« Prev Next »
Among researchers, it is common knowledge that transcription factors bring about changes in gene expression, at least in part, by binding directly to special sites within a cell's DNA. But how do scientists determine whether and where this binding occurs? Several techniques can be used to examine transcription factor binding, including DNA footprinting and electrophoretic mobility shift assays (EMSAs), which are also known as gel shift assays. Both of these techniques are fundamental to the analysis of gene regulation.
DNA Footprinting
DNA footprinting is an in vitro technique used to examine the binding of proteins to specific regions of DNA. This technique cleverly exploits the fact that when a transcription factor is bound to DNA with a certain affinity, the DNA is protected from degradation by nucleases. The transcription factor of interest thus leaves its "footprint" on the DNA.
A typical footprinting experiment involves radioactive labeling of the DNA region of interest—usually a larger chunk of DNA suspected to contain one or more transcription factor binding sites. This fragment is radioactively labeled on one end and then incubated in vitro both with and without the transcription factor of interest. Next, the DNA is treated with a nuclease, such as DNAse I, that digests only unprotected DNA. Finally, the DNA products resulting from the digestion are separated on a polyacrylamide gel, which is dried and placed against X-ray film to enable visualization. The resulting image is referred to as an autoradiograph.
Figure 1 shows an example of an autoradiograph resulting from a typical DNA footprinting assay. To see the footprint, compare the two leftmost lanes. A ladder of bands of continually decreasing size results from DNAse digestion of DNA only. However, in the presence of the transcription factor Sp1, a range of band sizes is missing from the lane, indicating a region of DNA that is protected by being bound to Sp1. It is important to note that DNAse digestion is typically not allowed to proceed to completion; such partial digestion results in a range of fragment lengths. The length of the DNA fragment containing the Sp1 binding site can be determined by including standard lanes (that is, lanes containing DNA fragments of known size).
The results depicted in Figure 1 also consider the role of zinc (Zn) in Sp1-DNA binding. Sp1 is a protein that has multiple zinc-finger motifs, or regions known to bind to zinc; however, the inclusion of EDTA, an agent that chelates metal ions, inhibits zinc binding. Thus, notice that in the lane with EDTA, the binding between Sp1 and DNA is lost. Moreover, note that when the investigators added back ions of zinc (lane 5), cobalt (lane 6), and nickel (lane 7), they saw that only the addition of zinc ions resulted in Sp1 binding.
Gel Shift Assays
Gel shift assays, or EMSAs, are another technique used to study transcription factor binding. Here, the basic concept is that a piece of DNA will migrate through a gel more slowly if it is bound to a protein, such as a transcription factor. A difference, or "shift," in the rate of migration in the presence and absence of transcription factor is thus taken as evidence of binding.
Scientists can use molecular biology techniques to create different protein fragments in order to determine which specific part of a protein binds to a piece of DNA. For instance, in a gel shift assay, plasmids encoding peptides can be induced to express truncated proteins. The only protein fragments that bound to the DNA used in this experiment were those that contained at a specific minimum size. Of course, the longer an amino acid chain is, the larger the resulting protein is, which means that the protein takes longer to travel through the gel. Thus, by examining the results and noting that binding did not occur when the protein contained n# of amino acids but did take place when the protein had N+100# of amino acids, the scientists were able to conclude that amino acids between N and N+100 were required for the protein-DNA interaction under consideration.
One important requirement for gel shift assays is that the gel-running conditions not disrupt protein-DNA interactions. These are called nondenaturing conditions, and they differ from the conditions used for routine DNA visualization.
Variations on the Gel Shift Theme: Competition and Supershift Assays
Several modifications can be incorporated into the basic gel shift assay process to strengthen the quality of the results. For instance, to provide further proof that a protein-DNA binding interaction is unique, or specific to a particular sequence, researchers often perform a so-called competition assay. Such an assay involves not only radioactively labeled ("hot") DNA, but also addition of an excess of unlabeled ("cold") DNA of identical sequence. Here, the cold DNA sequence should replace the hot DNA as the transcription factor binding partner, thus eliminating detectable band shift on the autoradiograph, which only detects the hot DNA. Reversal of the gel shift by cold DNA of known sequence provides evidence of the importance of that sequence in transcription factor binding.
