Abstract
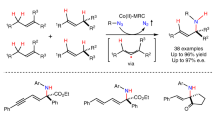
Metalloradical catalysis (MRC) exploits the metal-centred radicals present in open-shell metal complexes as one-electron catalysts for the generation of metal-stabilized organic radicals—key intermediates that control subsequent one-electron homolytic reactions. Cobalt(II) complexes of porphyrins, as stable 15e-metalloradicals with a well-defined low-spin d7 configuration, have dominated the ongoing development of MRC. Here, to broaden MRC beyond the use of Co(II)-based metalloradical catalysts, we describe systematic studies that establish the operation of Fe(III)-based MRC and demonstrate an initial application for asymmetric radical transformations. Specifically, we report that five-coordinate iron(III) complexes of porphyrins with an axial ligand, which represent another family of stable 15e-metalloradicals with a d5 configuration, are potent metalloradical catalysts for olefin cyclopropanation with different classes of diazo compounds via a stepwise radical mechanism. This work lays a foundation and mechanistic blueprint for future exploration of Fe(III)-based MRC towards the discovery of diverse stereoselective radical reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. The crystal structure data of compounds [Fe(P2)Cl], 1, (R)-3n, (1R,2R)-3ac, 8′ and 9 have been deposited in the Cambridge structural database under reference nos. CCDC 2128685, 2128686, 2128688, 2128687, 2128689 and 2043165, respectively. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Zard, S. Z. Radical Reactions in Organic Synthesis (Oxford Chemistry Masters, 2003).
Curran, D. P., Porter, N. A. & Giese, B. Stereochemistry of Radical Reactions: Concepts, Guidelines and Synthetic Applications (Wiley, 2008).
Bar, G. & Parsons, A. F. Stereoselective radical reactions. Chem. Soc. Rev. 32, 251–263 (2003).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Kern, N., Plesniak, M. P., McDouall, J. J. W. & Procter, D. J. Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals. Nat. Chem. 9, 1198–1204 (2017).
Wang, F., Chen, P. H. & Liu, G. S. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 51, 2036–2046 (2018).
Gu, Q. S., Li, Z. L. & Liu, X. Y. Copper(I)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 53, 170–181 (2020).
Li, Z. L., Fang, G. C., Gu, Q. S. & Liu, X. Y. Recent advances in copper-catalyzed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 49, 32–48 (2020).
Lee, W. C. C. & Zhang, X. P. Asymmetric radical cyclopropanation of alkenes. Trends Chem. 4, 850–851 (2022).
van Leest, N. P., de Zwart, F. J., Zhou, M. & de Bruin, B. Controlling radical-type single-electron elementary steps in catalysis with redox-active ligands and substrates. JACS Au 1, 1101–1115 (2021).
Rajanbabu, T. V. & Nugent, W. A. Selective generation of free-radicals from epoxides using a transition-metal radical. A powerful new tool for organic synthesis. J. Am. Chem. Soc. 116, 986–997 (1994).
Funken, N., Muhlhaus, F. & Gansauer, A. General, highly selective synthesis of 1,3- and 1,4 difunctionalized building blocks by regiodivergent epoxide opening. Angew. Chem. Int. Ed. 55, 12030–12034 (2016).
Yao, C. B., Dahmen, T., Gansauer, A. & Norton, J. Anti-Markovnikov alcohols via epoxide hydrogenation through cooperative catalysis. Science 364, 764–767 (2019).
Ye, K. Y., McCallum, T. & Lin, S. Bimetallic radical redox-relay catalysis for the isomerization of epoxides to allylic alcohols. J. Am. Chem. Soc. 141, 9548–9554 (2019).
Chan, Y. W. & Chan, K. S. Metalloradical-catalyzed aliphatic carbon−carbon activation of cyclooctane. J. Am. Chem. Soc. 132, 6920–6922 (2010).
Roy, S., Das, S. K. & Chattopadhyay, B. Cobalt(II)-based metalloradical activation of 2-(diazomethyl)-pyridines for radical transannulation and cyclopropanation. Angew. Chem. Int. Ed. 57, 2238–2243 (2018).
Roy, S., Khatua, H., Das, S. K. & Chattopadhyay, B. Iron(II)-based metalloradical activation: switch from traditional click chemistry to denitrogenative annulation. Angew. Chem. Int. Ed. 58, 11439–11443 (2019).
Das, S. K., Roy, S., Khatua, H. & Chattopadhyay, B. Iron-catalyzed amination of strong aliphatic C(sp3)–H bonds. J. Am. Chem. Soc. 142, 16211–16217 (2020).
Roy, S. et al. Road map for the construction of high-valued N-heterocycles via denitrogenative annulation. Acc. Chem. Res. 54, 4395–4409 (2021).
Roy, S. et al. Iron-catalyzed radical activation mechanism for denitrogenative rearrangement over C(sp3)–H amination. Angew. Chem. Int. Ed. 60, 8772–8780 (2021).
Das, S. K. et al. An iron(II)-based metalloradical system for intramolecular amination of C(sp2)–H and C(sp3)–H bonds: synthetic applications and mechanistic studies. Chem. Sci. 13, 11817–11828 (2022).
Lee, W.-C. C. et al. Asymmetric radical cyclopropanation of dehydroaminocarboxylates: stereoselective synthesis of cyclopropyl α-amino acids. Chem 7, 1588–1601 (2021).
Riart-Ferrer, X. et al. Metalloradical activation of carbonyl azides for enantioselective radical aziridination. Chem 7, 1120–1134 (2021).
Xie, J. J. et al. New catalytic radical process involving 1,4-hydrogen atom abstraction: asymmetric construction of cyclobutanones. J. Am. Chem. Soc. 143, 11670–11678 (2021).
Lang, K., Hu, Y., Lee, W.-C. C. & Zhang, X. P. Combined radical and ionic approach for the enantioselective synthesis of β-functionalized amines from alcohols. Nat. Synth. 1, 548–557 (2022).
Woggon, W. D. Metalloporphyrines as active site analogues—lessons from enzymes and enzyme models. Acc. Chem. Res. 38, 127–136 (2005).
Oohora, K., Onoda, A. & Hayashi, T. Hemoproteins reconstituted with artificial metal complexes as biohybrid catalysts. Acc. Chem. Res. 52, 945–954 (2019).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Wolf, J. R. et al. Shape and stereoselective cyclopropanation of alkenes catalyzed by iron porphyrins. J. Am. Chem. Soc. 117, 9194–9199 (1995).
Lai, T. S. et al. Alkene cyclopropanation catalyzed by Halterman iron porphyrin: participation of organic bases as axial ligands. Dalton Trans. 2006, 4845–4851 (2006).
Aggarwal, V. K., de Vicente, J. & Bonnert, R. V. Catalytic cyclopropanation of alkenes using diazo compounds generated in situ. A novel route to 2-arylcyclopropylamines. Org. Lett. 3, 2785–2788 (2001).
Li, Y. et al. Remarkably stable iron porphyrins bearing nonheteroatom-stabilized carbene or (alkoxycarbonyl)carbenes: isolation, X-ray crystal structures, and carbon atom transfer reactions with hydrocarbons. J. Am. Chem. Soc. 124, 13185–13193 (2002).
Adams, L. A. et al. Diastereoselective synthesis of cyclopropane amino acids using diazo compounds generated in situ. J. Org. Chem. 68, 9433–9440 (2003).
Morandi, B. & Carreira, E. M. Iron-catalyzed cyclopropanation in 6 M KOH with in situ generation of diazomethane. Science 335, 1471–1474 (2012).
Morandi, B., Dolva, A. & Carreira, E. M. Iron-catalyzed cyclopropanation with glycine ethyl ester hydrochloride in water. Org. Lett. 14, 2162–2163 (2012).
Zhu, S. F. & Zhou, Q. L. Iron-catalyzed transformations of diazo compounds. Natl Sci. Rev. 1, 580–603 (2014).
Allouche, E. M. D., Al-Saleh, A. & Charette, A. B. Iron-catalyzed synthesis of cyclopropanes by in situ generation and decomposition of electronically diversified diazo compounds. Chem. Commun. 54, 13256–13259 (2018).
Ning, Y. Q. et al. Difluoroacetaldehyde N-triftosylhydrazone (DFHZ-Tfs) as a bench-stable crystalline diazo surrogate for diazoacetaldehyde and difluorodiazoethane. Angew. Chem. Int. Ed. 59, 6473–6481 (2020).
Damiano, C., Sonzini, P. & Gallo, E. Iron catalysts with N-ligands for carbene transfer of diazo reagents. Chem. Soc. Rev. 49, 4867–4905 (2020).
Le Maux, P., Juillard, S. & Simonneaux, G. Asymmetric synthesis of trifluoromethylphenyl cyclopropanes catalyzed by chiral metalloporphyrins. Synthesis 2006, 1701–1704 (2006).
Chen, Y. & Zhang, X. P. Asymmetric cyclopropanation of styrenes catalyzed by metal complexes of D2-symmetrical chiral porphyrin: superiority of cobalt over iron. J. Org. Chem. 72, 5931–5934 (2007).
Intrieri, D. et al. Highly diastereoselective cyclopropanation of α-methylstyrene catalysed by a C2-symmetrical chiral iron porphyrin complex. Chem. Commun. 50, 1811–1813 (2014).
Carminati, D. M. et al. Designing ‘totem’ C2-symmetrical iron porphyrin catalysts for stereoselective cyclopropanations. Chem. Eur. J. 22, 13599–13612 (2016).
Carminati, D. M. et al. Synthesis, characterisation and catalytic use of iron porphyrin amino ester conjugates. New J. Chem. 41, 5950–5959 (2017).
Bos, M. et al. Recent progress toward the synthesis of trifluoromethyl- and difluoromethyl-substituted cyclopropanes. Chem. Eur. J. 23, 4950–4961 (2017).
Mykhailiuk, P. K. 2,2,2-Trifluorodiazoethane (CF3CHN2): a long journey since 1943. Chem. Rev. 120, 12718–12755 (2020).
Decaens, J. et al. Synthesis of fluoro-, monofluoromethyl-, difluoromethyl- and trifluoromethyl-substituted three-membered rings. Chem. Eur. J. 27, 2935–2962 (2021).
Pons, A. et al. Asymmetric synthesis of fluoro, fluoromethyl, difluoromethyl and trifluoromethylcyclopropanes. Acc. Chem. Res. 54, 2969–2990 (2021).
Morandi, B. & Carreira, E. M. Iron-catalyzed cyclopropanation with trifluoroethylamine hydrochloride and olefins in aqueous media: in situ generation of trifluoromethyl diazomethane. Angew. Chem. Int. Ed. 49, 938–941 (2010).
Morandi, B., Cheang, J. & Carreira, E. M. Iron-catalyzed preparation of trifluoromethyl substituted vinyl- and alkynylcyclopropane. Org. Lett. 13, 3080–3081 (2011).
Phelan, J. P. et al. Redox-neutral photocatalytic cyclopropanation via radical/polar crossover. J. Am. Chem. Soc. 140, 8037–8047 (2018).
Zhang, X. Y. et al. Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications. Nat. Commun. 10, 284 (2019).
Denton, J. R., Sukumaran, D. & Davies, H. M. L. Enantioselective synthesis of trifluoromethyl-substituted cyclopropanes. Org. Lett. 9, 2625–2628 (2007).
Zhang, X. Y. et al. Fluoroalkyl N-triftosylhydrazones as easily decomposable diazo surrogates for asymmetric [2 + 1] cycloaddition: synthesis of chiral fluoroalkyl cyclopropenes and cyclopropanes. ACS Catal. 11, 8527–8537 (2021).
Huang, W. S. et al. General catalytic enantioselective access to monohalomethyl and trifluoromethyl cyclopropanes. Chem. Eur. J. 24, 10339–10343 (2018).
Pagire, S. K., Kumagai, N. & Shibasaki, M. Highly enantio- and diastereoselective synthesis of 1,2,3-trisubstituted cyclopropanes from α,β-unsaturated amides and stabilized sulfur ylides catalyzed by a chiral copper(I) complex. ACS Catal. 11, 11597–11606 (2021).
Morandi, B., Mariampillai, B. & Carreira, E. M. Enantioselective cobalt-catalyzed preparation of trifluoromethyl-substituted cyclopropanes. Angew. Chem. Int. Ed. 50, 1101–1104 (2011).
Tinoco, A., Steck, V., Tyagi, V. & Fasan, R. Highly diastereo- and enantioselective synthesis of trifluoromethyl-substituted cyclopropanes via myoglobin-catalyzed transfer of trifluoromethylcarbene. J. Am. Chem. Soc. 139, 5293–5296 (2017).
Carminati, D. M. et al. Biocatalytic strategy for the highly stereoselective synthesis of CHF2-containing trisubstituted cyclopropanes. Angew. Chem. Int. Ed. 60, 7072–7076 (2021).
Kotozaki, M. et al. Highly enantioselective synthesis of trifluoromethyl cyclopropanes by using Ru(II)–Pheox catalysts. Chem. Commun. 54, 5110–5113 (2018).
Altarejos, J., Sucunza, D., Vaquero, J. J. & Carreras, J. Enantioselective copper-catalyzed synthesis of trifluoromethyl-cyclopropylboronates. Org. Lett. 23, 6174–6178 (2021).
Chen, Y., Fields, K. B. & Zhang, X. P. Bromoporphyrins as versatile synthons for modular construction of chiral porphyrins: cobalt-catalyzed highly enantioselective and diastereoselective cyclopropanation. J. Am. Chem. Soc. 126, 14718–14719 (2004).
Xu, X. et al. Highly asymmetric intramolecular cyclopropanation of acceptor-substituted diazoacetates by Co(II)-based metalloradical catalysis: iterative approach for development of new-generation catalysts. J. Am. Chem. Soc. 133, 15292–15295 (2011).
Kweon, J. & Chang, S. Highly robust iron catalyst system for intramolecular C(sp3)–H amidation leading to γ-lactams. Angew. Chem. Int. Ed. 60, 2909–2914 (2021).
Nakamura, M. Electronic structures of highly deformed Iron(III) porphyrin complexes. Coord. Chem. Rev. 250, 2271–2294 (2006).
Nakamura, M. in Fundamentals of Porphyrin Chemistry: A 21st Century Approach (eds Brothers, P. J. & Senge, M. O.) 631–659 (Wiley, 2022).
Cheng, R. J. et al. Control of spin state by ring conformation of Iron(III) porphyrins. A novel model for the quantum-mixed intermediate spin state of ferric cytochrome c′ from photosynthetic bacteria. J. Am. Chem. Soc. 119, 2563–2569 (1997).
Stuzhin, P. A. et al. Effects of solvation on the spin state of Iron(III) in 2,8,12,18-tetrabutyl-3,7,13,17-tetramethyl-5,10-diazaporphyrinatoiron(III) chloride. Inorg. Chem. 49, 4802–4813 (2010).
Sahoo, D., Quesne, M. G., de Visser, S. P. & Rath, S. P. Hydrogen-bonding interactions trigger a spin-flip in Iron(III) porphyrin complexes. Angew. Chem. Int. Ed. 54, 4796–4800 (2015).
Wei, Y. et al. Cyclopropanations via heme carbenes: basic mechanism and effects of carbene substituent, protein axial ligand, and porphyrin substitution. J. Am. Chem. Soc. 140, 1649–1662 (2018).
Acknowledgements
We are grateful for financial support by the NSF (CHE-2154885) and in part by NIH (R01-GM102554). W.-C.C.L. was supported by a LaMattina Graduate Fellowship and Dean’s Dissertation Fellowship. We thank B. Li (Boston College) for X-ray structure determination, J. Jin (Boston College) for EPR measurements, M. Graf (Boston College) for SQUID measurements and M. Kumar (Massachusetts Institute of Technology) for HRMS measurements. We thank J. Zhang (Johns Hopkins University) for helpful discussions and valuable suggestions. We also acknowledge financial support by NIH (S10-OD026910) and NSF (CHE-2117246) for the purchase of NMR spectrometers at the Magnetic Resonance Center of Boston College.
Author information
Authors and Affiliations
Contributions
W.-C.C.L. conducted the experiments. D.-S.W. and Y.Z. performed the DFT calculations. X.P.Z. conceived the work and directed the project. W.-C.C.L. and X.P.Z. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Buddhadeb Chattopadhyay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary information on experimental methods, synthetic procedures and compound characterizations, additional discussion, computational details, X-ray crystallographic data, NMR spectra and HPLC traces.
Supplementary Data 1
Crystallographic data for catalyst [Fe(P2)Cl]; CCDC reference 2128685.
Supplementary Data 2
Crystallographic data for compound 1; CCDC reference 2128686.
Supplementary Data 3
Crystallographic data for compound 3n; CCDC reference 2128688.
Supplementary Data 4
Crystallographic data for compound 3ac; CCDC reference 2128687.
Supplementary Data 5
Crystallographic data for compound 8′; CCDC reference 2128689.
Supplementary Data 6
Crystallographic data for compound 9; CCDC reference 2043165.
Supplementary Data 7
Energies and coordinates in XYZ format of DFT computations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, WC.C., Wang, DS., Zhu, Y. et al. Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism. Nat. Chem. 15, 1569–1580 (2023). https://doi.org/10.1038/s41557-023-01317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01317-8