, and post-KT 1 week urinary and serum MCP-1 or fractalkine were assessed using the Pearson R correlation. Internal validation of post-KT urinary MCP-1 and fractalkine was performed using bootstrapping (bootstrap B = 1000) by calculating AUROC.
Power analysis was performed using PASS software (PASS 2021: v21.0.2, NCSS Statistical Software, Kaysville, UT, USA)11. Reliability of measurements was evaluated with coefficient of variation (CV) and intraclass correlation coefficient (ICC)12,13.
Statistical significance was determined when the P value was < 0.05.
Data availability
The data that support the findings of this study are available on reasonable requests from the corresponding author.
Abbreviations
- AR:
-
Acute rejection
- AUROC:
-
Area under the receiver operating characteristic
- CKD:
-
Chronic kidney disease
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
- DSA:
-
Donor specific antibody
- eGFR:
-
Estimated glomerular filtration rate
- ESKD:
-
End-stage kidney disease
- ICC:
-
Intraclass correlation coefficient
- KT:
-
Kidney transplantation
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- PRA:
-
Panel reactive antibodies
References
Solez, K. I. M. et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int. 44, 411–422 (1993).
Solez, K. et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am. J. Transpl. 8, 753–760 (2008).
Loupy, A. et al. The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transpl. 17, 28–41 (2017).
Faddoul, G. et al. Analysis of biomarkers within the initial 2 years posttransplant and 5-year kidney transplant outcomes: Results from clinical trials in organ transplantation-17. Transplantation 102, 673–680 (2018).
Dallman, M. J. Cytokines and transplantation: Th1/Th2 regulation of the immune response to solid organ transplants in the adult. Curr. Opin. Immunol. 7, 632–638 (1995).
Noronha, I. L. et al. In situ expression of tumor necrosis factor-alpha, interferon-gamma, and interleukin-2 receptors in renal allograft biopsies. Transplantation 54, 1017–1024 (1992).
Hirt-Minkowski, P., De Serres, S. A. & Ho, J. Developing renal allograft surveillance strategies—Urinary biomarkers of cellular rejection. Can. J. Kidney Health Dis. 2, 28 (2015).
Ho, J., Rush, D. N. & Nickerson, P. W. Urinary biomarkers of renal transplant outcome. Curr. Opin. Organ Transpl. 20, 476–481 (2015).
Rotondi, M. et al. High pretransplant serum levels of CXCL9 are associated with increased risk of acute rejection and graft failure in kidney graft recipients. Transpl. Int. 23, 465–475 (2010).
Hricik, D. E. et al. Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am. J. Transpl. 13, 2634–2644 (2013).
Novikov, I., Fund, N. & Freedman, L. S. A modified approach to estimating sample size for simple logistic regression with one continuous covariate. Stat. Med. 29, 97–107 (2010).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163 (2016).
Vorosmarty, C. J., Douglas, E. M., Green, P. A. & Revenga, C. Geospatial indicators of emerging water stress: An application to Africa. Ambio 34, 230–236 (2005).
Furness, P. N. et al. Protocol biopsy of the stable renal transplant: A multicenter study of methods and complication rates. Transplantation 76, 969–973 (2003).
Ali, S. & Sheerin, N. S. Biomarkers of acute injury: Predicting the long-term outcome after transplantation. Kidney Int. 84, 1072–1074 (2013).
Goldfarb-Rumyantzev, A. S. & Naiman, N. Genetic predictors of acute renal transplant rejection. Nephrol. Dial. Transpl. 25, 1039–1047 (2010).
Tatapudi, R. R. et al. Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int. 65, 2390–2397 (2004).
Yamada, K. et al. Prediction of chronic renal allograft dysfunction from evaluations of TGFBeta1 and the renin-angiotensin system. Clin. Exp. Nephrol. 7, 238–242 (2003).
Mota, A. P. et al. Cytokines signatures in short and long-term stable renal transplanted patients. Cytokine 62, 302–309 (2013).
Karczewski, M. et al. Distinct cytokine patterns in different states of kidney allograft function. Transpl. Proc. 41, 4147–4149 (2009).
Wada, T. et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 58, 1492–1499 (2000).
Lee, I. et al. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J. Immunol. 171, 6929–6935 (2003).
Yan, Q. et al. Expression and significance of RANTES and MCP-1 in renal tissue with chronic renal allograft dysfunction. Transpl. Proc. 48, 2034–2039 (2016).
Prodjosudjadi, W. et al. Increased urinary excretion of monocyte chemoattractant protein-1 during acute renal allograft rejection. Nephrol. Dial. Transpl. 11, 1096–1103 (1996).
Dubinski, B. et al. Activated cells in urine and monocyte chemotactic peptide-1 (MCP-1)-sensitive rejection markers in renal graft recipients. Transpl. Immunol. 18, 203–207 (2008).
Boratynska, M. et al. Immunocytological urinalysis and monocyte chemotactic peptide-1 in renal transplant recipients with polyomavirus replication. Transpl. Proc. 38, 151–154 (2006).
Haskell, C. A. et al. Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection. J. Clin. Invest. 108, 679–688 (2001).
Cockwell, P., Chakravorty, S. J., Girdlestone, J. & Savage, C. O. Fractalkine expression in human renal inflammation. J. Pathol. 196, 85–90 (2002).
