Featured
-
-
News & Views |
 Copper catalysed asymmetric amination
Copper catalysed asymmetric amination
Chiral amines possessing a stereogenic carbon atom bearing three carbon substituents and one nitrogen substituent are challenging structural motifs to prepare enantioselectively. Now, such motifs have been accessed in high enantiopurities by asymmetric Cu-catalysed propargylic amination using sterically confined ligands.
- Joshua D. Sieber
-
Article |
 Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioenriched α-disubstituted α-ethynylamines are valuable synthons to chiral α-tertiary amines and azacycles, but their facile access remains challenging. Now, sterically confined pyridinebisoxazoline ligands have been developed to facilitate highly enantioselective Cu(I)-catalysed propargylic amination of both aliphatic and aryl ketone-derived propargylic carbonates to give α-tertiary ethynylamines. Related tandem sequences are reported to synthesize quaternary azacycles.
- Zheng Zhang
- , Ying Sun
- & Jian Zhou
-
Article
| Open Access Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Achieving selectivity control in allylic arylations is a long-standing challenge in catalysis. Now a rhodium-catalysed system demonstrates chemo-, regio- and enantioselectivity, enabling Suzuki–Miyaura-type arylation with racemic, non-symmetrical, acyclic allylic systems; chelation is speculated to facilitate oxidative addition and enable both enantiomers of the starting material to converge onto a single product.
- Violeta Stojalnikova
- , Stephen J. Webster
- & Stephen P. Fletcher
-
News & Views |
 Sulfur stereochemistry takes centre stage
Sulfur stereochemistry takes centre stage
Directional interactions and three-dimensional functional groups are crucial to medicinal compounds. Consequently, new functional groups require stereocontrolled synthetic methods. Now, an enantiopure building block provides controlled and divergent access to valuable sulfonimidoyl functional groups.
- James A. Bull
-
Article |
 Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent
Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent
The stereochemical control and bifunctional manipulation of chiral sulfur functional groups is a long-standing challenge. Now, an enantiopure bench-stable S(VI) fluoride exchange reagent enables the asymmetric synthesis of sulfoximines, sulfonimidamides and sulfonimidoyl fluorides. The bifunctional nature of this reagent provides a practical method for the introduction of S(VI) functionality.
- Shun Teng
- , Zachary P. Shultz
- & Justin M. Lopchuk
-
News & Views |
 Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Tetracoordinate boron molecules are the key intermediates in many organoboron-related chemical transformations. Now, using alkynyl tetracoordinate boron species, a nickel-catalysed asymmetric 1,3-metallate shift towards axial chirality has been developed, giving access to various axially chiral alkenes in high efficiency.
- Li-Qing Ren
- & Chuan He
-
Article |
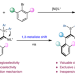 Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Enantioselective transformations based on tetracoordinate borons are elusive. Now, an enantioselective nickel-catalysed strategy for the construction of axially chiral alkenes has been reported, via a 1,3-metallate shift of alkynyl tetracoordinate boron species. The reaction uses readily accessible starting materials and a cheap transition-metal catalyst, and the chemoselectivity, regioselectivity and atroposelectivity could be well controlled.
- Xingxing Ma
- , Mengwei Tan
- & Qiuling Song
-
News & Views |
 An acid for an acid
An acid for an acid
Stereoselective decarboxylative protonation can produce diverse chiral molecules from widely available carboxylic acids. However, general and practical strategies are lacking. Now, a chiral spirocyclic phosphoric acid-catalysed decarboxylation of aminomalonic acids has enabled the modular synthesis of α-amino acids.
- Xufeng Lin
- & Alemayehu Gashaw Woldegiorgis
-
Article |
 Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Cobalt(II) complexes of porphyrins have dominated the development of metalloradical catalysts. Now it has been shown that five-coordinate iron(III) complexes of porphyrins with an axial ligand are also potent metalloradical catalysts for olefin cyclopropanation. They are shown to react with different classes of diazo compounds via a stepwise radical mechanism.
- Wan-Chen Cindy Lee
- , Duo-Sheng Wang
- & X. Peter Zhang
-
Article |
 Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Methods for the direct construction of 1,3-disubstituted planar chiral ferrocenes are elusive. Now, a modular platform enables the construction of planar chirality in 1,3-disubstituted ferrocenes/ruthenocenes via enantioselective relay remote C–H arylation. The strategy involves an initial enantiodetermining ortho-C‒H activation enabled by a Pd(II)/chiral amino-acid ligand, followed by relay to the remote meta-position by a bridgehead-substituted norbornene mediator.
