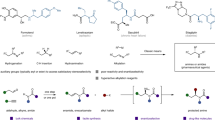
Abstract
Chiral α-tertiary amines and related azacycles are sought-after compounds for drug development. Despite progress in the catalytic asymmetric construction of aza-quaternary stereocentres, enantioselective synthesis of multifunctional α-tertiary amines remains underdeveloped. Enantioenriched α-disubstituted α-ethynylamines are attractive synthons for constructing chiral α-tertiary amines and azacycles, but methods for their catalytic enantioselective synthesis need to be expanded. Here we describe an enantioselective asymmetric Cu(I)-catalysed propargylic amination (ACPA) of simple ketone-derived propargylic carbonates to give both α-dialkylated and α-alkyl–α-aryl α-tertiary ethynylamines. Sterically confined pyridinebisoxazoline (PYBOX) ligands, with a C4 shielding group and relaying groups, play a key role in achieving excellent enantioselectivity. The syntheses of quaternary 2,5-dihydropyrroles, dihydroquinines, dihydrobenzoquinolines and dihydroquinolino[1,2-α]quinolines are reported, and the synthetic value is further demonstrated by the enantioselective catalytic total synthesis of a selective multi-target β-secretase inhibitor. Enantioselective Cu-catalysed propargylic substitutions with O- and C-centred nucleophiles are also realized, further demonstrating the potential of the PYBOX ligand.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2212823 (4aa), 2304114 (5e), 2304057 (5l), 2212826 (8h), 2212213 (9a) and 2226279 (22). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Royer, J. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley, 2010).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Li, M.-L., Yu, J.-H., Li, Y.-H., Zhu, S.-F. & Zhou, Q.-L. Highly enantioselective carbene insertion into N–H bonds of aliphatic amines. Science 366, 990–994 (2019).
Xi, Y.-M., Ma, S.-J. & Hartwig, J. F. Catalytic asymmetric addition of an amine N–H bond across internal alkenes. Nature 588, 254–260 (2020).
Chen, C.-Y., Peters, J. C. & Fu, G. C. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 596, 250–256 (2021).
Hameed, A., Al-Rashida, M. & Shah, M. R. α-Tertiary Amines en Route to Natural Products (Elsevier, 2021).
Hager, A. et al. Synthetic approaches towards alkaloids bearing α-tertiary amines. Nat. Prod. Rep. 33, 491–522 (2016).
Talele, T. T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 63, 13291–13315 (2020).
Pronin, S. V., Reiher, C. A. & Shenvi, R. A. Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles and amines. Nature 501, 195–199 (2013).
Shibasaki, M. & Kanai, M. Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C-C bond formation to ketones and ketimines. Chem. Rev. 108, 2853–2873 (2008).
Yin, Q., Shi, Y.-J., Wang, J.-X. & Zhang, X.-M. Direct catalytic asymmetric synthesis of α-chiral primary amines. Chem. Soc. Rev. 49, 6141–6153 (2020).
Xu, Y.-Z., Wang, J.-J., Deng, G.-J. & Shao, W. Recent advances in the synthesis of chiral α-tertiary amines via transition-metal catalysis. Chem. Commun. 59, 4099–4114 (2023).
Wu, Y.-W., Hu, L., Li, Z. & Deng, L. Catalytic asymmetric umpolung reactions of imines. Nature 523, 445–450 (2015).
Chen, J.-J. et al. Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines. Nature 618, 294–300 (2023).
Ye, C.-X., Shen, X., Chen, S.-M. & Meggers, E. Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids. Nat. Chem. 14, 566–573 (2022).
Xu, P., Xie, J.-J., Wang, D.-S. & Zhang, X. P. Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination. Nat. Chem. 15, 498–507 (2023).
Keith, J. M. & Jacobsen, E. N. Asymmetric hydrocyanation of hydrazones catalyzed by lanthanide-PYBOX complexes. Org. Lett. 6, 153–155 (2004).
