Abstract
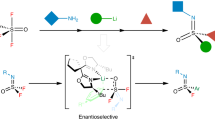
An increased interest to expand three-dimensional chemical space for the design of new materials and medicines has created a demand for isosteric replacement groups of commonly used molecular functionality. The structural and chemical properties of chiral S(VI) functional groups provide unique spatial and electronic features compared with their achiral sulfur- and carbon-based counterparts. Manipulation of the S(VI) centre to introduce structural variation with stereochemical control has remained a synthetic challenge. The stability of sulfonimidoyl fluorides and the efficiency of sulfur fluorine exchange chemistry has enabled the development of the enantiopure bifunctional S(VI) transfer reagent t-BuSF to overcome current synthetic limitations. Here, we disclose a reagent platform that serves as a chiral sulfur fluorine exchange template for the rapid asymmetric synthesis of over 70 sulfoximines, sulfonimidoyl fluorides and sulfonimidamides with excellent enantiomeric excess and good overall yields. Furthermore, the practical utility of the bifunctional S(VI) transfer reagent was demonstrated in the syntheses of enantiopure pharmaceutical intermediates and analogues.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data, including experimental procedures, compound characterization data and stability analysis data, are available within the article and its Supplementary Information. X-ray crystallographic data for the structures within this article and the Supplementary Information have been deposited with the Cambridge Crystallographic Data Centre. The data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/. Compounds with X-ray structures: (S)-t-BuSF (CCDC 2243804), 2a (CCDC 2243801), 3f (CCDC 2243803), 4a (CCDC 2243800), 7a (CCDC 2243808), 7d (CCDC 2243809) 7e (CCDC 2243805), 7f (CCDC 2243806), 7i (CCDC 2243802), 7k (CCDC 2243799), 7l (CCDC 2243807), 9a (CCDC 2243810) and S6a (CCDC 2243798).
References
Scott, K. A. & Njardarson, J. T. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 376, 5 (2018).
Devendar, P. & Yang, G. F. Sulfur-containing agrochemicals. Top. Curr. Chem. 375, 82 (2017).
Takimiya, K., Osaka, I., Mori, T. & Nakano, M. Organic semiconductors based on [1]benzothieno[3,2-b][1]benzothiophene substructure. Acc. Chem. Res. 47, 1493–1502 (2014).
Boyd, D. A. Sulfur and its role in modern materials science. Angew. Chem. Int. Ed. 55, 15486–15502 (2016).
Yue, T.-J., Wang, L.-Y. & Ren, W.-M. The synthesis of degradable sulfur-containing polymers: precise control of structure and stereochemistry. Polym. Chem. 12, 6650–6666 (2021).
Gege, C. et al. A helicase-primase drug candidate with sufficient target tissue exposure affects latent neural herpes simplex virus infections. Sci. Transl. Med. 13, eabf8668 (2021).
Walker, D. P. et al. Sulfoximine-substituted trifluoromethylpyrimidine analogs as inhibitors of proline-rich tyrosine kinase 2 (PYK2) show reduced hERG activity. Bioorg. Med. Chem. Lett. 19, 3253–3258 (2009).
Sehgelmeble, F. et al. Sulfonimidamides as sulfonamides bioisosteres: rational evaluation through synthetic, in vitro, and in vivo studies with γ-secretase inhibitors. ChemMedChem 7, 396–399 (2012).
Foote, K. M. et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and Rad3 related (ATR) kinase with application as an anticancer agent. J. Med. Chem. 61, 9889–9907 (2018).
Coleman, L., Farady, C., Gatlik, E. & Schieker, M. Dosing regimen for an NLRP3 inhibitor in the treatment of osteoarthritis. WO Patent 2023/002399 A1 (2023).
Lücking, U. Sulfoximines: a neglected opportunity in medicinal chemistry. Angew. Chem. Int. Ed. 52, 9399–9408 (2013).
Lücking, U. Neglected sulfur(VI) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front. 6, 1319–1324 (2019).