- Lan Zhou
- , Hong-Gang Cheng
- & Qianghui Zhou
-
News & Views |
 Chiral sulfinyls from sulfones
Chiral sulfinyls from sulfones
The selective removal of one oxygen atom from sulfones, without over-reduction to sulfide, is a challenging task. Now, through organocatalysis and incorporation of a cyano group into the sulfone, an asymmetric deoxygenation strategy has been developed, providing an efficient method for the synthesis of chiral sulfinyl compounds.
- Elżbieta Wojaczyńska
- & Jacek Wojaczyński
-
Article |
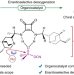 Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
The direct conversion of sulfones to chiral sulfinyl compounds is one of the major challenges in the fields of asymmetric synthesis and organosulfur chemistry. Now, through incorporation of a cyano group into the sulfone, an organocatalytic asymmetric deoxygenation strategy has been developed that enables the synthesis of chiral sulfinyl compounds.
- Shengli Huang
- , Zhen Zeng
- & Hailong Yan
-
Article |
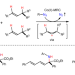 Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Controlling various selectivities in radical reactions presents both formidable challenges and great opportunities. Now, Co(II)-based metalloradical catalysis has enabled the concurrent control of multiple convergences and selectivities in intermolecular radical allylic C−H amination. The reaction provides access to valuable chiral α-tertiary amines directly from an isomeric mixture of alkenes.
- Pan Xu
- , Jingjing Xie
- & X. Peter Zhang
-
Article |
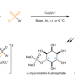 Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Heteroatom–heteroatom cross-couplings have so far remained elusive. Now, a copper-catalysed enantioselective S–O cross-coupling of diverse diols or triols with sulfonyl chlorides has been realized via a single-electron reductive elimination manifold. The reaction provides access to value-added chiral C3 building blocks and inositol phosphates through enantioselective desymmetrization of biomass-derived alcohols.
- Yong-Feng Cheng
- , Zhang-Long Yu
- & Xin-Yuan Liu
-
Article |
 Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage
Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage
The enantioselective functionalization of C60 is highly challenging, typically requiring complex chiral tethers or demanding chromatography. Fullerenes have now been shown to undergo Diels–Alder reactions in a chemo-, regio- and enantio-selective fashion through confinement within an enantiopure metal–organic cage functionalized with a chiral formylpyridine group.
- Zifei Lu
- , Tanya K. Ronson
- & Jonathan R. Nitschke
-
Article |
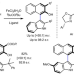 Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Direct oxidative methods for the enantioselective synthesis of heterobiaryl compounds that exhibit axial chirality remain elusive. Now, the use of an iron catalyst in the presence of a chiral PyBOX ligand and an oxidant enables the direct coupling of naphthols and indoles with high levels of enantio- and cross-selectivity.
- Richard R. Surgenor
- , Xiangqian Liu
- & Martin D. Smith
-
Article |
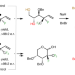 Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Methods to access organofluorine compounds with a trifluoromethyl- and fluoro-substituted carbon stereogenic centre are severely limited. Now, a flexible and stereodivergent approach has been developed for the efficient preparation of homoallylic alcohols containing this moiety. Conversion to polyfluoro furanosides and pyranosides has been demonstrated, which is relevant to antiviral drug development.
- Shibo Xu
- , Juan del Pozo
- & Amir H. Hoveyda
-
Article |
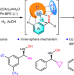 Nickel-catalysed asymmetric hydrogenation of oximes
Nickel-catalysed asymmetric hydrogenation of oximes
The asymmetric hydrogenation of oximes to chiral hydroxylamines is a long-standing challenge because of the labile N–O bond and inert C=N bond. Now, it has been shown that this reaction can be catalysed with a chiral nickel complex, and the weak interactions between catalyst and substrate are found to play a crucial role.
- Bowen Li
- , Jianzhong Chen
- & Wanbin Zhang
-
Article |
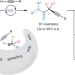 Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
A general copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of diverse racemic tertiary alkyl halides with terminal alkynes has been developed, forging all-carbon quaternary stereocentres. Key to the success is the rational design of chiral anionic N,N,N-ligands tailor-made for the computationally predicted outer-sphere radical group transfer pathway.
- Fu-Li Wang
- , Chang-Jiang Yang
- & Xin-Yuan Liu
-
Article |
 Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
A straightforward method for synthesizing optically active α-amino acids from abundant carboxylic acids has been developed. Based on a nitrene-mediated stereocontrolled 1,3-nitrogen shift, this approach provides access to a large variety of unnatural α-amino acids with aryl, allyl, propargyl and alkyl side chains and enables late-stage amination of carboxylic-acid-containing drugs.