Detz, R. J., Abiri, Z., Remi, G., Hiemstra, H. & van Maarseveen, J. H. Enantioselective copper-catalysed propargylic substitution: synthetic scope study and application in formal total syntheses of (+)-anisomycin and (−)-cytoxazone. Chem. Eur. J. 17, 5921–5930 (2011).
Nishikawa, D., Hirano, K. & Miura, M. Asymmetric synthesis of α‑aminoboronic acid derivatives by copper-catalyzed enantioselective hydroamination. J. Am. Chem. Soc. 137, 15620–15623 (2015).
Liu, R.-R. et al. Palladium/l‑proline-catalyzed enantioselective α‑arylative desymmetrization of cyclohexanones. J. Am. Chem. Soc. 138, 5198–5201 (2016).
Riart-Ferrer, X. et al. Metalloradical activation of carbonyl azides for enantioselective radical aziridination. Chem 7, 1120–1134 (2021).
Ma, J.-G. et al. Enantioselective synthesis of pyroglutamic acid esters from glycinate via carbonyl catalysis. Angew. Chem. Int. Ed. 60, 10588–10592 (2021).
Zhang, Y., Qiao, D.-Y., Duan, M., Wang, Y. & Zhu, S.-L. Enantioselective synthesis of α-aminoboronates by NiH-catalysed asymmetric hydroamidation of alkenyl boronates. Nat. Commun. 13, 5630–5637 (2022).
Cai, Q.-L. et al. Well-defined chiral dinuclear copper complexes in enantioselective propargylic substitution: for a long-standing supposition on binuclear mechanism. Chem 10, 265–282 (2024).
Lauder, K., Toscani, A., Scalacci, N. & Castagnolo, D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 117, 14091–14200 (2017).
Rokade, B. V., Barker, J. & Guiry, P. J. Development of and recent advances in asymmetric A3 coupling. Chem. Soc. Rev. 48, 4766–4790 (2019).
Zorba, L. P. & Vougioukalakis, G. C. The ketone-amine-alkyne (KA2) coupling reaction: transition metal-catalyzed synthesis of quaternary propargylamines. Coord. Chem. Rev. 429, 213603 (2021).
Pfeffer, C., Probst, P., Wannenmacher, N., Frey, W. & Peters, R. Direct enantioselective addition of alkynes to imines by a highly efficient palladacycle catalyst. Angew. Chem. Int. Ed. 61, e202206835 (2022).
Morisaki, K. et al. Mechanistic studies and expansion of the substrate scope of direct enantioselective alkynylation of α-ketiminoesters catalyzed by adaptable (phebox)rhodium(III) complexes. J. Am. Chem. Soc. 138, 6194–6230 (2016).
Huang, G.-C., Yang, J. & Zhang, X.-G. Highly enantioselective zinc/BINOL-catalyzed alkynylation of α-ketoimine ester: a new entry to optically active quaternary α-CF3 α-amino acids. Chem. Commun. 47, 5587–5589 (2011).
Hatano, M., Yamashita, K., Mizuno, M., Ito, O. & Ishihara, K. C-selective and diastereoselective alkyl addition to β,γ-alkynyl-imino esters with zinc(II)ate complexes. Angew. Chem. Int. Ed. 54, 2707–2711 (2015).
Trost, B. M., Hung, C.-I. & Scharf, M. J. Direct catalytic asymmetric vinylogous additions of α,β- and β,γ-butenolides to polyfluorinated alkynyl ketimines. Angew. Chem. Int. Ed. 57, 11408–11412 (2018).
Pan, Y.-K. et al. Kinetic resolution of α-tertiary propargylic amines through asymmetric remote aminations of anilines. ACS Catal. 11, 8443–8448 (2021).
Detz, R. J., Delville, M. M. E., Hiemstra, H. & van Maarseveen, J. H. Enantioselective copper-catalyzed propargylic amination. Angew. Chem. Int. Ed. 47, 3777–3780 (2008).
Hattori, G., Matsuzawa, H., Miyake, Y. & Nishibayashi, Y. Copper-catalyzed asymmetric propargylic substitution reactions of propargylic acetates with amines. Angew. Chem. Int. Ed. 47, 3781–3783 (2008).
