Abstract
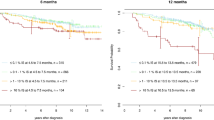
The European Treatment and Outcome Study (EUTOS) population-based registry includes data of all adult patients newly diagnosed with Philadelphia chromosome-positive and/or BCR-ABL1+ chronic myeloid leukemia (CML) in 20 predefined countries and regions of Europe. Registration time ranged from 12 to 60 months between January 2008 and December 2013. Median age was 55 years and median observation time was 29 months. Eighty percent of patients were treated first line with imatinib, and 17% with a second-generation tyrosine kinase inhibitor, mostly according to European LeukemiaNet recommendations. After 12 months, complete cytogenetic remission (CCyR) and major molecular response (MMR) were achieved in 57% and 41% of patients, respectively. Patients with high EUTOS risk scores achieved CCyR and MMR significantly later than patients with low EUTOS risk. Probabilities of overall survival (OS) and progression-free survival for all patients at 12, 24 and 30 months was 97%, 94% and 92%, and 95%, 92% and 90%, respectively. The new EUTOS long-term survival score was validated: the OS of patients differed significantly between the three risk groups. The probability of dying in remission was 1% after 24 months. The current management of patients with tyrosine kinase inhibitors resulted in responses and outcomes in the range reported from clinical trials. These data from a large population-based, patient sample provide a solid benchmark for the evaluation of new treatment policies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chereda B, Melo JV . Natural course and biology of CML. Ann Hematol 2015; 94: 107–121.
Hehlmann R, Hochhaus A, Baccarani M . Chronic myeloid leukaemia. Lancet 2007; 370: 342–350.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood 2013; 122: 872–884.
Hehlmann R, Grimwade D, Simonsson B, Apperley J, Baccarani M, Barbui T et al. The European LeukemiaNet: achievements and perspectives. Haematologica 2011; 96: 156–162.
Hoglund M, Sandin F, Hellstrom K, Bjoreman M, Bjorkholm M, Brune M et al. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood 2013; 122: 1284–1292.
Thielen N, Visser O, Ossenkoppele G, Janssen J . Chronic myeloid leukaemia in The Netherlands: a population-based study on incidence, treatment and survival in 3,585 patients from 1989–2012. Eur J Haematol 2016; 97: 145–154.
Smith AG, Painter D, Howell DA, Evans P, Smith G, Patmore R et al. Determinants of survival in patients with chronic myeloid leukaemia treated in the new era of oral therapy: findings from a UK population-based patient cohort. BMJ Open 2014; 4: e004266.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006; 108: 1809–1820.
Hoffmann VS, Baccarani M, Hasford J, Lindoerfer D, Burgstaller S, Sertic D et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia 2015; 29: 1336–1343.
Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood 2011; 118: 686–692.
Hoffmann VS, Baccarani M, Lindoerfer D, Castagnetti F, Turkina A, Zaritsky A et al. The EUTOS prognostic score: review and validation in 1288 patients with CML treated frontline with imatinib. Leukemia 2013; 27: 2016–2022.
Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016; 30: 48–56.
Kalbfleisch JD, Prentice RL . The Statistical Analysis of Failure Time Data, vol. 36. Wiley: New York, NY, USA, 2011.
Gray RJ . A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia 2015; 29: 1823–1831.
Saussele S, Silver RT . Management of chronic myeloid leukemia in blast crisis. Ann Hematol 2015; 94 (Suppl 2): S159–165.
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004.
Saglio G, Kim D-W, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259.
Hochhaus A, Rosti G, Cross NC, Steegmann JL, le Coutre P, Ossenkoppele G et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia 2016; 30: 57–64.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270.
Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 2012; 119: 1123–1129.
Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol 2011; 29: 1634–1642.
Cortes JE, Kim DW, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicius L et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol 2012; 30: 3486–3492.
Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol 2010; 28: 424–430.
Acknowledgements
For the contribution to patient registration, data collection and follow-up, we thank Hana Klamova, Prague and Olga Cerna, Prague. The contribution of Jiri Mayer, Brno, as a chairman of the Czech Leukemia Study Group for Life, is also acknowledged. For the Camelia and Infinity study groups, very important support was given by Institution of Biostatistics and analyses, Masaryk University, Brno, Czech Republic (Vladimir Dusek, head, Jan Muzik, Zuzana Zbozinkova, Tomas Pavlik). In addition, Barbara Braithwaite, Florence Tarantin and the KROHEM (Croatian Cooperative Group for Hematologic Diseases) are acknowledged for their valuable contributions. We thank Catherine Sodan-Boyer and Gabi Bartsch for For the administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
VSH, MB, and JH receive research funding from Novartis. FC has a consulting role and receives honoraria from Novartis, BMS, ARIAD and Pfizer. FR receives honoraria from BMS and has a consulting role at Novartis. LFC has a consulting role and receives funding for clinical research from Novartis, BMS and Pfizer. AT has a consulting role at Novartis, BMS and Pfizer. DZ receives funding of travel, accommodations or expenses from Novartis and BMS. AZ has a consulting or advisory role at and participated in a speakers’ bureau for Novartis and received research funding from BMS. BS has a consulting or advisory role at BMS. ZS has a consulting or advisory role at Novartis. TS has a consulting role at Novartis, BMS, Adamed and Angelini. RC has had research funding from Novartis, Bristol-Myers-Squibb and Pfizer, and has been on the speakers bureau of Novartis and Pfizer in the past 5 years. AB has participated in a speakers’ bureau and received research support from Novartis Pharma Services/Office in Serbia via the patient assistant support program. AH participated in a speakers’ bureau for Novartis and BMS, receives research funding and funding of travel, accommodations or expenses from Novartis and BMS, and provides expert testimony for Novartis and BMS. LG receives research grants from Novartis. SB receives honoraria from Novartis, Celgene and AOP, has a consulting or advisory role at Novartis and Celgene and receives funding of travel, accommodations or expenses from Novartis and AOP. PK has a consulting or advisory role at GSK, BMS and Novartis, receives funding from Novartis, provides expert testimony for Pfizer and Novartis and receives funding of travel, accommodations or expenses from BMS and Ariad. DL receives research funding from Novartis. SS has received research support from Novartis and BMS and receives honoraria from Novartis, BMS, Ariad and Pfizer. AH has received research funding from Novartis. RH has received research support from Novartis, Consultant for BMS. KI, GSF, DS, JG, SL, IZ, HE, PC, GO and GR have no conflict of financial interests to declare.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Hoffmann, V., Baccarani, M., Hasford, J. et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia 31, 593–601 (2017). https://doi.org/10.1038/leu.2016.246
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.246
This article is cited by
-
Long-term outcomes after upfront second-generation tyrosine kinase inhibitors for chronic myeloid leukemia: managing intolerance and resistance
Leukemia (2024)
-
Pathogenesis and management of accelerated and blast phases of chronic myeloid leukemia
Leukemia (2023)
-
Asciminib as a third line option in chronic myeloid leukemia
International Journal of Hematology (2023)
-
An Update on the Management of Advanced Phase Chronic Myeloid Leukemia
Current Hematologic Malignancy Reports (2023)
-
Mitochondrial Dysfunction in Cardiotoxicity Induced by BCR-ABL1 Tyrosine Kinase Inhibitors -Underlying Mechanisms, Detection, Potential Therapies
Cardiovascular Toxicology (2023)