- Chen-Xi Ye
- , Xiang Shen
- & Eric Meggers
-
Article |
 A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes
A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes
The synthesis of chiral interlocked molecules in which the mechanical bond provides the only source of stereochemistry remains challenging. Now, a chiral interlocked auxiliary approach to mechanically planar chiral rotaxanes has been developed and its potential demonstrated through the synthesis of a range of difficult targets with high enantioselectivity.
- Alberto de Juan
- , David Lozano
- & Stephen M. Goldup
-
Article |
 A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes
A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes
Complex substituted cyclobutanes are common motifs in bioactive molecules but are difficult to make enantioselectively. Now, it has been shown that cross-coupling reactions between cyclobutenes and arylboronic acids—initiated by Rh-catalysed asymmetric carbometallation—can provide access to a diverse range of chiral cyclobutanes.
- F. Wieland Goetzke
- , Alexander M. L. Hell
- & Stephen P. Fletcher
-
News & Views |
 The gains from breaking symmetry
The gains from breaking symmetry
All-carbon quaternary centres are challenging targets in organic synthesis. Now, the development of a zinc-catalysed desymmetrization method enables the synthesis of chiral alcohols with all-carbon quaternary centres by the selective reduction of symmetrical α,α-disubstituted malonates.
- Jadwiga Gajewy
- & Marcin Kwit
-
Article |
 Catalytic reductive desymmetrization of malonic esters
Catalytic reductive desymmetrization of malonic esters
The desymmetrization of easily accessible malonic esters represents an attractive approach towards the formation of chiral quaternary stereocentres, but is largely limited to enzymatic hydrolysis. Now, a zinc-catalysed asymmetric hydrosilylation reaction—that works with a broad scope of substrates—has been shown to reduce one of the esters to a primary alcohol with excellent enantiocontrol.
- Pengwei Xu
- & Zhongxing Huang
-
Article |
 Concise, scalable and enantioselective total synthesis of prostaglandins
Concise, scalable and enantioselective total synthesis of prostaglandins
Current methods for the synthesis of prostaglandins suffer from low yields and lengthy steps. Now, a strategy for their enantioselective synthesis has been developed with rhodium-catalysed enyne cycloisomerization as the key step. This concise route was scaled up, enabling the preparation of fluprostenol on a 20-gram scale.
- Fuhao Zhang
- , Jingwen Zeng
- & Xumu Zhang
-
Article |
 Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions
Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions
Using readily accessible tropones and γ-methylidene-δ-valerolactones, the divergent synthesis of three classes of challenging [5.5.0] or [4.4.1] bicyclic systems has been achieved—with high efficiency and stereoselectivity—through Pd-catalysed higher-order cycloaddition. Mechanistic studies and density functional theory calculations indicate that the divergent reactions arise from the different reactivity of two diastereomeric intermediates.
- Li-Cheng Yang
- , Ya-Nong Wang
- & Yu Zhao
-
Article |
 Time-dependent enantiodivergent synthesis via sequential kinetic resolution
Time-dependent enantiodivergent synthesis via sequential kinetic resolution
Access to both enantiomers of a chiral target compound typically relies on reversing the absolute configuration of the chiral component in the reaction system that is used to make them. A time-dependent enantiodivergent synthesis is reported in which the same enantiomer of a chiral catalyst can give both enantiomers of the product, depending on the reaction time.
- Hang-Fei Tu
- , Pusu Yang
- & Shu-Li You
-
News & Views |
 Teaching an old ligand new tricks
Teaching an old ligand new tricks
Tertiary amines are poor directing groups for C(sp3)–H activation using Pdii catalysts due to favourable β-hydride elimination pathways. Now, an N-acetyl amino acid ligand is shown to shut down this deleterious pathway, enabling facile arylation of a highly medicinally relevant group of compounds.
- Vinod G. Landge
- & Michael C. Young
-
Article |
 Catalytic C(sp3)–H bond activation in tertiary alkylamines
Catalytic C(sp3)–H bond activation in tertiary alkylamines
A practical and selective palladium-catalysed process for C(sp3)–H arylation of tertiary alkylamines is enabled by the use of a simple amino acid-derived ligand. The reaction combines a range of multifaceted tertiary alkylamines with readily available aryl-boronic acids to form γ-aryl tertiary alkylamines and can also be performed enantioselectively.
