Featured
-
-
Article |
 Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids
Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids
Alkene hydrofluoroalkylation offers a promising route to diverse fluoroalkylated compounds but current methods have limitations, such as needing expensive fluoroalkylating reagents. Now, leveraging iron photocatalysis and hydrogen-atom-transfer catalysis, a hydrofluoroalkylation method has been developed that utilizes feedstock chemicals such as trifluoroacetic acid as direct fluoroalkyl radical precursors, providing a redox-neutral, general protocol to introduce fluoroalkyl moieties.
- Kang-Jie Bian
- , Yen-Chu Lu
- & Julian G. West
-
Article |
 Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
The synthesis of optically enriched atropisomers has so far been limited to molecules containing aryl groups. Now a variant of non-aryl atropisomerism has been identified in vinyl sulfoxonium ylides, and an organocatalytic method has been developed to produce these molecules. This type of axial chirality is characterized by restricted rotation of the central C(sp2)–C(sp2) bond.
- Fengjin Wu
- , Yichi Zhang
- & Yong Huang
-
News & Views |
 An open-shell molecule that exhibits conformational dynamics
An open-shell molecule that exhibits conformational dynamics
Open-shell organic molecules with properties that can be modulated by external stimuli are of interest for spintronics applications. Now, an overcrowded alkene with open-shell tetraradical character has been synthesized in which the interaction between the π-conjugated subunits depends on the charge and spin state.
- Yoshito Tobe
-
News & Views |
 Enyne difluorination
Enyne difluorination
Fluorination strategies are important in assisting the synthesis of pharmaceuticals. Iodine(I/III) catalysis has become particularly useful for installing gem-difluoro groups but is limited to styrenes. Now, the hypervalent iodane-catalysed difluorination of enynes has enabled access to diverse homopropargylic difluorides.
- Rachel C. Epplin
- & Tanja Gulder
-
Meeting Report |
 Altogether changed, and yet the same
Altogether changed, and yet the same
Organic chemists meet biennially to present exciting developments in the realm of synthesis. Thomas Barber discusses the standout themes of this year’s international synthesis in organic chemistry symposium.
- Thomas Barber
-
Article |
 Stereocontrolled access to thioisosteres of nucleoside di- and triphosphates
Stereocontrolled access to thioisosteres of nucleoside di- and triphosphates
Nucleoside diphosphates and triphosphates impact nearly every aspect of biochemistry. Now, a modular, reagent-based platform has been developed to enable the stereocontrolled and scalable synthesis of a library of such molecules. This operationally simple approach provides access to pure stereoisomers of nucleoside α-thiodiphosphates and α-thiotriphosphates.
- Hai-Jun Zhang
- , Michał Ociepa
- & Phil S. Baran
-
Article
| Open Access Regioselective, catalytic 1,1-difluorination of enynes
Regioselective, catalytic 1,1-difluorination of enynes
Hypervalent iodine catalysis remains a powerful method to enable geminal difluoromethylenation of alkenes. However, the scope is mainly limited to styrene derivatives. Now, enynes have been validated as competent substrates where a formal 1,2-shift of the alkyne occurs, thereby enabling highly versatile homopropargylic difluorides to be generated.
- Zi-Xuan Wang
- , Keith Livingstone
- & Ryan Gilmour
-
Research Briefing |
 Rigid macrocycles with multiple hydrogen-bond donors for effective anion binding and transport
Rigid macrocycles with multiple hydrogen-bond donors for effective anion binding and transport
Aromatic oligoamide macrocycles have been developed in which the constrained backbone enforces hydrogen-bond donors to orient towards the macrocycle centre, forming a highly electropositive cavity. These macrocycles show strong binding for various anions and can partition into biomembranes to facilitate selective transmembrane anion transport.
-
Article |
 Aromatic pentaamide macrocycles bind anions with high affinity for transport across biomembranes
Aromatic pentaamide macrocycles bind anions with high affinity for transport across biomembranes
Effective synthetic anion receptors are challenging to design. Now, star-shaped macrocycles, with a cavity defined by multiple convergent amide NH and phenyl CH groups, have been synthesized in one pot from their monomeric building blocks. These macrocycles strongly bind a variety of anions, selectively transport chloride across cell membranes and restore the function of cystic fibrosis cells.