Although a competition assay provides confirmation of the DNA sequence involved in binding, confirmation of the identity of the protein causing the shift is provided by a supershift assay. A supershift control is especially important if the source of a transcription factor is nuclear extract, because multiple transcription factors will be present in such an extract; however, if a single, purified transcription factor is used in a gel shift experiment, a supershift control is less critical. The supershift assay exploits the fact that antibodies that specifically bind to proteins of interest can be isolated. Here, addition of an antibody specific to a known transcription factor of the DNA suspected to be in the DNA-protein complex should cause an even larger change in migration rate on a nondenaturing gel, therefore triggering a "supershift."
To better understand how this technique works, consider the example of a study in which investigators examined the regulation of a drug-metabolizing gene called DDR. Inspection of the promoter region of DDR revealed consensus sequences for hepatocyte nuclear factor (HNF) proteins (Figure 2; Ozeki et al., 2001). Specifically, the researchers suspected that one member of this protein family, HNF-4, was involved in gene regulation, while another family member, HNF-1, was not. The research team thus made a radioactive probe for the promoter region they were studying and called it Foot A; their evidence suggested that there was a protein in the cell nucleus that bound to this probe. Then, after combining the labeled probe with nuclear extract (denoted "N.E." in the figure), the investigators noted a clear shift in the location of the probe (lane 2).
Next, the researchers performed a competition experiment using an excess of Foot A that was unlabeled; in this case, the shift was no longer visible. In fact, mixing excess unlabeled probe (lane 3, Foot A) resulted in a complete loss of the shifted product. (It is important to note that the protein in the nuclear extract was still binding to the probe, but this specific protein-DNA complex could not be seen because the majority was binding to the unlabeled probe, which could not be detected in this experiment.) The investigators were also able to interfere, or compete, for the protein in the nuclear extract when they added a sequence that contained a known HNF-4 binding site (α1-AT-A), but this interference did not occur when they added a sequence with a known HNF-1 binding site (α1-AT-B).
Although this first experiment confirmed that there was something in the nuclear extract that bound to the probe, the specific interacting molecule was not identified. In order to definitively demonstrate that it was an HNF-4 protein binding to their promoter, the investigators next performed a supershift assay. By mixing the labeled probe and nuclear extract again, the research team reformed the complexes. Then, in order to identify specific protein components of the DNA-protein complex, they added antibodies that only recognized one specific protein. The resulting supershifts (shown in Figure 2B) revealed that both HNF-4α and HNF-4γ, but not HNF-1α or HNF-1β, bound to the Foot A probe. In these experiments, the antibody-protein-DNA complexes migrated even more slowly than the protein-DNA complexes alone (at the locations noted by arrows), thus allowing the investigators to track specific binding to the bands that were supershifted.
Together, DNA footprinting and gel shift assays provide essential information about physical interactions between transcription factors and DNA. As a result, these techniques have left a lasting footprint on the study of gene regulation.
References and Recommended Reading
Fried, M., & Crothers, D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Research 9, 6505–6525 (1981)
Garner, M. M., & Revzin, A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: Application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Research 9, 3047–3060 (1981)
Kadonaga, J. T., & Tjian, R. Affinity purification of sequence-specific DNA binding proteins. Proceedings of the National Academy of Sciences 83, 5889–5893 (1986)
Ozeki, T., et al. Co-operative regulation of the transcription of human dihydrodiol dehydrogenase (DD)4/aldo-keto reductase (AKR)1C4 gene by hepatocyte nuclear factor (HNF)-4a/g and HNF-1a. Biochemical Journal 355, 537–544 (2001)



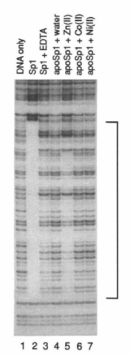
 Figure 1
Figure 1