- Jesus Rodrigalvarez
- , Manuel Nappi
- & Matthew J. Gaunt
-
Article |
 A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling
A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling
Asymmetric Sonogashira C(sp3)–C(sp) couplings provide complementary approaches to established C(sp3)–C(sp2/sp3) couplings for chiral C–C bond formation; however, relatively few reactions have been developed. Now, a versatile, enantioconvergent Sonogashira coupling via a radical intermediate has been developed. The approach uses a copper catalyst featuring a multidentate electron-rich cinchona alkaloid-derived ligand.
- Xiao-Yang Dong
- , Yu-Feng Zhang
- & Xin-Yuan Liu
-
Article |
 Enantioselective construction of remote tertiary carbon–fluorine bonds
Enantioselective construction of remote tertiary carbon–fluorine bonds
Although methods exist to construct secondary stereocentres containing both a C–F and C–H bond, the ability to form a tertiary C–F bond, remote from pre-existing activating groups, remains challenging. Now, C–F tertiary, benzylic stereocentres have been constructed through a Pd-catalysed enantioselective Heck reaction of acyclic alkenyl fluorides with arylboronic acids.
- Jianbo Liu
- , Qianjia Yuan
- & Matthew S. Sigman
-
Thesis |
 Double vision
Double vision
Michelle Francl wonders if a molecule can be just a little bit chiral?
- Michelle Francl
-
News & Views |
 Easy as one, two, three
Easy as one, two, three
The preparation of three-dimensional frameworks with multiple stereocentres from simple acyclic hydrocarbons represents a challenging transformation. Now, starting from simple and readily available reagents, formation of these complex targets can be achieved in just three catalytic transformations with high levels of stereocontrol.
- Laura Castoldi
- & Vittorio Pace
-
Article |
 Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals
Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals
Although samarium-mediated cyclizations have the potential to generate significant molecular complexity, historically it has not proven possible to exert enantiocontrol through the use of a chiral ligand in complex product synthesis. Now, an enantioselective SmI2-mediated radical cyclization has been developed using a chiral aminodiol ligand. Desymmetrizing 5-exo ketyl-alkene cyclizations and cyclization cascades of unsaturated ketoesters deliver complex products and typically proceed with high enantioselectivity and diastereoselectivity.
- Nicolas Kern
- , Mateusz P. Plesniak
- & David J. Procter
-
Article |
 Catalyst-controlled oligomerization for the collective synthesis of polypyrroloindoline natural products
Catalyst-controlled oligomerization for the collective synthesis of polypyrroloindoline natural products
The collective synthesis of several oligomeric polypyrroloindoline natural products, including hodgkinsine, hodgkinsine B, idiospermuline, quadrigemine H and isopsychotridines B and C, is accomplished through the iterative action of an asymmetric small molecule copper catalyst. This strategy also enables the synthesis of putatively unnatural quadrigemine H-type isomers.
- Christopher R. Jamison
- , Joseph J. Badillo
- & David W. C. MacMillan
-
-
Article |
 Asymmetric triplex metallohelices with high and selective activity against cancer cells
Asymmetric triplex metallohelices with high and selective activity against cancer cells
Water-soluble metallohelices containing an antiparallel head-to-head-to-tail arrangement of strands are reported. This amphipathic functional topology is akin to that of host-defence peptides. The metallohelices show high and selective toxicity to a cancer cell line, causing dramatic changes in the cell cycle without DNA damage. In contrast, there is no significant toxicity to MRSA and Escherichia coli.
- Alan D. Faulkner
- , Rebecca A. Kaner
- & Peter Scott
-
News & Views |
 A radical revolution in synthesis
A radical revolution in synthesis
A newly designed thiol catalyst for radical cyclization reactions is the result of a long and storied battle to control the reactivity of carbon-centred radicals.
- Andrew F. Parsons
-
Article |
 An organic thiyl radical catalyst for enantioselective cyclization
An organic thiyl radical catalyst for enantioselective cyclization
The ability of thiyl radicals to promote reactions has been known for decades although its extension to asymmetric catalysis has only rarely been explored. Now, an organic thiyl radical catalyst with a carefully structured chiral pocket has been designed as a means to achieve highly enantioselective radical cyclizations.
- Takuya Hashimoto
- , Yu Kawamata
- & Keiji Maruoka
-
News & Views |
 Rethinking cross-coupling
Rethinking cross-coupling
The palladium-catalysed cross-coupling of aryl- or alkenylboronates and aryl halides has proved phenomenally successful for the formation of Csp2–Csp2 bonds. Now, an alternative non-transition-metal-mediated coupling using similar reactants has been reported for the stereo-controlled formation of Csp2–Csp3 bonds.