Miyake, Y., Uemura, S. & Nishibayashi, Y. Catalytic propargylic substitution reactions. ChemCatChem 1, 342–356 (2009).
Detz, R. J., Hiemstra, H. & van Maarseveen, J. H. Catalyzed propargylic substitution. Eur. J. Org. Chem. 63, 6263–6276 (2009).
Ding, C.-H. & Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Zhang, D.-Y. & Hu, X.-P. Recent advances in copper-catalyzed propargylic substitution. Tetrahedron Lett. 56, 283–295 (2015).
Hattori, G., Yoshida, A., Miyake, Y. & Nishibayashi, Y. Enantioselective ring-opening reactions of racemic ethynyl epoxides via copper-allenylidene intermediates: efficient approach to chiral β-amino alcohols. J. Org. Chem. 74, 7603–7607 (2009).
Gómez, J. E., Guo, W.-S., Gaspa, S. & Kleij, A. W. Copper-catalyzed synthesis of γ-amino acids featuring quaternary stereocenters. Angew. Chem. Int. Ed. 56, 15035–15038 (2017).
Tian, L., Gong, L. & Zhang, X. Copper-catalyzed enantioselective synthesis of β-amino alcohols featuring tetrasubstituted tertiary carbons. Adv. Synth. Catal. 360, 2055–2059 (2018).
Guo, W.-S., Zuo, L.-H., Cui, M.-Y., Yan, B.-W. & Ni, S.-F. Propargylic amination enabled the access to enantioenriched acyclic α-quaternary α-amino ketones. J. Am. Chem. Soc. 143, 7629–7634 (2021).
Liu, T., Ni, S.-F. & Guo, W.-S. Practical asymmetric amine nucleophilic approach for the modular construction of protected α-quaternary amino acids. Chem. Sci. 13, 6806–6812 (2022).
Royer, J. Asymmetric Synthesis of Nitrogen Heterocycles (Wiley, 2009).
Phillips, A. M. F. Synthetic Approaches to Nonaromatic Nitrogen Heterocycles (Wiley, 2021).
Hattori, G. et al. Copper-catalyzed enantioselective propargylic amination of propargylic esters with amines: copper-allenylidene complexes as key intermediates. J. Am. Chem. Soc. 132, 10592–10608 (2010).
Sakata, K. & Nishibayashi, Y. Mechanism and reactivity of catalytic propargylic substitution reactions via metal–allenylidene intermediates: a theoretical perspective. Catal. Sci. Technol. 8, 12–25 (2018).
Roh, S. W., Choi, K. & Lee, C. Transition metal vinylidene- and allenylidene-mediated catalysis in organic synthesis. Chem. Rev. 119, 4293–4356 (2019).
Zhu, R.-Y., Chen, L., Hu, X.-S., Zhou, F. & Zhou, J. Enantioselective synthesis of P-chiral tertiary phosphine oxides with an ethynyl group via Cu(I)-catalyzed azide-alkyne cycloaddition. Chem. Sci. 11, 97–106 (2020).
Liao, K. et al. Highly enantioselective CuAAC of functional tertiary alcohols featuring an ethynyl group and their kinetic resolution. Angew. Chem. Int. Ed. 60, 8488–8493 (2020).
Gong, Y. et al. Sulfonyl-PYBOX ligands enable kinetic resolution of α-tertiary azides by CuAAC. Angew. Chem. Int. Ed. 62, e202301470 (2023).
Worrell, B. T., Malik, J. A. & Fokin, V. V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 340, 457–460 (2013).
Clayden, J., Lund, A., Vallverdú, L. & Helliwell, M. Ultra-remote stereocontrol by conformational communication of information along a carbon chain. Nature 431, 966–971 (2004).
Liao, S.-H., Sun, X.-L. & Tang, Y. Side arm strategy for catalyst design: modifying bisoxazolines for remote control of enantioselection and related. Acc. Chem. Res. 47, 2260–2272 (2014).
