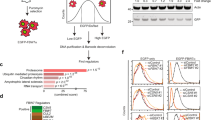
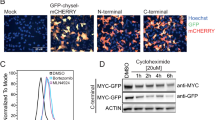
Abstract
The SCFFbw7 ubiquitin ligase mediates growth-factor-regulated turnover of the Myc oncoprotein. Here we show that SCFβ-TrCP binds to Myc by means of a characteristic phosphodegron and ubiquitylates Myc; this results in enhanced Myc stability. SCFFbw7 and SCFβ-TrCP can exert these differential effects through polyubiquitylation of the amino terminus of Myc. Whereas SCFFbw7 with the Cdc34 ubiquitin-conjugating enzyme specifically requires lysine 48 (K48) of ubiquitin, SCFβ-TrCP uses the UbcH5 ubiquitin-conjugating enzyme to form heterotypic polyubiquitin chains on Myc. Ubiquitylation of Myc by SCFβ-TrCP is required for Myc-dependent acceleration of cell cycle progression after release from an arrest in S phase. Therefore, alternative ubiquitylation events at the N terminus can lead to the ubiquitylation-dependent stabilization of Myc.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Levens, D. L. Reconstructing MYC. Genes Dev. 17, 1071–1077 (2003).
Oster, S. K., Ho, C. S., Soucie, E. L. & Penn, L. Z. The myc oncogene: MarvelouslY Complex. Adv. Cancer Res. 84, 81–154 (2002).
Eilers, M. & Eisenman, R. N. Myc's broad reach. Genes Dev. 22, 2755–2766 (2008).
Eilers, M., Schirm, S. & Bishop, J. M. The MYC protein activates transcription of the alpha-prothymosin gene. EMBO J. 10, 133–141 (1991).
Robinson, K., Asawachaicharn, N., Galloway, D. A. & Grandori, C. c-Myc accelerates S-phase and requires WRN to avoid replication stress. PLoS ONE 4, e5951 (2009).
Schorl, C. & Sedivy, J. M. Loss of protooncogene c-Myc function impedes G1 phase progression both before and after the restriction point. Mol. Biol. Cell 14, 823–835 (2003).
Wang, H. et al. c-Myc depletion inhibits proliferation of human tumor cells at various stages of the cell cycle. Oncogene 27, 1905–1915 (2008).
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl Acad. Sci. USA 101, 9085–9090 (2004).
Yada, M. et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 (2004).
Sears, R. et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14, 2501–2514. (2000).
Bhatia, K. et al. Point mutations in the c-Myc transactivation domain are common in Burkitt's lymphoma and mouse plasmacytomas. Nat. Genet. 5, 56–61 (1993).
Moberg, K. H., Bell, D. W., Wahrer, D. C., Haber, D. A. & Hariharan, I. K. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 413, 311–316 (2001).
Kim, S. Y., Herbst, A., Tworkowski, K. A., Salghetti, S. E. & Tansey, W. P. Skp2 regulates Myc protein stability and activity. Mol. Cell 11, 1177–1188 (2003).
von der Lehr, N. et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11, 1189–1200 (2003).
Choi, S. H., Wright, J. B., Gerber, S. A. & Cole, M. D. Myc protein is stabilized by suppression of a novel E3 ligase complex in cancer cells. Genes Dev. 24, 1236–1241 (2010).
Adhikary, S. et al. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 123, 409–421 (2005).
Zhao, X. et al. The N-Myc–DLL3 cascade is suppressed by the ubiquitin ligase Huwe1 to inhibit proliferation and promote neurogenesis in the developing brain. Dev. Cell 17, 210–221 (2009).
Otto, T. et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 15, 67–78 (2009).
Popov, N. et al. The ubiquitin-specific protease USP28 is required for MYC stability. Nat. Cell Biol. 9, 765–774 (2007).
Frescas, D. & Pagano, M. Deregulated proteolysis by the F-box proteins SKP2 and β-TrCP: tipping the scales of cancer. Nat. Rev. Cancer 8, 438–449 (2008).
Winston, J. T. et al. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 (1999).
Hattori, K., Hatakeyama, S., Shirane, M., Matsumoto, M. & Nakayama, K. Molecular dissection of the interactions among IκBα, FWD1, and Skp1 required for ubiquitin-mediated proteolysis of IκBα. J. Biol. Chem. 274, 29641–29647 (1999).
Wu, G. et al. Structure of a β-TrCP1-Skp1-β-catenin complex: destruction motif binding and lysine specificity of the SCFβ-TrCP1 ubiquitin ligase. Mol. Cell 11, 1445–1456 (2003).
Tang, X. et al. Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell 129, 1165–1176 (2007).
Gonen, H. et al. Identification of the ubiquitin carrier proteins, E2s, involved in signal-induced conjugation and subsequent degradation of IκBα. J. Biol. Chem. 274, 14823–14830 (1999).
Verma, R., Feldman, R. M. & Deshaies, R. J. SIC1 is ubiquitinated in vitro by a pathway that requires CDC4, CDC34, and cyclin/CDK activities. Mol. Biol. Cell 8, 1427–1437 (1997).
Kuras, L. et al. Dual regulation of the met4 transcription factor by ubiquitin-dependent degradation and inhibition of promoter recruitment. Mol. Cell 10, 69–80 (2002).