Zhu, Y. et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 59, 2950–2957 (2011).
Siemeister, G. et al. Bay 1000394, a novel cyclin-dependent kinase inhibitor, with potent antitumor activity in mono- and in combination treatment upon oral application. Mol. Cancer Ther. 11, 2265–2273 (2012).
Lücking, U. et al. Changing for the better: discovery of the highly potent and selective CDK9 inhibitor VIP152 suitable for once weekly intravenous dosing for the treatment of cancer. J. Med. Chem. 64, 11651–11674 (2021).
Agarwal, S. et al. Discovery of N-cyano-sulfoximineurea derivatives as potent and orally bioavailable NLRP3 inflammasome inhibitors. ACS Med. Chem. Lett. 11, 414–418 (2020).
Madurka, I. et al. DFV890: a new oral NLR3 inhibitor—tested in an early phase 2a randomised clinical trial in patients with COVID-19 pneumonia and impaired respiratory function. Infection 51, 641–654 (2023).
Mäder, P. & Kattner, L. Sulfoximines as rising stars in modern drug discovery? Current status and perspective on an emerging functional group in medicinal chemistry. J. Med. Chem. 63, 14243–14275 (2020).
Chinthakindi, P. K. et al. Sulfonimidamides in medicinal and agricultural chemistry. Angew. Chem. Int. Ed. 56, 4100–4109 (2017).
Frings, M., Bolm, C., Blum, A. & Gnamm, C. Sulfoximines from a medicinal chemist’s perspective: physicochemical and in vitro parameters relevant for drug discovery. Eur. J. Med. Chem. 126, 225–245 (2017).
Liang, D.-D. et al. Silicon-free SuFEx reactions of sulfonimidoyl fluorides: scope, enantioselectivity, and mechanism. Angew. Chem. Int. Ed. 59, 7494–7500 (2020).
Liang, D.-D., Pujari, S. P., Subramaniam, M., Besten, M. & Zuilhof, H. Configurationally chiral SuFEx-based polymers. Angew. Chem. Int. Ed. 61, e202116158 (2022).
Mukherjee, H. et al. A study of the reactivity of S(VI)–F containing warheads with nucleophilic amino-acid side chains under physiological conditions. Org. Biomol. Chem. 15, 9685–9695 (2017).
Luisi, R. & Bull, J. A. Synthesis of sulfoximines and sulfonimidamides using hypervalent iodine mediated NH transfer. Molecules 28, 1120 (2023).
Yu, H., Li, Z. & Bolm, C. Copper-catalyzed transsulfinamidation of sulfinamides as a key step in the preparation of sulfonamides and sulfonimidamides. Angew. Chem. Int. Ed. 57, 15602–15605 (2018).
Bizet, V., Hendriks, C. M. M. & Bolm, C. Sulfur imidations: access to sulfimides and sulfoximines. Chem. Soc. Rev. 44, 3378–3390 (2015).
Chen, Y. & Gibson, J. A convenient synthetic route to sulfonimidamides from sulfonamides. RSC Adv. 5, 4171–4174 (2015).
Matos, P. M. & Stockman, R. A. Synthetic approaches and applications of sulfonimidates. Org. Biomol. Chem. 18, 6429–6442 (2020).
Johnson, C. R. et al. Preparation and reactions of sulfonimidoyl fluorides. J. Org. Chem. 48, 1–3 (1983).
Lo, P. K. T. & Willis, M. C. Nickel(II)-catalyzed addition of aryl and heteroaryl boroxines to the sulfinylamine reagent TrNSO: the catalytic synthesis of sulfinamides, sulfonimidamides, and primary sulfonamides. J. Am. Chem. Soc. 143, 15576–15581 (2021).
Ding, M., Zhang, Z.-X., Davies, T. Q. & Willis, M. C. A silyl sulfinylamine reagent enables the modular synthesis of sulfonimidamides via primary sulfinamides. Org. Lett. 24, 1711–1715 (2022).
