« Prev Next »
Bacteria have an amazing capacity to break down, or catabolize, different carbohydrates and use them as sources of carbon and energy; these carbohydrates include both glucose and lactose. The classic bacterial regulatory system for lactose utilization, the lac operon, is governed by repression (i.e., negative transcriptional regulation); specifically, the product of the lac i gene represses this system whenever lactose is absent. But, remarkably, in the presence of both glucose and lactose, E. coli bacteria will break down all of the glucose before switching to lactose. Glucose is a good first choice because it enters a bacterium's metabolism more directly than lactose. But how does a bacterium "know" when to move from glucose to lactose? What is the molecular basis for this switch? And what about other carbohydrates? How is the metabolism of these substances regulated?
Lactose Metabolism Is Regulated by Glucose Catabolites
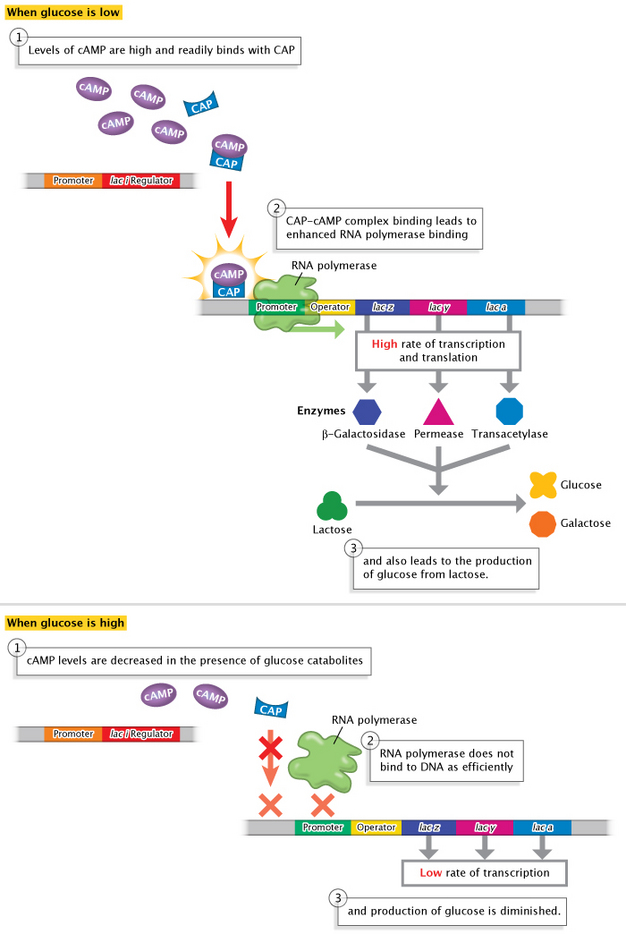
In bacteria such as E. coli, it appears that the products of carbohydrate metabolism themselves activate the switch between glucose and lactose use. Specifically, the catabolites produced by the breakdown of glucose prevent production of a signal molecule called cyclic AMP (cAMP). In turn, the absence of cAMP results in a failure to activate production of the enzymes necessary to metabolize lactose, even when lactose is available. Glucose therefore stops activation of the lac operon (a cluster of coordinately regulated genes involved in lactose catabolism), which prevents lactose use and leads to preferential use of glucose. However, in the absence of glucose, the lac operon can be activated if the lac i repressor is turned off by lactose binding.
More specifically, in E. coli, the switch from glucose use to lactose use depends on the presence of both cAMP and a molecule called catabolite activator protein (CAP). CAP binds with cAMP, and the CAP-cAMP complex then binds to a specific DNA sequence found upstream of the lac operon operator and promoter. CAP-cAMP complex binding leads to enhanced RNA polymerase binding and activation of gene expression from the lac operon. Importantly, this process is affected by glucose levels, because cAMP levels are decreased in the presence of glucose catabolites. Thus, an elevation in cAMP concentration signals the absence of glucose, because lower glucose levels lead to increased cAMP levels. In turn, increased cAMP levels lead to enhanced expression of the lac operon. In the presence of glucose, however, intracellular levels of cAMP fall, which leads to a lack of lac operon activation. The lac operon is therefore positively regulated by the absence of glucose catabolites (Figure 1).
Arabinose Metabolism Is Regulated by the araC Protein
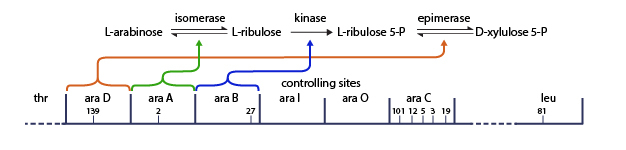
Not all forms of carbohydrate metabolism are activated and repressed in precisely this manner, however. For example, consider the process by which E. coli bacteria break down the carbohydrate arabinose. In E. coli, the L-arabinose operon directs expression of a variety of genes (araB, araA, and araD) that encode enzymes involved in arabinose metabolism (Figure 2). This operon has two transcription control regions, araI and araO, that correspond to the initiator and operator sequences, as well as a promoter region at which RNA polymerase binds to start operon expression. Expression of the L-arabinose operon is both positively and negatively regulated by the protein product of the araC gene.
In order to understand how one protein can be involved in both positive and negative regulation, it is first necessary to consider the protein's structure. All proteins have a specific three-dimensional shape that is intimately tied to their function. Moreover, many regulatory proteins, including the one encoded by araC, have a number of possible conformations, depending on the environment. In this case, the araC protein has one conformation in the presence of arabinose and a second conformation in its absence. In E. coli, the switch from one conformation to another, and therefore the commencement of gene transcription, happens within three seconds of the addition of arabinose to the environment (Hirsch & Schleif, 1973).
Thus, the araC regulatory gene produces one product that can exist in two physical states, or conformations. These two conformations, denoted P1 and P2, can repress or activate the L-arabinose operon, depending on which form is more prevalent. Acting as a homodimer, the product of araC can either bend the DNA or let it relax into a straighter form, which changes the ability of RNA polymerase to access the operator region (Figure 3). When arabinose is present, araC relaxes the DNA configuration so that transcription can occur, but when this carbohydrate is absent, the same protein constrains the DNA to reduce its ability to bind with RNA polymerase.
It is important to note that positive regulation of the L-arabinose operon by araC does not reach its maximal value unless there is no glucose present in the system; this is the same sort of catabolite sensitivity that is seen with the lac operon, in that cAMP receptor protein (CRP) also binds regulatory sequences in the L-arabinose operon (Greenblatt & Schleif, 1971). This mechanism allows bacteria to prioritize their energy sources, with a preference for the use of glucose.
References and Recommended Reading
Englesberg, E., Squires, C. & Meronk Jr., F. The L-Arabinose Operon in Escherichia coli. PNAS 62, 1100-1107 (1969).
Greenblatt, J., & Schleif, R. Arabinose C protein: Regulation of the arabinose operon in vitro. Nature New Biology 233, 166–170 (1971).
Hirsch, J., & Schleif, R. On the mechanism of action of L-arabinose C gene activator and lactose repressor. Journal of Molecular Biology 80, 433–444 (1973).



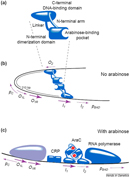
 Figure 3
Figure 3


