- Ruikai Cao
- , Robert B. Rossdeutcher
- & Bing Gong
-
Article |
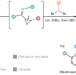 Modular synthesis of 1,2-azaborines via ring-opening BN-isostere benzannulation
Modular synthesis of 1,2-azaborines via ring-opening BN-isostere benzannulation
Preparation of monocyclic 1,2-azaborines, a unique class of benzene isosteres, has been challenging. Now, an efficient and modular method has been developed to access diverse multi-substituted 1,2-azaborines from readily available cyclopropyl imines/ketones and dibromoboranes. The reaction goes through an unusual ring-opening BN-isostere benzannulation mechanism.
- Hairong Lyu
- , Thomas H. Tugwell
- & Guangbin Dong
-
Article
| Open Access Tetrafluorenofulvalene as a sterically frustrated open-shell alkene
Tetrafluorenofulvalene as a sterically frustrated open-shell alkene
Tetrafluorenofulvalene (TFF) defies conventional rules of bond strength in organic chemistry. In particular, the central alkene bond of TFF becomes stronger in the quintet state and in the tetraanion. These changes arise from the unusual interplay between the twist, aromaticity and spin pairing in the π-electron system of TFF.
- Bibek Prajapati
- , Madan D. Ambhore
- & Marcin Stępień
-
News & Views |
 Hydrogenative double-bond deuteration
Hydrogenative double-bond deuteration
Deuterated compounds are used in many applications such as mass-spectrometry standards, drugs or in organic light-emitting diodes. Now, hydrogen-activated homogeneous pincer complex catalysts can be used to perform selective alkene deuteration with the cheapest available deuterium source, D2O.
- Anika Tarasewicz
- & Volker Derdau
-
News & Views |
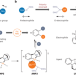 Aryl ortho methoxylation
Aryl ortho methoxylation
Aryl ethers are useful intermediates in organic synthesis and are found in countless biologically active compounds. Now, through palladium/norbornene cooperative catalysis and incorporation of a polarity-reversed N–O reagent as the O-electrophile, an efficient arene methoxylation approach has been successfully developed.
- Kun Zhao
- & Zhenhua Gu
-
Article |
 Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis
Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis
Merging carbonyls to form an alkene by removing their oxygens is rare, yet synthetically useful, and the selective combination of two different carbonyls is especially challenging. Now, two strategies for cross-metathesis of unbiased carbonyls have been developed. An Fe-catalysed carbene/ylide strategy affords Z-alkenes, while Cr-catalysed gem-di-metals yield E-alkenes.
- Lumin Zhang
- & David A. Nagib
-
Article |
 Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Cobalt(II) complexes of porphyrins have dominated the development of metalloradical catalysts. Now it has been shown that five-coordinate iron(III) complexes of porphyrins with an axial ligand are also potent metalloradical catalysts for olefin cyclopropanation. They are shown to react with different classes of diazo compounds via a stepwise radical mechanism.
- Wan-Chen Cindy Lee
- , Duo-Sheng Wang
- & X. Peter Zhang
-
Article |
 Hydrogenative alkene perdeuteration aided by a transient cooperative ligand
Hydrogenative alkene perdeuteration aided by a transient cooperative ligand
Deuterogenation methods typically introduce only two deuterium atoms per unsaturation. Now the single-step hydrogenative perdeuteration of alkenes has been achieved using H2 and D2O, with incorporation of up to 4.9 D atoms per C=C double bond. The reaction is catalysed by a ruthenium pincer complex with a catalytic amount of thiol, which serves as a transient cooperative ligand.
- Jie Luo
- , Lijun Lu
- & David Milstein
-
Article |
 Ortho-C–H methoxylation of aryl halides enabled by a polarity-reversed N–O reagent
Ortho-C–H methoxylation of aryl halides enabled by a polarity-reversed N–O reagent
Pd/norbornene cooperative catalysis provides a strategy for arene functionalization, but the electrophile scope is typically limited to ‘soft’ elements such as carbon, nitrogen and sulfur. Now the ortho-C–H methoxylation of aryl halides has been realized using a polarity-reversed N−O reagent and facilitated by a C7-bromo-substituted norbornene mediator.
