News & Views |
Featured
-
-
Letter |
 The SCF–FBXW5 E3-ubiquitin ligase is regulated by PLK4 and targets HsSAS-6 to control centrosome duplication
The SCF–FBXW5 E3-ubiquitin ligase is regulated by PLK4 and targets HsSAS-6 to control centrosome duplication
The PLK4 kinase and centrosomal protein HsSAS-6 both regulate centrosome duplication. PLK4 negatively controls an FBXW5-containing ubiquitin ligase, which targets SAS-6 for destruction to restrict centrosome re-duplication.
- Anja Puklowski
- , Yahya Homsi
- & Nisar P. Malek
-
Letter |
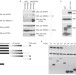 WWP2 is an E3 ubiquitin ligase for PTEN
WWP2 is an E3 ubiquitin ligase for PTEN
The lipid phosphatase and tumour suppressor PTEN is regulated by ubiquitylation. The E3 ligase WWP2/AIP-2 is found to mediate PTEN degradation and is suggested to function as an oncogene.
- Subbareddy Maddika
- , Sridhar Kavela
- & Junjie Chen
-
Letter |
 RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling
RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling
Axin negatively regulates Wnt signalling by promoting degradation of β-catenin. Poly(ADP)-ribosylation of axin induces its degradation to relieve this inhibition. The ubiquitin ligase RNF146 is now shown to recognize and ubiquitylate PARsylated axin2.
- Yue Zhang
- , Shanming Liu
- & Feng Cong
-
Article |
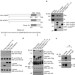 Control of PKA stability and signalling by the RING ligase praja2
Control of PKA stability and signalling by the RING ligase praja2
The activity of protein kinase A (PKA) is regulated by association and dissociation of its regulatory (R) subunits. The E3 ubiquitin ligase praja2 is now shown to ubiquitylate and stimulate the degradation of the R subunits in response to elevated cAMP levels.
- Luca Lignitto
- , Annalisa Carlucci
- & Antonio Feliciello
-
Letter |
 A tetrapyrrole-regulated ubiquitin ligase controls algal nuclear DNA replication
A tetrapyrrole-regulated ubiquitin ligase controls algal nuclear DNA replication
Organelle DNA replication is coupled to nuclear DNA replication in plant cells. In the red alga Cyanidioschyzon merolae, a plastid signalling molecule, Mg-ProtoIX, binds the F-box SCF ubiquitin ligase Fbx3 to inhibit cyclin 1 degradation and thus block nuclear DNA replication.
- Yuki Kobayashi
- , Sousuke Imamura
- & Kan Tanaka
-
Article |
 Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells
Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells
REST transcription factor is a master regulator of neural stem and progenitor cells, which is subjected to ubiquitylation-mediated proteasomal degradation during differentiation. In progenitor cells, degradation is inhibited by the action of the deubiquitylase enzyme HAUSP to prevent untimely neuronal differentiation.
- Zhi Huang
- , Qiulian Wu
- & Shideng Bao
-
Letter |
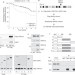 TSPYL5 suppresses p53 levels and function by physical interaction with USP7
TSPYL5 suppresses p53 levels and function by physical interaction with USP7
The genetic locus encompassing TSPYL5 is frequently amplified in breast cancer. TSPYL5 is now shown to repress p53 accumulation by interacting with and inhibiting the p53 de-ubiquitylating enzyme USP7.
- Mirjam T. Epping
- , Lars A.T. Meijer
- & René Bernards
-
Article |
 Fates-shifted is an F-box protein that targets Bicoid for degradation and regulates developmental fate determination in Drosophila embryos
Fates-shifted is an F-box protein that targets Bicoid for degradation and regulates developmental fate determination in Drosophila embryos
A Bicoid gradient drives patterning along the anterior–posterior axis in Drosophila embryos. Degradation of Bicoid by the F-box protein Fsd is shown to be required for the correct gradient profile of bicoid and developmental fate determination.
- Junbo Liu
- & Jun Ma
-
Article |
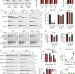 CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity
CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity
A chaperone complex containing CSPα, Hsc70 and SGT binds to monomeric SNAP-25 and prevents its aggregation and degradation. Loss of CSPα inhibits SNARE complex formation.
- Manu Sharma
- , Jacqueline Burré
- & Thomas C. Südhof
-
News & Views |
 Working on a chain: E3s ganging up for ubiquitylation
Working on a chain: E3s ganging up for ubiquitylation
Substrate specificity in ubiquitylation is conferred by ubiquitin ligases (E3s). Now, several ways that E3s can interact to mediate ubiquitylation are illustrated for Ubr1 (a RING finger E3) and Ufd4 (a HECT domain E3), in Saccharomyces cerevisiae. These interactions and the related concept of E4 activity are discussed.
