« Prev Next »
The Hox genes are a set of transcription factor genes that exhibit an unusual property: They provide a glimpse of one way in which gene expression is translated into the many different forms that animals (metazoans) exhibit. For the most part, the genome seems to be a welter of various genes scattered about randomly, with no order present in their arrangement on a chromosome—the order only becomes apparent in their expression through the process of development. The Hox genes, in contrast, seem like an island of comprehensible structure. These are genes that specify segment identity—whether a segment of the embryo will form part of the head, thorax, or abdomen, for instance—and they are all clustered together in one (usually) tidy spot. Within that cluster, there is even further evidence of order.
Hox Genes in Drosphila
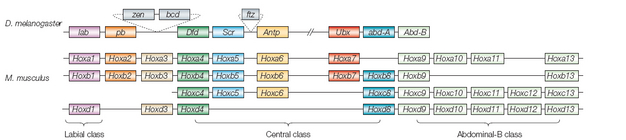
To better understand the arrangement and role of Hox genes, take a look at the Drosophila portion of Figure 1. As shown, in Drosophila there are eight Hox genes in a row, and the genes' order within that row reflects their order of expression in the fly body. The gene found on the left or 3' end of the DNA strand, denoted lab (labial), is expressed in the head; on the other hand, the gene at the right end of the DNA strand, Abd-B (Abdominal-B), is expressed at the end of the fly's abdomen.
Knocking out individual Hox genes in Drosophila causes homeotic transformations—in other words, one body part develops into another. A famous example is the Antennapedia mutant, in which legs develop on the fly's head instead of antennae. The Hox genes are early actors in the cascade of interactions that enable the development of morphologically distinct regions in a segmented animal. Indeed, the activation of a Hox gene from the 3' end is one of the earliest triggers that lead the segment to develop into part of the head.
Hox Genes in Mice and Other Vertebrates
Now examine the mouse portion of Figure 1. Vertebrates, including mice, have Hox genes that are homologous to those of the fly, and these genes are clustered in discrete locations with a 3'-to-5' order reflecting an anterior to posterior order of expression. There are several differences between the mouse and fly Hox genes, however. One obvious difference is that there are more Hox genes on the 5' side of the mouse segment; these correspond to expression in the tail, and flies do not have anything homologous to the chordate tail. Another difference is that, in the mouse, there are four banks of Hox genes: HoxA, HoxB, HoxC, and HoxD. Vertebrates have these parallel, overlapping sets of Hox genes, which suggest that morphology could be a product of a combinatorial expression of the genes in the four Hox clusters. This means that there could be a Hox code, in which identity can be defined with more gradations by mixing up the bounds of expression of each of the genes.
In the fly, the situation is much simpler. Because each segment more or less expresses only one Hox gene, mutating or knocking out a single Hox gene will have an effect on the corresponding body segment. In vertebrates, though, each segment has at least two, and in some cases four, Hox genes that may be involved in its development. As a result, there is the possibility of redundancy.
For instance, in mice, the HoxA3 gene is expressed in the anterior cervical vertebrae, near the region where the first neck vertebra articulates with the skull. Deleting HoxA3 has no detectable effects on that joint; either its influence is too subtle to measure, it affects some other aspect of cervical specification, or it has a partner gene that takes over its job in its absence. Notice in Figure 1 that HoxA3 has a paralog, or copy, called HoxD3, which is expressed in a very similar place. When HoxD3 is mutated all by itself, there is a serious abnormality; here, the first neck vertebra has a partial fusion with the base of the skull. However, knocking out both HoxA3 and HoxD3 shows that HoxA3 is important after all; without it, the first neck vertebra doesn't form. In fact, in this instance, it is thought that the initial mesodermal tissue for the bone is so thoroughly respecified that it fuses completely with the skull instead, becoming part of the base of the skull.
These results tell us that a combination of Hox genes is required for the proper development of the first cervical vertebra. They also complicate analyses by indicating that knocking out the Hox genes one at a time in the mouse will result in cases in which no phenotype or a partial phenotype will be seen, even when the gene has an important role to play in that segment. Ultimately, all of the paralogous genes need to be knocked out. That is, in order to see what the third Hox gene does in the cluster, for instance, you need to carry out a paralogous deletion that destroys the function of HoxA3, HoxB3, and HoxD3 (there is no HoxC3) to assess the phenotype.
