« Prev Next »
You might think more genes are needed to make more complex organisms, but that is not the case. For example, we humans are thought to have between 25,000 and 30,000 genes that encode the proteins for all the parts of our bodies. On the other hand, the tiny roundworm Caenorhabditis elegans has nearly as many genes as we do—approximately 20,000—but far fewer body parts.
Given this situation and others like it, the variety of cell types in a given organism may be a better barometer for complexity in life than a strict count of the number of genes. The number of gene products can also greatly exceed the number of genes through such mechanisms as alternative splicing. For instance, scientists estimate that mammals have as many as 1014 different types of cells; one billion of those are different kinds of neurons in the brain alone. In contrast, C. elegans has a total of 302 neurons.
Cell Differentiation and Gene Expression
When "born" through cell division, cells are not static creatures; rather, they must transition to a mature phase. Cells start out "naïve" (in an early embryo, for example), and then, by means of expressing particular genes and proteins, differentiate into various mature cell types, whether sensory neurons, muscle cells, or red blood cells, to name a few.
So how do the different cell types develop? If you take a snapshot to capture a particular cell state, the picture you see is determined by how the genes contained in that cell are expressed. In fact, scientists think that the regulation of genes via transcription factors and chromatin is as important as the presence and nature of the genes themselves. Because there are fewer transcription factors than there are cell types, the key to cell differentiation lies in the combination of chromatin structures and transcription factors at particular genes during specific points of transition. This means that an entire photo gallery of phenotypes can result from different combinations of transcription factor activity.
Gene expression is controlled by multiple molecular systems, which become more elaborate as one moves up the evolutionary chain. Transcription factors are proteins that bind to specific DNA sequences, working either independently or in a concerted fashion. Further, chromatin remodeling can allow or prevent transcriptional apparatuses from navigating to their binding sites on DNA.
Elements in DNA That Regulate Gene Expression
Nucleotide sequences located immediately 5' of the transcription start site usually serve as regulatory regions involved in gene expression. In eukaryotes, expression also depends on enhancer sequences or silencer sequences located near genes. Many promoters contain an element called a TATA box. A specific protein binds to this sequence, and this protein interacts with RNA polymerase II, thereby promoting transcription initiation. The regulation of this binding depends on activating sequences within the promoter or enhancer. These sequences serve as binding sites for several similar and several different sequence-specific transcription factors.
The majority of genes in simple eukaryotic yeast contain a single upstream activating sequence located within a few hundred base pairs of the TATA box (Figure 1). In contrast, a typical animal gene is regulated by its adjacent promoter plus several enhancers that can be located in 5' and 3' regulatory regions, as well as within introns. Enhancers are, on average, 500 base pairs in length and contain as many as 10 binding sites for multiple transcription factors. For example, a gene might be regulated by two different activators and one repressor.
Tissue-specific enhancers can work over long distances. For instance, the enhancer sequences that regulate embryonic expression of Igf-2 in both mice and humans are found more than 100 kilobases from the transcription start site. Enhancers can also work independently of one another to direct composite patterns of gene expression when linked within a common region on the same DNA strand.
The Role of Chromatin Structure in Differentiation
Histones, or the proteins in chromatin around which DNA is packaged, also perform a function in gene regulation. In general, they act as "bouncers" that determine whether transcription factors gain access. However, modifications of histones can open up a gene, while modifications of DNA can shut it down. In general, methylation of DNA makes chromatin more tightly bound and results in downregulation or inhibition of gene transcription. Conversely, acetylation of histones loosens bindings and generally promotes transcription and translation. Methylation of histones can increase or decrease their acetylation, and it therefore acts as part of the regulatory "histone code."
Histone methylation was recently identified as an early determinant of cell type in embryonic development. At a very early stage of cell division, daughter cells are no longer all equal and symmetrical. When this happens, the smaller daughter cells move to the interior of the embryo and become the inner cell mass of stem cells. Upon observing this phenomenon, researchers hypothesized that the differences between cells (at least in mammalian embryos) first emerged at this point in development. They thus decided to test their prediction through a series of experiments beginning at the point of egg fertilization. Specifically, the investigators wondered how early in the process of embryonic cleavage the cells would become different from each other.
Upon carrying out their study, the researchers found differences between mouse embryonic cells as early as the four-cell stage, before cells had segregated to either the inside or outside of the embryo (Torres-Pallida et al., 2007). Moreover, these differences were dependent on the cleavage plane and sequence of the first cell divisions of the embryo. Thus, while we often think of cell division in early embryos as being completely symmetrical, it is apparent that the two cells that result from the initial cleavage begin to accumulate differences very early.
This 2007 study also showed that cell fate and transcription activity were determined by the level of histone methylation. The higher the levels of methylated histone H3 at specific arginine residues, the more inclined the cells were to gain qualities of inner embryonic cells, which would be expected to have stem-cell-like properties. Thus, manipulating epigenetic information in this way can influence cell fate determination in mammals.
Protein Interactions and Systems Biology
Thus, in a 2008 study, biologist Michael Stumpf and colleagues tried to determine just how large protein interaction networks were in different organisms (Stumpf et al., 2008). Using bioinformatics, the team estimated that 650,000 protein interactions occur in humans; this number is approximately three times more than that in the roundworm and 10 times more than that in the fruit fly. Moreover, it seems that a single protein can have dozens, if not hundreds, of different interactions. Consider the protein calmodulin, for example, whose interactions have been mapped using the Database of Interacting Proteins (DIP). (See Figure 2.)
In a commentary that accompanied Stumpf's article, Luis Nunes Amaral (2008) wrote, "These numbers provide a sobering view of where we stand in our cataloging of the human interactome. At present, we have identified <0.3% of all estimated interactions among human proteins. We are indeed at the dawn of systems biology." Research into intracellular protein reactions is thus certain to continue and expand in the future.
References and Recommended Reading
Levine, M., & Tijan, R. Transcription regulation and animal diversity. Nature 424, 147–151 (2003) doi:10.1038/nature01763 (link to article)
Luger, K., et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251–260 (1997) doi:10.1038/38444 (link to article)
Nunes Amaral, L. A. A truer measure of our ignorance. Proceedings of the National Academy of Sciences 105, 6795–6796 (2008) doi:10.1073/pnas.0802459105
Stumpf, M. P. H., et al. Estimating the size of the human interactome. Proceedings of the National Academy of Sciences 105, 6959–6964 (2008) doi:10.1073/pnas.0708078105
Torres-Padilla, M. E., et al. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445, 214–218 (2007) doi:10.1038/nature05458 (link to article)



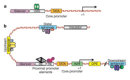
 Figure 1
Figure 1



