Nishibayashi, Y., Onodera, G., Inada, Y., Hidai, M. & Uemura, S. Synthesis of diruthenium complexes containing chiral thiolate-bridged ligands and their application to catalytic propargylic alkylation of propargylic alcohols with acetone. Organometallics 22, 873–876 (2003).
Nakajima, K., Shibata, M. & Nishibayashi, Y. Copper-catalyzed enantioselective propargylic etherification of propargylic esters with alcohols. J. Am. Chem. Soc. 137, 2472–2475 (2015).
Tsuchida, K., Senda, Y., Nakajima, K. & Nishibayashi, Y. Construction of chiral tri- and tetra-arylmethanes bearing quaternary carbon centers: copper-catalyzed enantioselective propargylation of indoles with propargylic esters. Angew. Chem. Int. Ed. 55, 9728–9732 (2016).
Zhang, Y.-C., Zhang, B.-W., Geng, R.-L. & Song, J. Enantioselective [3 + 2] cycloaddition reaction of ethynylethylene carbonates with malononitrile enabled by organo/metal cooperative catalysis. Org. Lett. 20, 7907–7911 (2018).
Gómez, J. E., Cristòfol, À. & Kleij, A. W. Copper-catalyzed enantioselective construction of tertiary propargylic sulfones. Angew. Chem. Int. Ed. 58, 3903–3907 (2019).
Zhang, Z.-J. et al. N-heterocyclic carbene/copper cooperative catalysis for the asymmetric synthesis of spirooxindoles. Angew. Chem. Int. Ed. 58, 12190–12194 (2019).
Li, R.-Z., Liu, D.-Q. & Niu, D.-W. Asymmetric O-propargylation of secondary aliphatic alcohols. Nat. Catal. 3, 672–680 (2020).
Xu, Y.-W., Li, L. & Hu, X.-P. Enantioselective copper-catalyzed [3 + 3] cycloaddition of tertiary propargylic esters with 1H‑pyrazol-5(4H)‑ones toward optically active spirooxindoles. Org. Lett. 22, 9534–9538 (2020).
Liu, S.-Y., Tanabe, Y., Kuriyama, S., Sakata, K. & Nishibayashi, Y. Ruthenium-catalyzed enantioselective propargylic phosphinylation of propargylic alcohols with phosphine oxides. Angew. Chem. Int. Ed. 60, 11231–11236 (2021).
Shen, L., Lin, Z., Guo, B. & Zi, W. Synthesis of cycloheptanoids through catalytic enantioselective (4 + 3)-cycloadditions of 2-aminoallyl cations with dienol silyl ethers. Nat. Synth. 1, 883–891 (2022).
Gong, F. et al. Asymmetric semipinacol rearrangement enabled by copper-catalyzed propargylic alkylation. ACS Catal. 12, 12036–12044 (2022).
Zhang, Y., Tanabe, Y., Kuriyama, S., Sakata, K. & Nishibayashi, Y. Interplay of diruthenium catalyst in controlling enantioselective propargylic substitution reactions with visible light-generated alkyl radicals. Nat. Commun. 14, 859 (2023).
Arachchi, M. K., Schaugaard, R. N., Schlegel, H. B. & Nguyen, H. M. Scope and mechanistic probe into asymmetric synthesis of α‑trisubstituted-α-tertiary amines by rhodium catalysis. J. Am. Chem. Soc. 145, 19642–19654 (2023).
Nguyen, A. T. & Kim, H. K. Recent advances in synthetic routes to azacycles. Molecules 28, 2737–2781 (2023).
Zhou, J. Multicatalyst System in Asymmetric Catalysis (Wiley, 2014).
Lu, L.-Q., Chen, J.-R. & Xiao, W.-J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 45, 1278–1293 (2012).
Sánchez-Roselló, M., Aceña, J. L., Simón-Fuentesa, A. & del Pozo, C. A general overview of the organocatalytic intramolecular aza-Michael reaction. Chem. Soc. Rev. 43, 7430–7453 (2014).
