« Prev Next »

Natural selection, genetic drift, and gene flow are the mechanisms that cause changes in allele frequencies over time. When one or more of these forces are acting in a population, the population violates the Hardy-Weinberg assumptions, and evolution occurs. The Hardy-Weinberg Theorem thus provides a null model for the study of evolution, and the focus of population genetics is to understand the consequences of violating these assumptions.
Natural selection occurs when individuals with certain genotypes are more likely than individuals with other genotypes to survive and reproduce, and thus to pass on their alleles to the next generation. As Charles Darwin (1859) argued in On the Origin of Species, if the following conditions are met, natural selection must occur:
- There is variation among individuals within a population in some trait.
- This variation is heritable (i.e., there is a genetic basis to the variation, such that offspring tend to resemble their parents in this trait).
- Variation in this trait is associated with variation in fitness (the average net reproduction of individuals with a given genotype relative to that of individuals with other genotypes).
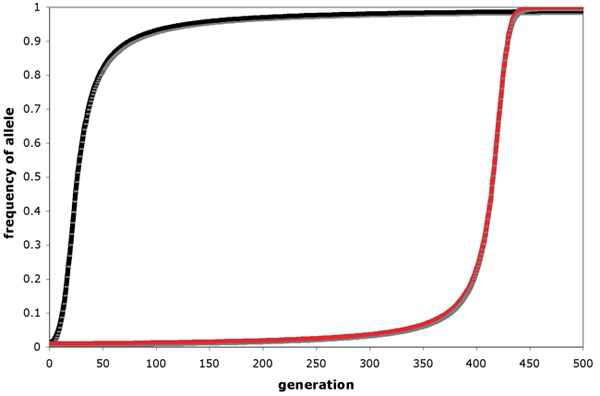
Directional selection leads to increase over time in the frequency of a favored allele. Consider three genotypes (AA, Aa and aa) that vary in fitness such that AA individuals produce, on average, more offspring than individuals of the other genotypes. In this case, assuming that the selective regime remains constant and that the action of selection is the only violation of Hardy-Weinberg assumptions, the A allele would become more common each generation and would eventually become fixed in the population. The rate at which an advantageous allele approaches fixation depends in part on the dominance relationships among alleles at the locus in question (Figure 1). The initial increase in frequency of a rare, advantageous, dominant allele is more rapid than that of a rare, advantageous, recessive allele because rare alleles are found mostly in heterozygotes. A new recessive mutation therefore can't be "seen" by natural selection until it reaches a high enough frequency (perhaps via the random effects of genetic drift — see below) to start appearing in homozygotes. A new dominant mutation, however, is immediately visible to natural selection because its effect on fitness is seen in heterozygotes. Once an advantageous allele has reached a high frequency, deleterious alleles are necessarily rare and thus mostly present in heterozygotes, such that the final approach to fixation is more rapid for an advantageous recessive than for an advantageous dominant allele. As a consequence, natural selection is not as effective as one might naively expect it to be at eliminating deleterious recessive alleles from populations.
Balancing selection, in contrast to directional selection, maintains genetic polymorphism in populations. For example, if heterozygotes at a locus have higher fitness than homozygotes (a scenario known as heterozygote advantage or overdominance), natural selection will maintain multiple alleles at stable equilibrium frequencies. A stable polymorphism can also persist in a population if the fitness associated with a genotype decreases as that genotype increases in frequency (i.e., if there is negative frequency-dependent selection). It is important to note that heterozygote disadvantage (underdominance) and positive frequency-dependent selection can also act at a locus, but neither maintains multiple alleles in a population, and thus neither is a form of balancing selection.
Genetic drift results from the sampling error inherent in the transmission of gametes by individuals in a finite population. The gamete pool of a population in generation t is the total pool of eggs and sperm produced by the individuals in that generation. If the gamete pool were infinite in size, and if there were no selection or mutation acting at a locus with two alleles (A and a), we would expect the proportion of gametes containing the A allele to exactly equal the frequency of A, and the proportion of gametes containing a to equal the frequency of a. Compare this situation to tossing a fair coin. If you were to toss a coin an infinite number of times, the proportion of heads would be 0.50, and the proportion of tails would be 0.50. If you toss a coin only 10 times, however, you shouldn't be too surprised to get 7 heads and 3 tails. This deviation from the expected head and tail frequencies is due to sampling error. The more times you toss the coin, the closer these frequencies should come to 0.50 because sampling error decreases as sample size increases.
In a finite population, the adults in generation t will pass on a finite number of gametes to produce the offspring in generation t + 1. The allele frequencies in this gamete pool will generally deviate from the population frequencies in generation t because of sampling error (again, assuming there is no selection at the locus). Allele frequencies will thus change over time in this population due to chance events — that is, the population will undergo genetic drift. The smaller the population size (N), the more important the effect of genetic drift. In practice, when modeling the effects of drift, we must consider effective population size (Ne), which is essentially the number of breeding individuals, and may differ from the census size, N, under various scenarios, including unequal sex ratio, certain mating structures, and temporal fluctuations in population size.
