Abstract
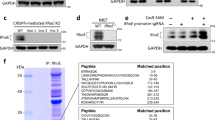
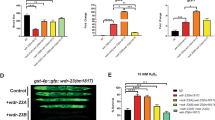
At steady state, most Rho GTPases are bound in the cytosol to Rho guanine nucleotide dissociation inhibitors (RhoGDIs)1. RhoGDIs have generally been considered to hold Rho proteins passively in an inactive state within the cytoplasm. Here we describe an evolutionarily conserved mechanism by which RhoGDI1 controls the homeostasis of Rho proteins in eukaryotic cells. We found that depletion of RhoGDI1 promotes misfolding and degradation of the cytosolic geranylgeranylated pool of Rho GTPases while activating the remaining membrane-bound fraction. Because RhoGDI1 levels are limiting, and Rho proteins compete for binding to RhoGDI1, overexpression of an exogenous Rho GTPase displaces endogenous Rho proteins bound to RhoGDI1, inducing their degradation and inactivation. These results raise important questions about the conclusions drawn from studies that manipulate Rho protein levels. In many cases the response observed may arise not simply from the overexpression itself but from additional effects on the levels and activity of other Rho GTPases as a result of competition for binding to RhoGDI1; this may require a re-evaluation of previously published studies that rely exclusively on these techniques.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
DerMardirossian, C. & Bokoch, G. M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 (2005).
Dransart, E., Morin, A., Cherfils, J. & Olofsson, B. Uncoupling of inhibitory and shuttling functions of rho GDP dissociation inhibitors. J. Biol. Chem. 280, 4674–4683 (2005).
Gorovoy, M. et al. RhoGDI-1 modulation of the activity of monomeric RhoGTPase RhoA regulates endothelial barrier function in mouse lungs. Circ. Res. 101, 50–58 (2007).
Abe, M., Qadota, H., Hirata, A. & Ohya, Y. Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae. J. Cell Biol. 162, 85–97 (2003).
Tong, Z. et al. Adjacent positioning of cellular structures enabled by a Cdc42 GTPase-activating protein-mediated zone of inhibition. J. Cell Biol. 179, 1375–1384 (2007).
Togawa, A. et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIα. Oncogene 18, 5373–5380 (1999).
Shibata, S. et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nature Med. 14, 1370–1376 (2008).
Bielek, H., Anselmo, A. & Dermardirossian, C. Morphological and proliferative abnormalities in renal mesangial cells lacking RhoGDI. Cell Signal. 21, 1974–1983 (2009).
Tiedje, C., Sakwa, I., Just, U. & Hofken, T. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol. Biol. Cell 19, 2885–2896 (2008).
Winter-Vann, A. M. & Casey, P. J. Post-prenylation-processing enzymes as new targets in oncogenesis. Nature Rev. Cancer 5, 405–412 (2005).
Doye, A. et al. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111, 553–564 (2002).
Vega, F. M. & Ridley, A. J. SnapShot: Rho family GTPases. Cell 129, 1430 (2007).
Shao, F. et al. Biochemical characterization of the Yersinia YopT protease: cleavage site and recognition elements in Rho GTPases. Proc. Natl Acad. Sci. USA 100, 904–909 (2003).
Young, J. C., Agashe, V. R., Siegers, K. & Hartl, F. U. Pathways of chaperone-mediated protein folding in the cytosol. Nature Rev. Mol. Cell Biol. 5, 781–791 (2004).
Michaelson, D. et al. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152, 111–126 (2001).
Hart, M. J. et al. A GDP dissociation inhibitor that serves as a GTPase inhibitor for the Ras-like protein CDC42Hs. Science 258, 812–815 (1992).
Ueda, T., Kikuchi, A., Ohga, N., Yamamoto, J. & Takai, Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J. Biol. Chem. 265, 9373–9380 (1990).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Guthrie, C., Fink, G., Simon, M. I. & Abelson, J. N. Guide to yeast genetics and molecular biology. Methods Enzymol. 194, 1–863 (1991).
Adamo, J. E. et al. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 155, 581–592 (2001).
Garcia-Mata, R. et al. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 406, 425–437 (2006).
Wennerberg, K. et al. RhoG signals in parallel with Rac1 and Cdc42. J. Biol. Chem. 277, 47810–47817 (2002).
Arthur, W. T., Noren, N. K. & Burridge, K. Regulation of Rho family GTPases by cell–cell and cell–matrix adhesion. Biol. Res. 35, 239–246 (2002).
van Buul, J. D. et al. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J. Cell Biol. 178, 1279–1293 (2007).
Huesken, D. et al. Design of a genome-wide siRNA library using an artificial neural network. Nature Biotechnol. 23, 995–1001 (2005).
Vouret-Craviari, V., Boulter, E., Grall, D., Matthews, C. & Van Obberghen-Schilling, E. ILK is required for the assembly of matrix-forming adhesions and capillary morphogenesis in endothelial cells. J. Cell Sci. 117, 4559–4569 (2004).
Bagrodia, S., Taylor, S. J., Jordon, K. A., Van Aelst, L. & Cerione, R. A. A novel regulator of p21-activated kinases. J. Biol. Chem. 273, 23633–23636 (1998).
Ren, X. D., Kiosses, W. B. & Schwartz, M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 (1999).
Acknowledgements
We thank Lisa Sharek for her technical support, and Channing Der, Jim Bear and Chloé Féral for comments. This study was supported by National Institutes of Health Grants GM029860 (to K.B.) and GM054712 (to P.B.), a Department of Defense Breast Cancer Predoctoral Fellowship (BC051092 to A.D.), a Susan Komen Foundation Postdoctoral Fellowship and a AHA Beginning Grant in Aid (5-40078 to R.G.M.) and a Fondation pour la Recherche Médicale Fellowship (to E.B.), an AHA Postdoctoral Fellowship (0825333E to E.B.) and an Allocation INSERM InCa/AVENIR (R08227AS to E.B.).
Author information
Authors and Affiliations
Contributions
E.B. and R.G.M. designed, performed experiments and wrote the manuscript. C.G. and A.D. helped with experimental design and procedures. G.R. and P.B. designed and performed the experiments in S. cerevisiae. K.B. directed the project and revised the manuscript. All authors provided detailed comments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1262 kb)
Supplementary Information
Supplementary Movie 1 (AVI 11723 kb)
Rights and permissions
About this article
Cite this article
Boulter, E., Garcia-Mata, R., Guilluy, C. et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol 12, 477–483 (2010). https://doi.org/10.1038/ncb2049
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2049
This article is cited by
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
NF-κB signaling in neoplastic transition from epithelial to mesenchymal phenotype
Cell Communication and Signaling (2023)
-
Mutant p53 murine oviductal epithelial cells induce progression of high-grade serous carcinoma and are most sensitive to simvastatin therapy in vitro and in vivo
Journal of Ovarian Research (2023)
-
Low-dose radiation induces unstable gene expression in developing human iPSC-derived retinal ganglion organoids
Scientific Reports (2023)
-
RhoGDIα regulates spermatogenesis through Rac1/cofilin/F-actin signaling
Communications Biology (2023)