- Xin Liu
- , Yue Fu
- & Guangbin Dong
-
News & Views |
 How polymers dance to the pulses of ultrasound
How polymers dance to the pulses of ultrasound
Scientists have been studying how polymers break in solutions for decades, but the mechanism by which chains are stretched to the point of covalent bond scission is not trivial. Now, an experiment series provides ample support for a dynamic model in which chains uncoil from end to middle, while concurrently relaxing.
- Charles E. Diesendruck
-
Article
| Open Access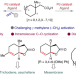 Access to unsaturated bicyclic lactones by overriding conventional C(sp3)–H site selectivity
Access to unsaturated bicyclic lactones by overriding conventional C(sp3)–H site selectivity
Bicyclic lactones are valuable motifs for the synthesis of natural products and bioactive molecules. Now, a palladium-catalysed protocol has been developed to access unsaturated bicyclic lactones in one step from corresponding carboxylic acids. The method demonstrates reverse site selectivity for C(sp3)–H activation to form diverse bicyclic cores.
- Jayabrata Das
- , Wajid Ali
- & Debabrata Maiti
-
News & Views |
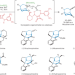 Total synthesis of strychnan alkaloids
Total synthesis of strychnan alkaloids
The synthesis of natural products with important biological properties has always fascinated chemists, but the development of rapid, efficient and stereoselective transformations remains challenging. Now, a strategy has been developed to produce several strychnan alkaloids through formation of a common bridged morphan core structure.
- Sylvain Canesi
-
Article
| Open Access Thiophosphate photochemistry enables prebiotic access to sugars and terpenoid precursors
Thiophosphate photochemistry enables prebiotic access to sugars and terpenoid precursors
The streamlined synthesis of multiple (proto)biomolecules from common starting materials is a key goal of prebiotic chemistry. Now, a one-pot synthesis of ribo-aminooxazoline (a precursor for prebiotic nucleotide synthesis) from HCN has been achieved. Additionally, the two moieties used in extant terpenoid biosynthesis have been accessed, with all carbon atoms also originating from HCN.
- Dougal J. Ritson
- & John D. Sutherland
-
Article
| Open Access Synthesis of a glycan hairpin
Synthesis of a glycan hairpin
Inspired by the design of peptide and nucleic acid sequences to adopt particular three-dimensional shapes, natural glycan motifs have now been combined to construct a glycan that adopts a hairpin conformation in water. Thus a designed glycan can now autonomously fold into a stable secondary structure absent in nature.
- Giulio Fittolani
- , Theodore Tyrikos-Ergas
- & Martina Delbianco
-
Article |
 Time-resolved imaging and analysis of the electron beam-induced formation of an open-cage metallo-azafullerene
Time-resolved imaging and analysis of the electron beam-induced formation of an open-cage metallo-azafullerene
Visualizing single-molecule reactions using electron microscopy can be difficult because of potential radiation damage from the electron beam. Now, however, it has been shown that a high-energy electron beam can be used to synthesize metallo-azafullerenes. Atomic-resolution, time-resolved transmission electron microscopy, with the help of computational calculations, is used to monitor the metal-encapsulation dynamics.
- Helen Hoelzel
- , Sol Lee
- & Dominik Lungerich
-
News & Views |
 Diradical ring formation
Diradical ring formation
Lactams and pyridines are privileged scaffolds, but strategies for combining these groups into one molecule are lacking. Now, N–N pyridinium ylides have been shown to form triplet state diradicals under photoinduced energy transfer, and subsequent [3+2] cycloaddition with the tethered alkene enables the synthesis of diverse ortho-pyridyl lactams.
- Peng-Zi Wang
- & Jia-Rong Chen
-
Article |
 A bridged backbone strategy enables collective synthesis of strychnan alkaloids
A bridged backbone strategy enables collective synthesis of strychnan alkaloids
The morphan core is central to strychnan alkaloid synthesis and is typically formed during the middle or later stages of the process. Now it has been shown that an allene/ketone-equipped morphan core can be constructed early in the synthesis through ketone α-allenylation and then used to introduce other rings and functionalities, enabling access to nine targets including strychnine and geissolosimine.
- Wenqiang Zhou
- , Song Xi
- & Min Zhang
-
Article |
 Energy-transfer-induced [3+2] cycloadditions of N–N pyridinium ylides
Energy-transfer-induced [3+2] cycloadditions of N–N pyridinium ylides
There is currently a lack of effective synthetic strategies for combining lactams and pyridines within a single molecular structure. Now, diastereoselective pyridyl lactamization has been developed using a photoinduced [3+2] cycloaddition of triplet diradical N–N pyridinium ylides with pendant alkenes. This method provides a useful synthon for preparing pyridyl γ- and δ-lactam scaffolds with syn-configuration.