- Ho-Yan Sun
- & Dennis G. Hall
-
News & Views |
 Cascades of catalytic selectivity
Cascades of catalytic selectivity
A combination of catalytic asymmetric diboration of terminal alkenes and Suzuki–Miyaura cross-coupling has been exploited in the synthesis of a variety of important medicinal agents. The process overcomes a number of problems in the application of these important catalytic processes.
- Rian D. Dewhurst
- & Todd B. Marder
-
Article |
 Rapid assembly of complex cyclopentanes employing chiral, α,β-unsaturated acylammonium intermediates
Rapid assembly of complex cyclopentanes employing chiral, α,β-unsaturated acylammonium intermediates
Despite their appearance in a number of bioactive natural products, the synthesis of 5-membered carbocycles has received much less attention than synthesis of their 6-membered counterparts. Here, a Michael-aldol-β-lactonization cascade is used to forge two C-C bonds, one C-O bond, two rings and up to three contiguous stereocentres and deliver complex cyclopentanes with high levels of relative and absolute stereocontrol.
- Gang Liu
- , Morgan E. Shirley
- & Daniel Romo
-
News & Views |
 Exploiting intramolecularity
Exploiting intramolecularity
Selective reaction of one alcohol among many in complex molecules can be achieved by the use of a catalyst that forms a single covalent bond to a nearby functional group.
- André M. Beauchemin
-
Article |
 Enantioselective synthesis of tatanans A–C and reinvestigation of their glucokinase-activating properties
Enantioselective synthesis of tatanans A–C and reinvestigation of their glucokinase-activating properties
Tatanans A, B and C, members of a family of complex sesquilignan natural products, were recently reported to possess potent anti-diabetic, glucokinase-activating properties. Here, a convergent enantioselective total synthesis of these tatanans enabled by catalytic allylic dearomatization is described. Contrary to previous reports, biological assays show that tatanans A–C are not allosteric activators of glucokinase.
- Qing Xiao
- , Jeffrey J. Jackson
- & Armen Zakarian
-
Article |
 Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes
Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes
Substituted allenes with axial chirality are of great utility in organic chemistry owing to their unique structure and reactivity, but synthetic methods to access them are limited. Here, a catalytic asymmetric synthesis of tetrasubstituted allenes is described that builds on the use of phase-transfer-catalysed asymmetric functionalization of 1-alkylallene-1,3-dicarboxylates.
- Takuya Hashimoto
- , Kazuki Sakata
- & Keiji Maruoka
-
News & Views |
 The power of pairing
The power of pairing
Supramolecular catalysts that combine an anionic chiral scaffold, a cationic coordinating structure and a metal centre have been shown to be highly effective for asymmetric synthesis. The success opens a new avenue for the design of new catalysts with a wide variety of chiral environments.
- Hajime Ito
-
Article |
 Engineering methylaspartate ammonia lyase for the asymmetric synthesis of unnatural amino acids
Engineering methylaspartate ammonia lyase for the asymmetric synthesis of unnatural amino acids
Substituted aspartic acids are highly valuable as tools for biological research and as chiral building blocks for pharmaceuticals. Here, engineering of the enzyme methylaspartate ammonia lyase to accept a large variety of substituted amines and fumarates and catalyse the asymmetric synthesis of aspartic acid derivatives is described.
- Hans Raj
- , Wiktor Szymański
- & Gerrit J. Poelarends
-
Article |
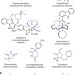 Enantioselective construction of quaternary N-heterocycles by palladium-catalysed decarboxylative allylic alkylation of lactams
Enantioselective construction of quaternary N-heterocycles by palladium-catalysed decarboxylative allylic alkylation of lactams
Nitrogen-containing heterocycles are ubiquitous in natural products, pharmaceuticals and in materials science. Here, the stereoselective synthesis of a wide array of structurally diverse, functionalized lactams by palladium-catalysed enantioselective enolate alkylation is described.
- Douglas C. Behenna
- , Yiyang Liu
- & Brian M. Stoltz
-

 Enantioselective construction of stereogenic-at-sulfur(IV) centres via catalytic acyl transfer sulfinylation
Enantioselective construction of stereogenic-at-sulfur(IV) centres via catalytic acyl transfer sulfinylation Back in the ring
Back in the ring