This phenomenon is also one reason why homeotic mutations in vertebrates are so rarely seen. In flies, one gene can be mutated, resulting in a haltere being transformed into a wing, or an antenna turning into a leg; in the mouse, two to four genes must be simultaneously removed to get a similar complete transformation.
Paralogous Knockouts in Mice
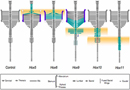
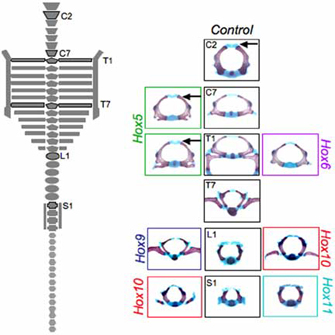
Technology has now progressed to the point at which researchers are beginning to publish descriptions of mice that have had complete paralogous gene sets knocked out. Cross-sections of vertebrae from such mice are shown on the right side of Figure 2. The middle column shows the normal control, or what the vertebrae are supposed to look like in a nonmutant mouse. On either side are the mutant forms for each of the paralogous mutants.
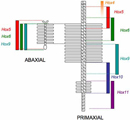
The image on the left side of Figure 2 illustrates the skeletal morphology that was assessed. At the top are the cervical vertebrae, C1 through C7, which are not associated with ribs. Next are the ribbed thoracic vertebrae, T1 through T13. T1 through T7 also wrap around to connect to the sternum, which is part of the abaxial skeleton (the vertebrae are part of the primaxial skeleton). Then come the lumbar vertebrae, L1 through L6, the sacral vertebrae, S1 through S4 (which articulate with the pelvis), and the many small tail vertebrae. Each of these bones has a discrete, recognizable morphology.
Look at T1 in the control. In addition to the oval profile of the vertebra, there is supposed to be a stout pair of ribs. To the left, bordered in green, is the effect of a complete knockout of all of the Hox5 genes—HoxA5, HoxB5, and HoxC5. Here, the ribs have started to form, but they are incomplete. This is a partial transformation toward a more cervical morphology. To the right, bordered in purple, is what happens to T1 when all of the Hox6 genes (HoxA6, HoxB6, and HoxC6) are taken out; as you can see, it looks almost exactly like the control C7 vertebra. This is a complete homeotic transformation of T1 to C7.
Each Hox paralog holds sway over a particular morphological domain. Notice that not only are there combinatorial arrangements within a bank of paralogs (those subtleties are not illustrated in Figure 4), but there are also combinations of sets of paralogs. The sacral segments, for instance, are defined by the expression of both Hox10 and Hox11 genes—one can imagine a kind of logical gate in the regulatory circuitry that switches on the downstream genes that signal the specific morphology required for joining to the pelvis only in the presence of both sets of Hox genes. Other experiments suggest that the ground state for a segment is to be thoracic-like and develop limbs; Hox10 and Hox11 may also have functions to suppress rib formation.
Understanding Body Patterning Through Hox Genes
What the Hox code represents is a somewhat digital mechanism for regulating axial patterning. By mixing and matching combinations of the expression of a small number of Hox genes, organisms generate a greater range of morphological possibilities. Wellick's experiments described in this summary (Wellick, 2007) are at a rather coarse level, revealing broad chunks of the Hox regulatory scheme, but future work should distill out the details, including the specific and finer aspects of morphological regulation. Genetic control of body shape is a difficult process to comprehend—but the Hox system is one place in which researchers are getting closer to comprehending this process.
References and Recommended Reading
Pearson, J. C., et al.
Modulating Hox gene functions during
animal body patterning. Nature Reviews
Genetics 6, 893–904 (2005) (link to article)
Wellik, D. M. Hox patterning of the vertebrate axial skeleton. Developmental Dynamics 236, 2454–2463 (2007)



 Figure 1: Genomic organization of the Hox gene cluster.
Figure 1: Genomic organization of the Hox gene cluster.