- Wooseok Lee
- , Yejin Koo
- & Sungwoo Hong
-
Article |
 Capturing primary ozonides for a syn-dihydroxylation of olefins
Capturing primary ozonides for a syn-dihydroxylation of olefins
Traditionally, ozone has been primarily used to oxidatively deconstruct carbon–carbon bonds. Now, it has been shown that ozone can be used for the construction of carbon–oxygen bonds without oxidative cleavage of the olefin substrate through capturing primary ozonides. Furthermore, intercepting primary ozonides with nucleophiles in continuous flow enabled the green, syn-dihydroxylation of olefins to be realized.
- Danniel K. Arriaga
- & Andy A. Thomas
-
Article |
 Efficient platform for synthesizing comprehensive heparan sulfate oligosaccharide libraries for decoding glycosaminoglycan–protein interactions
Efficient platform for synthesizing comprehensive heparan sulfate oligosaccharide libraries for decoding glycosaminoglycan–protein interactions
Large collections of defined glycosaminoglycan (GAG) structures have been synthetically challenging to obtain but are required to understand this important class of biomolecules. Now, an efficient platform for synthesizing large libraries of heparan sulfate oligosaccharides has been developed, providing a detailed view into the sulfation code of GAGs.
- Lei Wang
- , Alexander W. Sorum
- & Linda C. Hsieh-Wilson
-
Article |
 Copper-catalysed difluorocarbene transfer enables modular synthesis
Copper-catalysed difluorocarbene transfer enables modular synthesis
Although several transition-metal carbene complexes have been isolated and used for catalytic carbene transfer reactions, few metal difluorocarbene complexes have been reported. Now, the synthesis, characterization and reactivity of isolable copper(I) difluorocarbene complexes has been reported, which has enabled the development of a copper-catalysed difluorocarbene transfer reaction to access fluorinated compounds from simple chemical feedstocks.
- Xin Zeng
- , Yao Li
- & Xingang Zhang
-
Article
| Open Access 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
The ortho-substituted phenyl ring is a basic structural element in chemistry. Now, 2-oxabicyclo[2.1.1]hexanes have been developed as saturated bioisosteres of the ortho-substituted phenyl ring with improved physicochemical properties. Replacement of the phenyl ring with 2-oxabicyclo[2.1.1]hexanes in marketed agrochemicals fluxapyroxad and boscalid improved water solubility, reduced lipophilicity and retained bioactivity.
- Aleksandr Denisenko
- , Pavel Garbuz
- & Pavel K. Mykhailiuk
-
News & Views |
 Arene additions
Arene additions
Dipolar cycloadditions are excellent processes for generating heterocyclic systems from simple starting materials, but arenes as dipolarophiles have not been extensively explored. Now, the intramolecular dipolar cycloaddition of aromatic rings has been achieved using in situ generated diazoalkenes to produce bicyclic or tricyclic heterocycles.
- Abraham Ustoyev
- & Mitchell P. Croatt
-
Article |
 Arenes participate in 1,3-dipolar cycloaddition with in situ-generated diazoalkenes
Arenes participate in 1,3-dipolar cycloaddition with in situ-generated diazoalkenes
1,3-Dipolar cycloadditions are well-known transformations in organic synthesis. However, the reactivity of benzene rings in these processes is underexplored. In situ-generated diazoalkenes have now been shown to undergo intramolecular 1,3-dipolar cycloadditions with aromatic rings. The transformation results in an unaromatized benzene ring that enables the synthesis of functionalized heterocycles.
- Shubhangi Aggarwal
- , Alexander Vu
- & Valery V. Fokin
-
Article |
 Multi-site programmable functionalization of alkenes via controllable alkene isomerization
Multi-site programmable functionalization of alkenes via controllable alkene isomerization
The diverse site-selective functionalization, including multi-functionalization, of C=C double bonds and C(sp3)–H bonds remains a largely unmet challenge. Now, a palladium-catalysed aerobic oxidative method has been developed for the multi-site programmable functionalization of terminal olefins via a strategy that controls the reaction sequence between alkene isomerization and oxidative functionalization.