Bremerich, M., Conrads, C. M., Langletz, T. & Bolm, C. Additions to N-sulfinylamines as an approach for the metal-free synthesis of sulfonimidamides: O-benzotriazolyl sulfonimidates as activated intermediates. Angew. Chem. Int. Ed. 58, 19014–19020 (2019).
Zasukha, S. V. et al. Sulfonimidamides and imidosulfuric diamides: compounds from an underexplored part of biologically relevant chemical space. Chem. Eur. J. 25, 6928–6940 (2019).
Gao, B., Li, S., Wu, P., Moses, J. E. & Sharpless, K. B. SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew. Chem. Int. Ed. 57, 1939–1943 (2018).
Davies, T. Q. & Willis, M. C. Rediscovering sulfinylamines as reagents for organic synthesis. Chem. Eur. J. 27, 8918–8927 (2021).
Wang, L. & Cornella, J. A unified strategy for arylsulfur(VI) fluorides from aryl halides: access to Ar-SOF3 compounds. Angew. Chem. Int. Ed. 59, 23510–23515 (2020).
Liu, Y. et al. Rapid access to N-protected sulfonimidoyl fluorides: divergent synthesis of sulfonamides and sulfonimidamides. Org. Lett. 23, 3975–3980 (2021).
Passia, M. T. et al. Acid-mediated imidazole-to-fluorine exchange for the synthesis of sulfonyl and sulfonimidoyl fluorides. Org. Lett. 24, 8802–8805 (2022).
Passia, M. T., Amer, M. M., Demaerel, J. & Bolm, C. Synthesis of sulfonyl, sulfonimidoyl, and sulfoxyl fluorides under solvent-free mechanochemical conditions in a mixer mill by imidazole-to-fluorine exchange. ACS Sustain. Chem. Eng. 11, 6838–6843 (2023).
Kleymann, G. & Gege, C. Preparation of enantiomers of substituted thiazoles as antiviral compounds. WO Patent 2019/068817 A1 (2019).
Brandt, J. & Gais, H.-J. An efficient resolution of (±)-S-methyl-S-phenylsulfoximine with (+)-10-camphorsulfonic acid by the method of half-quantities. Tetrahedron: Asymmetry 8, 909–912 (1997).
Zhang, X., Wang, F. & Tan, C.-H. Asymmetric synthesis of S(IV) and S(VI) stereogenic centers. JACS Au 3, 700–714 (2023).
García-Cárceles, J. et al. 2-(fluoromethoxy)-4′-(s-methanesulfonimidoyl)-1,1′-biphenyl (UCM-1306), an orally bioavailable positive allosteric modulator of the human dopamine D1 receptor for Parkinson’s disease. J. Med. Chem. 65, 12256–12272 (2022).
Zhang, X., Ang, E. C. X., Yang, Z., Kee, C. W. & Tan, C.-H. Synthesis of chiral sulfinate esters by asymmetric condensation. Nature 604, 298–303 (2022).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines via the S-arylation of sulfinamides. J. Am. Chem. Soc. 141, 19263–19268 (2019).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines through the S-alkylation of sulfinamides. Angew. Chem. Int. Ed. 58, 17661–17665 (2019).
Maeda, Y. et al. Practical asymmetric synthesis of chiral sulfoximines via sulfur-selective alkylation. J. Org. Chem. 87, 3652–3660 (2022).
Mendonça Matos, P., Lewis, W., Argent, S. P., Moore, J. C. & Stockman, R. A. General method for the asymmetric synthesis of N–H sulfoximines via C–S bond formation. Org. Lett. 22, 2776–2780 (2020).
Yang, G.-F. et al. Synthesis of chiral sulfonimidoyl chloride via desymmetrizing enantioselective hydrolysis. J. Am. Chem. Soc. 145, 5439–5446 (2023).
Greed, S., Symes, O. & Bull, J. A. Stereospecific reaction of sulfonimidoyl fluorides with Grignard reagents for the synthesis of enantioenriched sulfoximines. Chem. Commun. 58, 5387–5390 (2022).