Ikeda, F. & Dikic, I. Atypical ubiquitin chains: new molecular signals. 'Protein Modifications: Beyond the Usual Suspects' review series. EMBO Rep. 9, 536–542 (2008).
Kim, H. T. et al. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J. Biol. Chem. 282, 17375–17386 (2007).
Zaaroor-Regev, D. et al. Regulation of the polycomb protein Ring1B by self-ubiquitination or by E6-AP may have implications to the pathogenesis of Angelman syndrome. Proc. Natl Acad. Sci. USA (2010).
Chakraborty, A. A. & Tansey, W. P. Inference of cell cycle-dependent proteolysis by laser scanning cytometry. Exp. Cell Res. 315, 1772–1778 (2009).
Seki, A., Coppinger, J. A., Jang, C. Y., Yates, J. R. & Fang, G. Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science 320, 1655–1658 (2008).
Macurek, L. et al. Polo-like kinase-1 is activated by Aurora A to promote checkpoint recovery. Nature 455, 119–123 (2008).
Steegmaier, M. et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 17, 316–322 (2007).
Otto, T. et al. Stabilization of N-Myc is a critical function of Aurora-A in human neuroblastoma. Cancer Cell 15, 67–78 (2009).
Petroski, M. D. & Deshaies, R. J. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin-RING ubiquitin-ligase complex SCF-Cdc34. Cell 123, 1107–1120 (2005).
Brzovic, P. S. & Klevit, R. E. Ubiquitin transfer from the E2 perspective: why is UbcH5 so promiscuous? Cell Cycle 5, 2867–2873 (2006).
Brzovic, P. S., Lissounov, A., Christensen, D. E., Hoyt, D. W. & Klevit, R. E. A UbcH5/ubiquitin noncovalent complex is required for processive BRCA1-directed ubiquitination. Mol. Cell 21, 873–880 (2006).
Blanco-Bose, W. E. et al. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology 48, 1302–1311 (2008).
Hann, S. R., Thompson, C. B. & Eisenman, R. E. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. Nature 314, 366–369 (1985).
Herold, S., Herkert, B. & Eilers, M. Facilitating replication under stress: an oncogenic function of MYC? Nat. Rev. Cancer 9, 441–444 (2009).
Trenz, K., Errico, A. & Costanzo, V. Plx1 is required for chromosomal DNA replication under stressful conditions. EMBO J. 27, 876–885 (2008).
Dominguez-Sola, D. et al. Non-transcriptional control of DNA replication by c-Myc. Nature 448, 445–451 (2007).
Koch, H. B. et al. Large-scale identification of c-MYC-associated proteins using a combined TAP/MudPIT approach. Cell Cycle 6, 205–217 (2007).
Peschiaroli, A. et al. SCFβ-TrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol. Cell 23, 319–329 (2006).
Mailand, N., Bekker-Jensen, S., Bartek, J. & Lukas, J. Destruction of Claspin by SCFβ-TrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol. Cell 23, 307–318 (2006).
Herold, S. et al. Miz1 and HectH9 regulate the stability of the checkpoint protein, TopBP1. EMBO J. 27, 2851–2861 (2008).
Fong, A. & Sun, S. C. Genetic evidence for the essential role of β-transducin repeat-containing protein in the inducible processing of NF-κB2/p100. J. Biol. Chem. 277, 22111–22114 (2002).
DiRenzo, J. et al. Growth factor requirements and basal phenotype of an immortalized mammary epithelial cell line. Cancer Res. 62, 89–98 (2002).
Acknowledgements
We thank Markus Welcker and Bruce Clurman for expression vectors encoding Fbw7 and β-TrCP2; Victoria Cowling for IMECs; and Axel Behrens for anti-Fbw7 antibody. This study was supported by grants from the Deutsche Forschungsgemeinschaft through the Transregio 17 ('Ras-dependent pathways in human tumours') project and the Wilhelm Sander Stiftung für Krebsforschung.
Author information
Authors and Affiliations
Contributions
N.P., C.S. and L.A.J. performed the experimental work. N.P. and M.E. planned the experiments. M.E. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1564 kb)
Rights and permissions
About this article
Cite this article
Popov, N., Schülein, C., Jaenicke, L. et al. Ubiquitylation of the amino terminus of Myc by SCFβ-TrCP antagonizes SCFFbw7-mediated turnover. Nat Cell Biol 12, 973–981 (2010). https://doi.org/10.1038/ncb2104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2104
This article is cited by
-
New insights in ubiquitin-dependent Wnt receptor regulation in tumorigenesis
In Vitro Cellular & Developmental Biology - Animal (2024)
-
O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N6-methyladenosine-dependent manner
Signal Transduction and Targeted Therapy (2023)
-
Targeting the MYC interaction network in B-cell lymphoma via histone deacetylase 6 inhibition
Oncogene (2022)
-
Disruption of male fertility-critical Dcaf17 dysregulates mouse testis transcriptome
Scientific Reports (2022)
-
Ubiquitin-specific peptidase 37: an important cog in the oncogenic machinery of cancerous cells
Journal of Experimental & Clinical Cancer Research (2021)