« Prev Next »
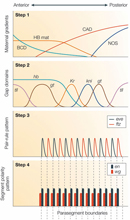
Within most differentiated cells, several levels of induction produce a multitude of transcription factors. Moreover, during embryonic development, the actual process of differentiation itself requires an integrated system of transcription factors that turn gene expression on and off with strict precision and timing. This is particularly true in the case of segmentation.
Segmentation is the term applied to the differentiation patterns of vertebrates and arthropods, in which serial repetition of similar anatomical modules creates a body axis. In vertebrates, segmentation results in the development of the spine, head, and limbs; in insects, it results in formation of the abdomen, head, and thorax. Segmentation is believed to be the result of the rhythmic activation and deactivation of certain signals that dictate specific relevant cellular processes. This oscillating process is called the segmentation clock. As scientists learn more about the timing and mechanisms of the segmentation clock, they also continue to uncover information about regulatory interactions and signal cascades in general.
The Role of Cascades in Insect Segmentation
A large portion of the existing research on genetic control of segmentation has been conducted using fruit flies. For instance, over the past 25 years, more than 40 genes have been identified as playing a role in segmentation in Drosophila melanogaster (Peel et al., 2005). Segmentation in fruit flies is controlled by a cascade of transcription factors, some of which have been found in other arthropods. Typically, cascade systems occur within individual cells; however, during segmentation processes, signal cascades are carried across multiple cells within specific regions of a developing embryo.
Pre-Existing Transcripts in Eggs Promote Formation of Transcription Factor Gradients
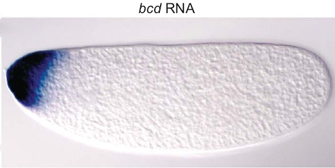
Expression of messenger RNA (mRNA) transcripts is controlled in time and space so that the protein products of these transcripts appear in gradients along each fertilized egg. Some of these proteins repress translation of other proteins, which results in higher concentrations of different polypeptides in the anterior and posterior parts of the developing embryo. For example, nanos inhibits hunchback translation; thus, while hunchback mRNA is ubiquitous, hunchback protein is more concentrated in the anterior portion of the embryo. Bicoid and hunchback are two important gene products involved in this process (Figure 2). In fact, mutations in the genes that code for these products have been shown to result in abnormal larval development. Larvae that carry these mutations may feature missing heads, thoracic regions, or abdominal regions, depending on the exact mutation.
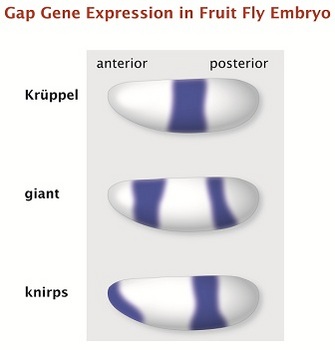
Early Gradients Activate Gap Genes
Pair-rule gene expression is not stable, however, and many of these genes serve as transcription factors to induce the expression of segment polarity genes, like engrailed (en) and wingless (wg). Boundaries between developing segments are defined by en or wg expression patterns that are established through the aperiodic expression of the pair-rule factors.
Vertebrate Somitogenesis and Notch Signaling
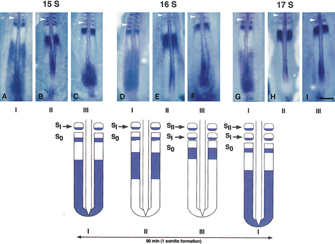
In vertebrates, the development of the precursor to an animal's skeletal system is called somitogenesis. Because the vertebrate spine consists of repeating units, the development of these units can be compared to the segmentation process in arthropods. Spinal development, however, is controlled by an oscillating process that is dependent on a group of genes in the Notch pathway, only some of which have homologues in the Drosophila segmentation control machinery.
During vertebrate spinal development, oscillations in gene expression occur on a temporal basis. Specifically, a signal in the Notch pathway is "on" when the spinal precursor, or somite, begins to form, but is "off" by the time somite formation is complete. Because Notch serves as a transcription factor, it alters the concentrations of its targets at the front of the somite as compared to the rear, thus establishing different segments.