Smith, S. D., Wheeler, M. A., Lorber, M. I. & Weiss, R. M. Temporal changes of cytokines and nitric oxide products in urine from renal transplant patients. Kidney Int. 58, 829–837 (2000).
Di Paolo, S., Gesualdo, L., Stallone, G., Ranieri, E. & Schena, F. P. Renal expression and urinary concentration of EGF and IL-6 in acutely dysfunctioning kidney transplanted patients. Nephrol. Dial. Transpl. 12, 2687–2693 (1997).
Kaden, J. & Priesterjahn, R. Increasing urinary IL-6 levels announce kidney graft rejection. Transpl. Int. 13(Suppl 1), S34-41 (2000).
Ma, C., Mishra, S., Demel, E. L., Liu, Y. & Zhang, N. TGF-beta controls the formation of kidney-resident T Cells via promoting effector T cell extravasation. J. Immunol. 198, 749–756 (2017).
van Aalderen, M. C. et al. Clinically relevant reactivation of polyomavirus BK (BKPyV) in HLA-A02-positive renal transplant recipients is associated with impaired effector-memory differentiation of BKPyV-specific CD8+ T cells. PLoS Pathog. 12, e1005903 (2016).
Kanzaki, G. & Shimizu, A. Currently available useful immunohistochemical markers of renal pathology for the diagnosis of renal allograft rejection. Nephrology (Carlton) 20(Suppl 2), 9–15 (2015).
Kanmaz, T. et al. Surveillance of acute rejection in baboon renal transplantation by elevation of interferon-gamma inducible protein-10 and monokine induced by interferon-gamma in urine. Transplantation 78, 1002–1007 (2004).
Roodnat, J. I. et al. Proteinuria after renal transplantation affects not only graft survival but also patient survival. Transplantation 72, 438–444 (2001).
Fernandez-Fresnedo, G. et al. The risk of cardiovascular disease associated with proteinuria in renal transplant patients. Transplantation 73, 1345–1348 (2002).
Weiner, D. E. et al. Albuminuria and allograft failure, cardiovascular disease events, and all-cause death in stable kidney transplant recipients: A cohort analysis of the FAVORIT trial. Am. J. Kidney Dis. 73, 51–61 (2019).
Knoll, G. A. Proteinuria in kidney transplant recipients: Prevalence, prognosis, and evidence-based management. Am. J. Kidney Dis. 54, 1131–1144 (2009).
Parikh, C. R. et al. Kidney biomarkers of injury and repair as predictors of contrast-associated AKI: A substudy of the PRESERVE trial. AM. J. Kidney Dis. 75, 187–194 (2020).
Valdimarsson, S., Jodal, U., Barregard, L. & Hansson, S. Urine neutrophil gelatinase-associated lipocalin and other biomarkers in infants with urinary tract infection and in febrile controls. Pediatr. Nephrol. 32, 2079–2087 (2017).
O’Brien, K. M., Upson, K. & Buckley, J. P. Lipid and creatinine adjustment to evaluate health effects of environmental exposures. Curr. Environ. Health Rep. 4, 44–50 (2017).
Kozakowski, N. et al. Monocytes/macrophages in kidney allograft intimal arteritis: No association with markers of humoral rejection or with inferior outcome. Nephrol. Dial. Transpl. 24, 1979–1986 (2009).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Jeon, J. et al. Dietary modification alters the intrarenal immunologic micromilieu and susceptibility to ischemic acute kidney injury. Front. Immunol. 12, 621176 (2021).
Acknowledgements
We thank the staff of the organ transplantation center and the nursing team of Samsung Medical Center, Sungkyunkwan University School of Medicine. We deeply appreciate the great technical support from Ji Hyun Kang, Kyungyi Choi, Ji Woo Kim, and Wu Hyun Lee at the Samsung Biomedical Research Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine. Hye Ryoun Jang was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HC20C0085). Kyungho Lee was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HI19C1337). Wooseong Huh was supported by the Samsung Biomedical Research Institute (Grant number: OTC 1600781) and the National Research Foundation of Republic of Korea (Grant number: 2017R1D1A1B04032172).
Funding
This study was supported by the Samsung Biomedical Research Institute (Grant number: OTC 1600781) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HC20C0085).
Author information
Authors and Affiliations
Contributions
H.R.J. and W.H. conceived and designed the experiments. H.R.J. and M.K. performed the experiments and wrote the manuscript. H.R.J., M.K., S.H., K.L., M.Y.P., K.E.Y., C.J.L., J.J., K.K., and G.Y.K. analyzed the data. K.W.L., J.E.L., J.B.P., Y.G.K., and D.J.K. contributed reagents/materials/analytical tool. All named authors have read the journal’s authorship agreement and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jang, H.R., Kim, M., Hong, S. et al. Early postoperative urinary MCP-1 as a potential biomarker predicting acute rejection in living donor kidney transplantation: a prospective cohort study. Sci Rep 11, 18832 (2021). https://doi.org/10.1038/s41598-021-98135-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98135-0
This article is cited by
-
Renal arterial resistive index, monocyte chemotactic protein 1 and neutrophil gelatinase-associated lipocalin, for predicting acute kidney injury in critically ill cancer patients
International Urology and Nephrology (2023)
-
Clinical value of urinary cytokines/chemokines as prognostic markers in patients with crescentic glomerulonephritis
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.