- Zhengxing Wu
- , Jingjie Meng
- & Wanbin Zhang
-
Research Briefing |
 Not all catenanes with oriented rings are topologically chiral
Not all catenanes with oriented rings are topologically chiral
Catenanes that are chiral owing to the relative orientation of their rings have always been referred to as ‘topologically chiral’. It is now shown that although in specific cases the stereochemistry is a topological property of the structure, the underlying stereogenic unit itself is not inherently topological in nature.
-
Article |
 A catenane that is topologically achiral despite being composed of oriented rings
A catenane that is topologically achiral despite being composed of oriented rings
Catenanes are topologically non-trivial and, perhaps for this reason, molecules composed of two oriented rings have always simply been referred to as ‘topologically chiral’. Now it has been shown that the same stereogenic unit can arise in systems whose stereochemistry is Euclidean, suggesting a need to rethink this fundamental form of mechanical chirality.
- Noel Pairault
- , Federica Rizzi
- & Stephen M. Goldup
-
Article
| Open Access Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective approaches to access chiral organophosphorus compounds are rare. A two-stage catalytic strategy for the synthesis of diverse enantioenriched P(V) compounds has now been developed: a bifunctional iminophosphorane superbase enables enantioselective nucleophilic desymmetrization, followed by downstream enantiospecific diversification of the resulting intermediate by substitution with medicinally relevant O-, N- and S-based nucleophiles.
- Michele Formica
- , Tatiana Rogova
- & Darren J. Dixon
-
Article |
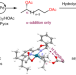 Regio- and enantioselective remote dioxygenation of internal alkenes
Regio- and enantioselective remote dioxygenation of internal alkenes
Functionalization of unsymmetrical internal alkenes usually takes place with low regioselectivity, giving a mixture of isomers. Now, a highly regio- and enantioselective remote 1,n-dioxygenation of internal alkenes using a palladium catalyst has been developed for the synthesis of chiral 1,n-diols. Regioselectivity tuning was demonstrated by altering the rate-determining step, enabled by the phenyl-substituted Pyox ligand.
- Xiaonan Li
- , Tilong Yang
- & Guosheng Liu
-
Article |
 Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Methods for the direct construction of 1,3-disubstituted planar chiral ferrocenes are elusive. Now, a modular platform enables the construction of planar chirality in 1,3-disubstituted ferrocenes/ruthenocenes via enantioselective relay remote C–H arylation. The strategy involves an initial enantiodetermining ortho-C‒H activation enabled by a Pd(II)/chiral amino-acid ligand, followed by relay to the remote meta-position by a bridgehead-substituted norbornene mediator.
- Lan Zhou
- , Hong-Gang Cheng
- & Qianghui Zhou
-
News & Views |
 Amino amide assembly
Amino amide assembly
Enantioenriched β-amino acid derivatives are attractive synthetic targets, considering the significance of these motifs in medicinal and material chemistry. Now, using ambiphilic ynamides as two-carbon synthons in a four-component reaction, three classes of β-amino amides with well-defined stereocentres can be accessed.
- Sunliang Cui
- & Linwei Zeng
-
Article |
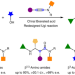 Modular enantioselective access to β-amino amides by Brønsted acid-catalysed multicomponent reactions
Modular enantioselective access to β-amino amides by Brønsted acid-catalysed multicomponent reactions
β-Amino acids are broadly found in biomimetic drugs and therapeutics, but the general synthesis of chiral β-amino amides remains limited. An organocatalytic four-component reaction has now been developed for their asymmetric synthesis with notable efficiency, chemoselectivity and stereoselectivity. This protocol shows broad product scope and provides a shortcut to other important structures.
- Jun Wei
- , Jian Zhang
- & Bin Tan
-
Article |
 Indole-5,6-quinones display hallmark properties of eumelanin
Indole-5,6-quinones display hallmark properties of eumelanin
Indole-5,6-quinone (IQ) is a long-sought intermediate and structural subunit of eumelanin pigments whose instability has precluded isolation and characterization. It has now been shown that a sterically shielded derivative of IQ exhibits hallmark eumelanin properties, including near-infrared absorption, ultrafast nonradiative decay and a persistent semiquinone radical formed by comproportionation.
