Article
|
Open Access
Featured
-
-
News & Views |
 Interlocked polyynes towards stable carbynes
Interlocked polyynes towards stable carbynes
Interlocking unstable motifs is a useful way to enhance their stability through shielding protection. Now, stable interlocked polyynes bearing several macrocycles have been prepared, including a [5]rotaxane having 34 contiguous alkynes with properties similar to those of carbyne.
- Adrian Saura-Sanmartin
-
Article
| Open Access Masked alkynes for synthesis of threaded carbon chains
Masked alkynes for synthesis of threaded carbon chains
Long polyynes have fascinating properties but they are difficult to synthesize as a consequence of their high reactivity. Now, it has been shown that cobalt carbonyl complexes can be used as masked alkyne equivalents, enabling the preparation of stable polyyne polyrotaxanes with up to 34 contiguous triple bonds.
- Connor W. Patrick
- , Yueze Gao
- & Harry L. Anderson
-
Article |
 A catenane that is topologically achiral despite being composed of oriented rings
A catenane that is topologically achiral despite being composed of oriented rings
Catenanes are topologically non-trivial and, perhaps for this reason, molecules composed of two oriented rings have always simply been referred to as ‘topologically chiral’. Now it has been shown that the same stereogenic unit can arise in systems whose stereochemistry is Euclidean, suggesting a need to rethink this fundamental form of mechanical chirality.
- Noel Pairault
- , Federica Rizzi
- & Stephen M. Goldup
-
News & Views |
 Interlocked structures on active duty
Interlocked structures on active duty
Interlocking macrocyclic carbon nanomaterials is an exciting way to tune their molecular properties, but all-conjugated catenanes and rotaxanes are extremely challenging to make. Now, fully π-conjugated [2]- and [3]catenanes as well as a [3]rotaxane have been prepared through an ‘active metal template’ approach.
- Satyajit Das
- & Fredrik Schaufelberger
-
Article
| Open Access Dimeric and trimeric catenation of giant chiral [8 + 12] imine cubes driven by weak supramolecular interactions
Dimeric and trimeric catenation of giant chiral [8 + 12] imine cubes driven by weak supramolecular interactions
Interlocked shape-persistent organic cages are rare structures and the majority are formed using π-stacking as the driving force. Now it is shown that weak dispersion interactions—which are modulated by changing the 1,4-substituents of the constituent dialdehyde linkers—can be used to form interlocked dimeric and trimeric catenated cages.
- Bahiru Punja Benke
- , Tobias Kirschbaum
- & Michael Mastalerz
-
Article |
 Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes
Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes
Despite mechanically axially chiral (MAC) catenanes being recognized in 1961, their stereoselective synthesis had not been disclosed until now. Closer inspection of the MAC stereogenic unit has also led to the identification of an analogous, but unremarked upon, form of rotaxane stereochemistry and the conceptualization of a general approach to prepare MAC molecules stereoselectively.
- John R. J. Maynard
- , Peter Gallagher
- & Stephen M. Goldup
-
News & Views |
 When push comes to shove
When push comes to shove
The adsorption of molecules onto a surface from solution generally proceeds spontaneously by means of an equilibrium process. Now, it has been shown that macrocycles can be pumped onto a MOF substrate through the formation of mechanical bonds in a ratcheting mechanism that results in an out-of-equilibrium state.
- Liang Zhang
-
Article |
 A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes
A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes
The synthesis of chiral interlocked molecules in which the mechanical bond provides the only source of stereochemistry remains challenging. Now, a chiral interlocked auxiliary approach to mechanically planar chiral rotaxanes has been developed and its potential demonstrated through the synthesis of a range of difficult targets with high enantioselectivity.
- Alberto de Juan
- , David Lozano
- & Stephen M. Goldup
-
News & Views |
 Tying peptide ropes
Tying peptide ropes
Although the natural lasso peptide microcin J25 remains an elusive target for total chemical synthesis itself, this topologically non-trivial building block has now been used to construct a range of interlocked molecular architectures including rotaxanes, catenanes and daisy chains.
- Jan H. van Maarseveen
-
Article |
 Dynamic covalent self-assembly of mechanically interlocked molecules solely made from peptides
Dynamic covalent self-assembly of mechanically interlocked molecules solely made from peptides
The construction of mechanically interlocked molecules solely made from peptides is a great synthetic challenge because of a lack of effective templating strategies. Now it has been shown that by combining self-assembly and dynamic covalent chemistry, catenanes, daisy chains and other interlocked peptides can be synthesized from genetically engineered building blocks.
- Hendrik V. Schröder
- , Yi Zhang
- & A. James Link
-
News & Views |
 A ravel alliance
A ravel alliance
A new class of interwoven metal–organic containers, including one with a cubic architecture, twelve crossing points and a large internal volume, has now been reported. Interconversion between different self-assembled structures can be triggered by simply exchanging the associated anions.
- Andrew W. Heard
- , Natasha M. A. Speakman
- & Jonathan R. Nitschke
-
News & Views |
 Untangling knotty problems
Untangling knotty problems
The synthesis of molecular knots has been a major achievement in the field of chemical topology, but only a few relatively simple ones have been made so far. A route based on a weaving approach has now been used to make a seven-crossing knot and could offer a route to more complicated structures.
- Dan Preston
- & Paul E. Kruger
-
Article |
 A molecular endless (74) knot
A molecular endless (74) knot
A combination of metal- and anion-template synthesis directs the weaving of molecular weft and warp strands in the assembly of a 3 × 3 interwoven grid. Connection of the ligand strands by alkene metathesis produces the topology of a seven-crossing endless knot, an important cultural and religious symbol.
- David A. Leigh
- , Jonathan J. Danon
- & Steffen L. Woltering
-
Article |
 Knotting a molecular strand can invert macroscopic effects of chirality
Knotting a molecular strand can invert macroscopic effects of chirality
Reversible nanoscale knotting and unknotting of a molecular strand can be used to control the handedness of helical organizations at macroscopic length scales. Dopant knotted and unknotted strands induce supramolecular helical structures of opposite handedness in achiral liquid crystals, and the left- and right-handed forms can be switched in situ.
- Nathalie Katsonis
- , Federico Lancia
- & Fredrik Schaufelberger
-
News & Views |
 Chirality makes a move
Chirality makes a move
Interlocked molecules can exhibit chiral stereogenic elements that are not found in covalently bound systems. Now, the shuttling of the ring in a [2]rotaxane has been shown to result in enantiomeric co-conformations that selectively bind chiral guests.
- Ellen M. G. Jamieson
- & Stephen M. Goldup
-
Article |
 Braiding, branching and chiral amplification of nanofibres in supramolecular gels
Braiding, branching and chiral amplification of nanofibres in supramolecular gels
Helical structures play important roles in biological processes, yet their aggregation into fibres—which can in turn form gels—is poorly understood. Now, the self-assembly of a linear pentakis (urea) peptidomimetic compound into helices that further intertwine into well-defined braided structures has been described and analysed through braid theory. Homochiral gels may be formed by exposing the precursor sol to a chiral material.
- Christopher D. Jones
- , Henry T. D. Simmons
- & Jonathan W. Steed
-
News & Views |
 What tangled webs we weave
What tangled webs we weave
Knots have been rigorously studied since the 1860s, but only in the past 30 years have they been made in the laboratory in molecular form. Now, the most complex small-molecule examples so far — a composite knot and an isomeric link, each with nine crossings — have been prepared.
- Edward E. Fenlon
-
Article |
 Stereoselective synthesis of a composite knot with nine crossings
Stereoselective synthesis of a composite knot with nine crossings
A composite knot with nine crossings of the same handedness has been prepared from a hexameric circular helicate in 41% yield in a two-step synthesis. An isomeric cyclic [3]catenane topologically constrained to always have at least three twists within the links is also formed. Both topologies have a high degree of writhe, analogous to that of supercoiled DNA.
- Liang Zhang
- , Alexander J. Stephens
- & David A. Leigh
-
Article |
 Ring-through-ring molecular shuttling in a saturated [3]rotaxane
Ring-through-ring molecular shuttling in a saturated [3]rotaxane
Controlled motion in mechanically interlocked molecules, such as a macrocycle moving back and forth along the axle of a rotaxane, forms the basis of complex functions in molecular machinery. Now, ring-through-ring shuttling has been achieved using two macrocycles that switch position between two anchoring sites, which involves the smaller ring passing through the larger one.
- Kelong Zhu
- , Giorgio Baggi
- & Stephen J. Loeb
-
News & Views |
 Supramolecular basketry
Supramolecular basketry
Both the topology and the mechanical strength of woven materials have inspired great synthetic efforts to replicate their structures at the nanoscale. Now, a triaxial weave has been prepared by self-assembly of a judiciously designed organic molecule through π–π and CH–π interactions.
- Yi Liu
-
Article |
 A triaxial supramolecular weave
A triaxial supramolecular weave
Woven topologies endow macroscopic objects with mechanical stability, but their molecular counterparts have remained difficult to prepare. Now, an extended triaxial supramolecular weave has been formed by the self-assembly of a judiciously shaped organic building block — a rigid oligoproline segment featuring two perylene-monoimide moieties — through π–π stacking and CH–π interactions.