- Meredith B. Metzger
- & Allan M. Weissman
-
Article |
 The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases
The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases
Two pathways in ubiquitylation are linked, as the RING domain E3 ligase Ubr1, from the N-end rule pathway, is found to bind and cooperate with the HECT domain E3 Ufd4 from the ubiquitin-fusion degradation pathway.
- Cheol-Sang Hwang
- , Anna Shemorry
- & Alexander Varshavsky
-
Article |
 Ubiquitylation of the amino terminus of Myc by SCFβ-TrCP antagonizes SCFFbw7-mediated turnover
Ubiquitylation of the amino terminus of Myc by SCFβ-TrCP antagonizes SCFFbw7-mediated turnover
SCFFbw7 mediates the ubiquitylation and degradation of Myc. Now, Myc is also shown to be stabilized by ubquitylation through SCFβ-TrCP. During recovery from S phase arrest, SCFβ-TrCP mediates the addition of a polyubiquitin chain on the Myc amino terminus that is functionally distinct from that formed by Fbw7.
- Nikita Popov
- , Christina Schülein
- & Martin Eilers
-
Perspective |
 Selective autophagy: ubiquitin-mediated recognition and beyond
Selective autophagy: ubiquitin-mediated recognition and beyond
- Claudine Kraft
- , Matthias Peter
- & Kay Hofmann
-
Article |
 Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation
Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation
The significance of autophagy for signal transduction has been unclear. Autophagy negatively regulates Wnt signalling by promoting Dishevelled (Dvl) degradation. The von Hippel-Lindau protein ubiquitylates Dvl to faciliatate its recruitment to autophagosomes through p62.
- Chan Gao
- , Weipeng Cao
- & Ye-Guang Chen
-
Article |
 Interplay between Cdh1 and JNK activity during the cell cycle
Interplay between Cdh1 and JNK activity during the cell cycle
The stress-activated kinase, JNK, is regulated by the anaphase promoting complex (APC) ubiquitin ligase. Conversely, JNK also negatively controls the APC by directly phosphorylating the Cdh1 component of the APC and decreasing its affinity for the APC core subunits.
- Gustavo J. Gutierrez
- , Toshiya Tsuji
- & Ze'ev A. Ronai
-
Letter |
 Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1
Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1
Rho guanine nucleotide dissociation inhibitors (RhoGDIs) bind to inactive Rho GTPases in the cytosol, but their function remains unclear. Several Rho GTPases are now shown to compete for RhoGDI binding and this is crucial for regulating Rho GTPase turnover and activation.
- Etienne Boulter
- , Rafael Garcia-Mata
- & Keith Burridge
-
Letter |
 Shugoshin–PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase
Shugoshin–PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase
Before meiosis II, centromeric sister chromatid cohesion is protected by shugoshin and protein phosphatase 2A, but the identity of the antagonising kinase has remained unclear. Casein kinase 1 is found to phosphorylate cohesin to promote its cleavage during fission yeast meiosis.
- Tadashi Ishiguro
- , Koichi Tanaka
- & Yoshinori Watanabe
-
Letter |
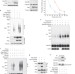 Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A
Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A
The levels of the cell cycle phosphatase Cdc25A are controlled by proteolysis. A de-ubiquitylation enzyme, Dub3, stabilizes Cdc25A, is required for Cdc25A-mediated activation of Cdk1, and may contribute to oncogenic transformation by Cdc25A.
- Yaron Pereg
- , Bob Y. Liu
- & Vishva M. Dixit
-
News & Views |
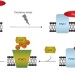 Mitochondria get a Parkin' ticket
Mitochondria get a Parkin' ticket
Recent studies have revealed a prominent role of mitochondrial dysfunction in the development of one of the most common neurodegenerative disorders, Parkinson's disease. The ubiquitin ligase Parkin and the protein kinase PINK1, whose mutations are associated with Parkinson's disease, function in a pathway that links ubiquitylation with selective autophagy of damaged mitochondria.
- Philipp Wild
- & Ivan Dikic
-
Letter |
 Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei
Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei
Whether cohesion-independent forces hold chromosomes together in metaphase is a debated issue. Artificial cleavage of cohesin is sufficient to induce chromosome disjunction in Drosophila syncytical embryos but cdk1 inactivation is required for normal subsequent chromosome separation.
- Raquel A. Oliveira
- , Russell S. Hamilton
- & Kim Nasmyth

 FBXW5 controls centrosome number
FBXW5 controls centrosome number