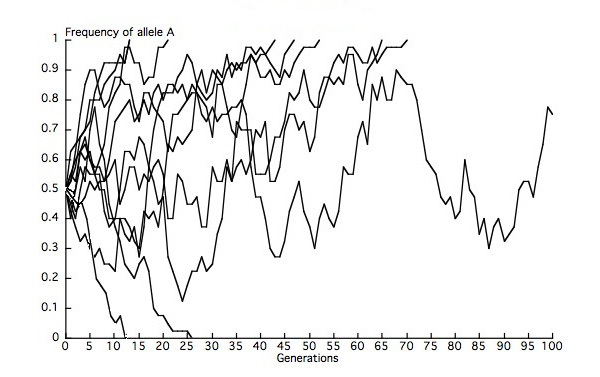
At a locus with multiple neutral alleles (alleles that are identical in their effects on fitness), genetic drift leads to fixation of one of the alleles in a population and thus to the loss of other alleles, such that heterozygosity in the population decays to zero. At any given time, the probability that one of these neutral alleles will eventually be fixed equals that allele's frequency in the population. We can think about this issue in terms of multiple replicate populations, each of which represents a deme (subpopulation) within a metapopulation (collection of demes). Given 10 finite demes of equal Ne, each with a starting frequency of the A allele of 0.5, we would expect eventual fixation of A in 5 demes, and eventual loss of A in 5 demes. Our observations are likely to deviate from those expectations to some extent because we are considering a finite number of demes (Figure 2). Genetic drift thus removes genetic variation within demes but leads to differentiation among demes, completely through random changes in allele frequencies.
Gene flow is the movement of genes into or out of a population. Such movement may be due to migration of individual organisms that reproduce in their new populations, or to the movement of gametes (e.g., as a consequence of pollen transfer among plants). In the absence of natural selection and genetic drift, gene flow leads to genetic homogeneity among demes within a metapopulation, such that, for a given locus, allele frequencies will reach equilibrium values equal to the average frequencies across the metapopulation. In contrast, restricted gene flow promotes population divergence via selection and drift, which, if persistent, can lead to speciation.
Natural selection, genetic drift and gene flow do not act in isolation, so we must consider how the interplay among these mechanisms influences evolutionary trajectories in natural populations. This issue is crucially important to conservation geneticists, who grapple with the implications of these evolutionary processes as they design reserves and model the population dynamics of threatened species in fragmented habitats. All real populations are finite, and thus subject to the effects of genetic drift. In an infinite population, we expect directional selection to eventually fix an advantageous allele, but this will not necessarily happen in a finite population, because the effects of drift can overcome the effects of selection if selection is weak and/or the population is small. Loss of genetic variation due to drift is of particular concern in small, threatened populations, in which fixation of deleterious alleles can reduce population viability and raise the risk of extinction. Even if conservation efforts boost population growth, low heterozygosity is likely to persist, since bottlenecks (periods of reduced population size) have a more pronounced influence on Ne than periods of larger population size.
We have already seen that genetic drift leads to differentiation among demes within a metapopulation. If we assume a simple model in which individuals have equal probabilities of dispersing among all demes (each of effective size Ne) within a metapopulation, then the migration rate (m) is the fraction of gene copies within a deme introduced via immigration per generation. According to a commonly used approximation, the introduction of only one migrant per generation (Nem = 1) constitutes sufficient gene flow to counteract the diversifying effects of genetic drift in a metapopulation.
Natural selection can produce genetic variation among demes within a metapopulation if different selective pressures prevail in different demes. If Ne is large enough to discount the effects of genetic drift, then we expect directional selection to fix the favored allele within a given focal deme. However, the continual introduction, via gene flow, of alleles that are advantageous in other demes but deleterious in the focal deme, can counteract the effects of selection. In this scenario, the deleterious allele will remain at an intermediate equilibrium frequency that reflects the balance between gene flow and natural selection.
Conclusion
The common conception of evolution focuses on change due to natural selection. Natural selection is certainly an important mechanism of allele-frequency change, and it is the only mechanism that generates adaptation of organisms to their environments. Other mechanisms, however, can also change allele frequencies, often in ways that oppose the influence of selection. A nuanced understanding of evolution demands that we consider such mechanisms as genetic drift and gene flow, and that we recognize the error in assuming that selection will always drive populations toward the most well adapted state.
References and Recommended Reading
Carroll, S. P. & Fox, C. W., eds. Conservation Biology: Evolution in Action. New York, NY: Oxford University Press, 2008.
Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London, England: John Murray, 1859.
Futuyma, D. J. Evolutionary Biology, 3rd ed. Sunderland, MA: Sinauer Associates, 1998.
Gillespie, J. H. Population Genetics: A Concise Guide, 2nd ed. Baltimore, MD: The Johns Hopkins University Press, 2004.
Haldane, J. B. S. A mathematical theory of natural and artificial selection, Part I. Transactions of the Cambridge Philosophical Society 23, 19–41 (1924).
Hedrick, P. W. Genetics of Populations, 3rd ed. Sudbury, MA: Sinauer & Associates, 2005.
Wright, S. Evolution: Selected Papers. Chicago, IL: University of Chicago Press, 1986.






