- Urszula Lewandowska
- , Wojciech Zajaczkowski
- & Helma Wennemers
-
News & Views |
 Moving into another dimension
Moving into another dimension
Molecular daisy-chain structures are typically made up of two interlocked components and can exhibit muscle-like contraction and extension in one dimension. Zinc-based multicomponent systems that can operate in two and three dimensions have now been designed and synthesized.
- Karine Fournel-Marotte
- & Frédéric Coutrot
-
Article |
 Mechanically interlocked daisy-chain-like structures as multidimensional molecular muscles
Mechanically interlocked daisy-chain-like structures as multidimensional molecular muscles
By exploiting structural rigidity, coordination geometries and bond rotational barriers that disfavour the formation of smaller homologues, molecular switches based on [c3] and [c4]daisy chains have been assembled selectively; they display muscle-like motion in multiple dimensions with changes in length of approximately 23% and 36%, respectively.
- Jia-Cheng Chang
- , Shin-Han Tseng
- & Sheng-Hsien Chiu
-
Article |
 Creating complex molecular topologies by configuring DNA four-way junctions
Creating complex molecular topologies by configuring DNA four-way junctions
The synthesis of topologically non-trivial compounds requires the manipulation of molecular recognition with an extraordinarily high level of control. Now, DNA four-way junctions have been configured to construct synthetic DNA knots and links, which can then be used to investigate important DNA-processing enzymes.
- Di Liu
- , Gang Chen
- & Yossi Weizmann
-
News & Views |
 A chiral catalyst with a ring to it
A chiral catalyst with a ring to it
A chiral [2]rotaxane in which the asymmetry is derived from the way in which the two components are mechanically interlocked — rather than being encoded in the covalent connectivity of the components themselves — has been shown to act as an enantioselective organocatalyst.
- Stephen M. Goldup
-
News & Views |
 A rotaxane with the golden touch
A rotaxane with the golden touch
The catalytic activity of a rotaxane incorporating a gold(I) centre can be switched on by the addition of a guest ion that can bind inside the macrocyclic cavity of the system. The nature of the guest can also influence the selectivity of the catalyst, reminiscent of allosteric modulation in enzymes.
- Ai-Lan Lee
-
News & Views |
 Shuttling in the solid state
Shuttling in the solid state
Incorporating mechanically interlocked molecular shuttles within a metal–organic framework that has enough free space in the crystal lattice to permit volume-conserving translational motion sets the stage for defect-free molecular-electronic device fabrication and more.
- Mark A. Olson
-
Article |
 A molecular shuttle that operates inside a metal–organic framework
A molecular shuttle that operates inside a metal–organic framework
The piston-like, translational motion of a molecular shuttle — a process that is fundamental to many mechanically interlocked molecular switches and machines — has now been demonstrated to occur inside the highly organized and dense structure (containing approximately 1021 shuttles per cm3) of a metal–organic framework material.
- Kelong Zhu
- , Christopher A. O'Keefe
- & Stephen J. Loeb
-
Article |
 An infinite chainmail of M6L6 metallacycles featuring multiple Borromean links
An infinite chainmail of M6L6 metallacycles featuring multiple Borromean links
Metallacycles made up of six copper ions and six cyclotriguaiacylene-based ligands form a unique topological entanglement in the solid state. Individual metallacycles are interwoven into an infinite 2D chainmail network where each one forms multiple Borromean-ring-like associations with its neighbours. Crystals of the complex grow in an unusual tubular morphology.
- Flora L. Thorp-Greenwood
- , Alexander N. Kulak
- & Michaele J. Hardie
-
News & Views |
 The I's have it
The I's have it
Rotaxanes with cyclodextrin end groups have been used as a platform to investigate anion binding in water, revealing that halogen bonding can serve as the basis for molecular recognition in aqueous solvents, which may have implications in medicinal chemistry and beyond.
- Mark S. Taylor
-
Article |
 Halogen bonding in water results in enhanced anion recognition in acyclic and rotaxane hosts
Halogen bonding in water results in enhanced anion recognition in acyclic and rotaxane hosts
The ability to achieve strong molecular recognition in water is a key challenge for supramolecular chemistry. Now, halogen bonding — the attractive interaction between an electron-deficient halogen atom and a Lewis base — has been shown to be superior to hydrogen bonding for strong anion binding in water. Ripple image: © PhotoDisc/Getty Images.
- Matthew J. Langton
- , Sean W. Robinson
- & Paul D. Beer
-
News & Views |
 Star-crossed self-assembly
Star-crossed self-assembly
Interwoven supramolecular structures are often held up as examples of beauty in chemistry, but these assemblies can be fragile depending on the environments they are exposed to. Post-assembly covalent modification can, however, trap them in robust molecular form, and a triply entwined [2]catenane is one of the most sophisticated examples so far.