Wink, M. Quinolizidine alkaloids: biochemistry, metabolism and function in plants and cell suspension cultures. Planta Med. 53, 509–514 (1987).
Edwards, P. D. et al. Application of fragment-based lead generation to the discovery of novel, cyclic amidine β-secretase inhibitors with nanomolar potency, cellular activity and high ligand efficiency. J. Med. Chem. 50, 5912–5925 (2007).
Sakata, K., Goto, Y., Yoshikawa, T. & Nishibayashi, Y. Enantioselectivity in ruthenium-catalyzed propargylic substitution reactions of propargylic alcohols with acetone: a DFT study. Chem. Asian J. 16, 3760–3766 (2021).
Sakata, K., Uehara, Y., Kohara, S., Yoshikawa, T. & Nishibayashi, Y. Effect of propargylic substituents on enantioselectivity and reactivity in ruthenium-catalyzed propargylic substitution reactions: a DFT study. ACS Omega 7, 36634–36642 (2022).
Acknowledgements
We are grateful for financial support from the NSFC (21725203 and 21971067, J.Z.; 21871090, F.Z.), the Shanghai Science and Technology Innovation Action Plan (no. 20JC1416900, J.Z.), the Innovation Program of Shanghai Municipal Education Commission (2023ZKZD37, J.Z.) and the Fundamental Research Funds for the Central Universities. Li-Xin Dai on the occasion of his 100th birthday.
Author information
Authors and Affiliations
Contributions
J.Z. and F.Z. conceived the idea. Z.Z. performed the experiments. Z.Z., Y.S. and H.L. collected and analysed the data. Z.Z., Y.S., Y.G., D.-L.T., H.L. and Z.-P.Z. prepared the starting materials. X.W. performed the DFT calculation studies. F.Z. and J.Z. directed the project and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Qin Yin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Less successful examples of challenging substrates.
Despite the broad substrate scope shown in Table 1, there still had some challenging substrates unable to afford the desired amines in satisfactory enantiomeric excess, including carbonates derived from ketones with lesser steric dissimilarities between two substituents, primary amines such as cyclohexyl amine, and acyclic secondary amines such as N-methylaniline. However, the use of our sterically confined PYBOX ligands could still afford obviously better enantioselectivity (for details, see Section 5.3 in SI). Condition A: α-alkyl ester 1 (0.05 mmol), amine 3 (0.06 mmol), CuBr2 (10 mol%), L (12 mol%), N,N-dimethylpiperazine (0.2 mmol) in n-PrOH (0.5 mL) at –20 °C for 2.5 days. Condition B: α-aryl ester 2 (0.05 mmol), amine 3 (0.06 mmol), CuCl2·2H2O (10 mol%), L15 (12 mol%), N-methylpiperidine (0.2 mmol) in MeOH (0.5 mL) at –20 °C for 2.5 days. Yield was determined by 1H NMR with 1,3,5-trimethoxybenzene as internal standard. The e.e. values were determined by chiral HPLC. aAt 0 °C. bRun for 5 days.
Supplementary information
Supplementary Information
Supplementary Tables 1–18, Figs. 1–8, Chiral ligand and starting material preparation, Experimental procedures, Synthetic transformations, Mechanistic studies and Product characterization.
Supplementary Data 1
Crystallographic data for compound 4aa; CCDC reference 2212823.
Supplementary Data 2
Crystallographic data for compound 5e; CCDC reference 2304114.
Supplementary Data 3
Crystallographic data for compound 5l; CCDC reference 2304057.
Supplementary Data 4
Crystallographic data for compound 8h; CCDC reference 2212826.
Supplementary Data 5
Crystallographic data for compound 9a; CCDC reference 2212213.
Supplementary Data 6
Crystallographic data for compound 22; CCDC reference 2226279.
Supplementary Data 7
Computational details and Cartesian coordinates.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Sun, Y., Gong, Y. et al. Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles. Nat. Chem. 16, 521–532 (2024). https://doi.org/10.1038/s41557-024-01479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-024-01479-z