Greed, S. et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur–fluorine exchange (SuFEx) reaction. Chem. Eur. J. 26, 12533–12538 (2020).
Shultz, Z. P., Scattolin, T., Wojtas, L. & Lopchuk, J. M. Stereospecific α-(hetero)arylation of sulfoximines and sulfonimidamides. Nat. Synth. 1, 170–179 (2022).
Booms, R. E. & Cram, D. J. Stereochemistry of sulfur compounds. III. Radical-chain mechanism for racemization of sulfinamides. J. Am. Chem. Soc. 94, 5438–5446 (1972).
Clarke, V. & Cole, E. R. Sulfenamides and sulfinamides IX exchange reactions of aryl sulfinamides. Phosphorus Sulf Silicon Relat. Elem. 92, 45–50 (1994).
Li-Yuan Bao, R., Zhao, R. & Shi, L. Progress and developments in the turbo Grignard reagent i-PrMgCl·LiCl: a ten-year journey. Chem. Commun. 51, 6884–6900 (2015).
Acknowledgements
We thank H. Lawrence (H. Lee Moffitt Cancer Center and Research Institute) and L. Calcul (University of South Florida) for NMR and high-resolution mass spectrometry support, and Q. Tang (USF) for assistance with X-ray crystallographic analysis. We gratefully acknowledge the National Institutes of Health (National Institute of General Medical Sciences; R35-GM142577, J.M.L.) for support of this research. This work has also been supported in part by the Chemical Biology Core Facility at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute designated Comprehensive Cancer Center (P30-CA076292) and the University of South Florida’s Chemical Purification Analysis and Screening Core Facility.
Author information
Authors and Affiliations
Contributions
S.T., Z.P.S. and J.M.L. designed the study. S.T., Z.P.S. and C.S. performed the experiments and interpreted the results. C.S. and L.W. performed the X-ray crystallographic analysis. Z.P.S. and J.M.L. prepared the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
A patent application naming J.M.L., Z.P.S. and S.T. as inventors has been filed by H. Lee Moffitt Cancer Center and Research Institute, which covers the synthetic methods and development regarding a chiral bifunctional S(VI) reagent for the asymmetric synthesis of sulfur-containing functional groups. The remaining authors declare no competing interest.
Peer review
Peer review information
Nature Chemistry thanks Yantao Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussions, Figs. 1–27, Tables 1–18, NMR spectra and HPLC chromatograms.
Supplementary Data 1
Crystallographic data for compound t-BuSF; CCDC reference 2243804.
Supplementary Data 2
Crystallographic data for compound 2a; CCDC reference 2243801.
Supplementary Data 3
Crystallographic data for compound 3f; CCDC reference 2243803.
Supplementary Data 4
Crystallographic data for compound 4a; CCDC reference 2243800.
Supplementary Data 5
Crystallographic data for compound S6a; CCDC reference 2243798.
Supplementary Data 6
Crystallographic data for compound 7k; CCDC reference 2243799.
Supplementary Data 7
Crystallographic data for compound 7a; CCDC reference 2243808.
Supplementary Data 8
Crystallographic data for compound 7i; CCDC reference 2243802.
Supplementary Data 9
Crystallographic data for compound 7f; CCDC reference 2243806.
Supplementary Data 10
Crystallographic data for compound 7l; CCDC reference 2243807.
Supplementary Data 11
Crystallographic data for compound 7e; CCDC reference 2243805.
Supplementary Data 12
Crystallographic data for compound 7d; CCDC reference 2243809.
Supplementary Data 13
Crystallographic data for compound 9a; CCDC reference 2243810.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teng, S., Shultz, Z.P., Shan, C. et al. Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent. Nat. Chem. 16, 183–192 (2024). https://doi.org/10.1038/s41557-023-01419-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01419-3
This article is cited by
-
Sulfur stereochemistry takes centre stage
Nature Chemistry (2024)