The Notch pathway is activated when a protein ligand signal from adjacent cells, called Delta, binds to Notch on a cell's surface. In this regard, Notch serves as a transmembrane receptor. Upon Delta binding, the part of Notch that is found in the cell's cytoplasm is released from the membrane. This part of Notch serves as a transcription factor, and it plays a major role in the segmentation clock. A cascade then activates several downstream genes, including the hairy/enhancer of split (hes) gene, which encodes another transcription factor. Indeed, the hairy1 transcription factor was the first such factor that was determined to be regulated in a temporal fashion; in investigations using chickens, researchers noted that the expression of this protein was consistent with the time it takes to form a single somite (90 minutes) (Figure 4; Palmeirim et al., 1997). The negative feedback loop of the Notch pathway results in waves of gene expression and, ultimately, the patterns of body development observed during embryonic growth. Notch signaling appears to be primarily found in vertebrates, and there is no evidence that it plays a role in early insect segmentation (Peel et al., 2005).
Several models have been proposed to explain the oscillation of gene expression in different organisms. In zebrafish, it seems that the Her1 and Her7 transcription factors can repress their own expression periodically, thus creating an oscillating effect on gene expression (Dequeant & Pourquie, 2008). A similar negative feedback system controlled by the HES protein family has also been observed in mice. However, in mice, expression DNA microarray analyses also identified a host of additional genes that are expressed in a cyclical pattern and appear to be associated with the segmentation clock. Whether these genes are controlled directly by the HES family of transcription factors or indirectly through HES-mediated induction of transcription factors acting downstream is not yet entirely clear.
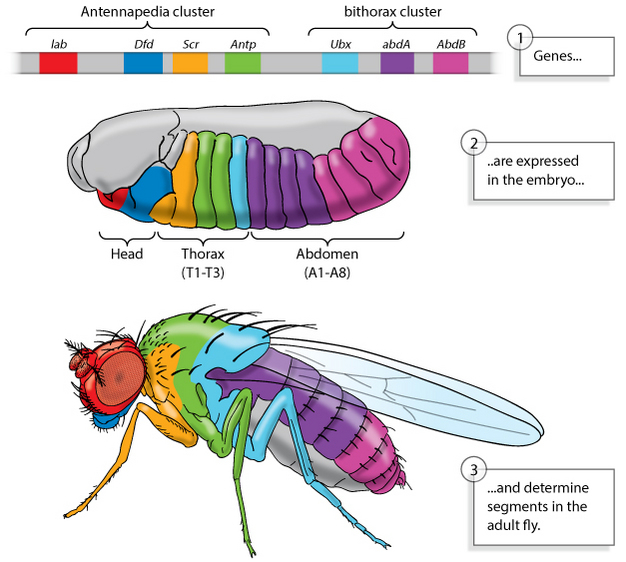
The Role of Hox Genes
It appears that the role of maternal factors in fruit flies is not universal among all insects, nor is it common to vertebrates. However, later in the cascades of nearly all insects and vertebrates, the Hox genes play a role in identifying each segment and defining what part of the body each segment will become (Figure 5). In fruit flies, the discovery of Hox genes was facilitated by observations of spontaneous mutants. Mutations in specific Hox genes caused legs to grow out of a fly's head, wings to grow where they shouldn't be, and a number of other physical deformities.
Future Directions
Thanks to extensive experimentation with fruit flies, much is known about the genetic factors that control body patterns. Similar experimentation with vertebrates is much more complicated and introduces a host of ethical issues. For these reasons, there is still little data about vertebrate segmentation and the oscillating clock. However, information regarding certain patterns, such as those discussed above, might help researchers develop strategies to treat diseases caused by abnormal segmental patterning during human development.
References and Recommended Reading
Dequeant, M., & Pourquie, O. Segmental patterning of the vertebrate embryonic axis. Nature Reviews Genetics 9, 370–382 (2008) doi:10.1038/nrg2320 (link to article)
Kageyama, R., et al. The hes gene family: Repressors and oscillators that orchestrate embryogenesis. Development 134, 1243–1251 (2007) doi:10.1242/dev.000786
Nibu, Y., et al. dCtBP mediates transcriptional repression by Knirps, Kruppel and Snail in the Drosophila embryo. EMBO Journal 17, 7009–7020 (1998) doi:10.1093/emboj/17.23.7009
Palmeirim, I., et al. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 91, 639–648 (1997)
Peel, A.,
et al. Arthropod segmentation: Beyond the Drosophila paradigm. Nature
Reviews Genetics 6, 905–916 (2005) doi:10.1038/nrg1724 (link to article)
Sadava, D., et al. Life: The Science of Biology, 8th ed. (Sunderland, MA/Freeman, New York, Sinauer Associates, 2006)



 Figure 1: Bicoid mRNA in a fruit fly (Drosophila) embryo.
Figure 1: Bicoid mRNA in a fruit fly (Drosophila) embryo.