- Guido H. Clever
-
Article |
 A Star of David catenane
A Star of David catenane
The Star of David topology is an iconic symbol that has been used in religious and cultural contexts for thousands of years. Now it is assembled in molecular form through a hexameric circular helicate generated by six tris(bipyridine) ligands entwined about six iron(II) cations. The structure of the two triply-entwined 114-membered rings is revealed by X-ray crystallography.
- David A. Leigh
- , Robin G. Pritchard
- & Alexander J. Stephens
-
-
News & Views |
 A star is born
A star is born
Macrocycles have been a mainstay of supramolecular chemistry since its beginnings. The latest addition to this rank of host compounds is the result of a simple and high-yielding one-step method that produces a star-shaped macrocycle able to bind anionic guests — and has the potential for generating a wide range of anion-responsive structures.
- James A. Wisner
-
Article |
 A pentagonal cyanostar macrocycle with cyanostilbene CH donors binds anions and forms dialkylphosphate [3]rotaxanes
A pentagonal cyanostar macrocycle with cyanostilbene CH donors binds anions and forms dialkylphosphate [3]rotaxanes
Macrocycles are key compounds in supramolecular chemistry, yet their efficient preparation is an ever present challenge. Now, it has been shown that a C5-symmetric macrocycle, termed ‘cyanostar’, can be formed in high yields on multigram scales in one pot. Cyanostars form unusually strong sandwich complexes with large and weakly coordinating anions and can template the formation of a dialkylphosphate [3]rotaxane.
- Semin Lee
- , Chun-Hsing Chen
- & Amar H. Flood
-
-
News & Views |
 A molecular production line
A molecular production line
A small molecule that mimics the sequence-specific peptide synthesis of nature's ribosomes paves the way for more elaborate artificial molecular synthesizers.
- Paul R. McGonigal
- & J. Fraser Stoddart
-
-
Article |
 Metal–organic frameworks with dynamic interlocked components
Metal–organic frameworks with dynamic interlocked components
The dynamics of mechanically interlocked molecules such as catenanes and rotaxanes have been studied in solution as examples of rudimentary molecular switches and machines. A metal–organic framework with a [2]rotaxane as a building block demonstrates that such dynamic processes can also operate inside a solid-state material.
- V. Nicholas Vukotic
- , Kristopher J. Harris
- & Stephen J. Loeb
-
-
News & Views |
 One-pot pentaknot
One-pot pentaknot
The most complex non-DNA synthetic molecular knot so far has been made in a single step by combining a number of reversible chemical interactions, including metal-directed self-assembly, anion templation and imine bond formation.
- Michaele J. Hardie
-
Article |
 Redox-responsive molecular helices with highly condensed π-clouds
Redox-responsive molecular helices with highly condensed π-clouds
Phenylene oligomers are shown to form left- and right-handed helices in solution, but a chiral symmetry-breaking process occurs on crystallization to give a non-racemic mixture of crystals that each contains only one enantiomer. One-electron oxidation of the oligomers suppresses the interconversion of the mirror-image helices in solution, locking-in one conformation and leading to chiral memory effects.
- Eisuke Ohta
- , Hiroyasu Sato
- & Takuzo Aida
-
News & Views |
 Linking rings without templates
Linking rings without templates
Interlocking molecules in solution usually requires recognition motifs that direct the assembly of the building blocks. Triply interlocked catenanes have now been constructed just relying on the interpenetration of structures typical of the solid state and slow reversible covalent bond formation.
- Jonathan E. Beves
- & David A. Leigh
-
Perspective |
 Robust dynamics
Robust dynamics
A new concept termed 'robust dynamics' is presented as the intellectual centerpiece to the union between metal–organic frameworks (MOFs) and mechanically interlocking molecules. Robust dynamics allows highly flexible entities, which are bound covalently to MOF backbones, to carry out repeated movements without affecting the integrity of the overall structure.
- Hexiang Deng
- , Mark A. Olson
- & Omar M. Yaghi
-
Article |
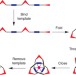 Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template
Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template
The synthesis of interlocked compounds such as catenanes and rotaxanes has undergone much development in recent years, but molecular knots are still relatively hard to make. It has now been shown that a linear bipyridine oligomer can fold around a single zinc-ion template to form a complex that can be cyclized to give a molecular trefoil knot.
- Jun Guo
- , Paul C. Mayers
- & Christopher A. Hunter
-
News & Views |
 Radical attraction of like charges
Radical attraction of like charges
Although it may seem counter-intuitive, the attraction between positively charged radical ions offers a new approach to driving controlled motion in molecular machines.
- Harry L. Anderson

 Mechanical scission of a knotted polymer
Mechanical scission of a knotted polymer Triple-clipped links
Triple-clipped links Tangled tetrahedra
Tangled tetrahedra Protecting polyynes
Protecting polyynes Switching spin
Switching spin