- Xueqing Wang
- , Lilia Kinziabulatova
- & Jean-Philip Lumb
-
Article
| Open Access A C–H activation-based enantioselective synthesis of lower carbo[n]helicenes
A C–H activation-based enantioselective synthesis of lower carbo[n]helicenes
A simple and general enantioselective method for the synthesis of non-fused lower carbo[n]helicenes (n = 4–6) is reported. The helicene scaffold is constructed with high enantioselectivity by Pd0-catalysed C–H arylation with aryl bromides. A bifunctional ligand provides a precise chiral environment that allows fine control of the enantioselectivity.
- Shu-Min Guo
- , Soohee Huh
- & Olivier Baudoin
-
News & Views |
 Lighting up hot stuff
Lighting up hot stuff
Plasmonic heating by nanoparticles has been used to promote a range of chemical reactions. Now, thermoplasmonic activation has been applied to latent ruthenium catalysts, enabling olefin metathesis initiated by visible and infrared light.
- Leah N. Appelhans
-
Article |
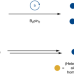 Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes
Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes
Borylated bicyclopentanes and bicyclohexanes are valuable compounds in drug research but are difficult to prepare. Now, an iridium-catalysed method has been developed for the borylation of the bridgehead tertiary C–H bonds in bicyclopentanes and bicyclohexanes, providing access to a variety of highly decorated bicyclic cores.
- Isaac F. Yu
- , Jenna L. Manske
- & John F. Hartwig
-
Article |
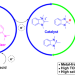 Metal-free reduction of CO2 to formate using a photochemical organohydride-catalyst recycling strategy
Metal-free reduction of CO2 to formate using a photochemical organohydride-catalyst recycling strategy
Photochemical reduction of CO2 is a significant challenge and many existing methods use catalysts containing rare metals. Now a metal-free version of this reaction—with high selectivity for formate generation over H2 or CO—has been achieved that features a combination of carbazole photosensitizer and organohydride catalyst.
- Weibin Xie
- , Jiasheng Xu
- & Ryosuke Matsubara
-
Article
| Open Access Structural resolution of a small organic molecule by serial X-ray free-electron laser and electron crystallography
Structural resolution of a small organic molecule by serial X-ray free-electron laser and electron crystallography
The structural analysis of small crystals has remained challenging. Now, the structure of a small organic molecule, rhodamine-6G, has been resolved from microcrystals using an X-ray free-electron laser and electron diffraction. The former showed better reliability for atomic coordinates, whereas the latter was more sensitive to charges; both techniques accurately determined the position of hydrogen atoms.
- Kiyofumi Takaba
- , Saori Maki-Yonekura
- & Koji Yonekura
-
Article
| Open Access Control of dynamic sp3-C stereochemistry
Control of dynamic sp3-C stereochemistry
Stereogenic sp3-hybridized carbon centres are the principal building blocks of chiral organic molecules. Usually, these centres are configurationally fixed. Now, low-energy pericyclic rearrangements have been used to create rigid cage molecules with fluxional sp3-stereochemistry, influencing chiral information transfer. The sp3-carbon stereochemistry of the cages is inverted through strain-assisted Cope rearrangements.
- Aisha N. Bismillah
- , Toby G. Johnson
- & Paul R. McGonigal
-
Article |
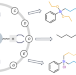 Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y
Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y
The controlled functionalization of multihydrosilanes is challenging. Now, using a hydrogen-atom-transfer photocatalyst based on neutral eosin Y, a method for the diverse functionalization of hydrosilanes has been developed, enabling the stepwise on-demand decoration of silicon atoms. This approach is distinguished by its atom-, step-, redox- and catalyst-economy, metal-free nature, its versatility (>150 examples), modularity, selectivity and scalability.
- Xuanzi Fan
- , Muliang Zhang
- & Jie Wu
-
Article
| Open Access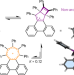 Rupturing aromaticity by periphery overcrowding
Rupturing aromaticity by periphery overcrowding
Stabilization from aromatic electron delocalization is highly favourable so it is typically preserved in even grossly distorted molecules. Now, peripheral overcrowding of an aromatic tropylium has been shown to cause sufficient geometric strain to rupture aromaticity, forming a non-aromatic bicyclic system that is in rapid equilibrium with its aromatic counterpart.
- Promeet K. Saha
- , Abhijit Mallick
- & Paul R. McGonigal

 Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids