« Prev Next »

Imagine an organism capable of producing infinite numbers of offspring and living forever. This hypothetical organism is referred to as a Darwinian demon, and one has never evolved into being (Law 1979). Instead, evolution has resulted in a diversity of life histories including all combinations of reproduction, life span, and life stages. Why has this diversity evolved?
Life Histories as Evolutionary Responses
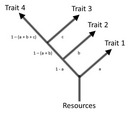
Intrinsic factors include relationships among genes, as well as among physiological, behavioral, and demographic traits. Such relationships are commonly referred to as trade-offs or costs because of the commonness of negative relationships among traits, particularly under the physical limits imposed by finite stores of resources. Trade-offs imply that the evolution of a trait both involves impacts on other traits and is constrained by these relationships. For example, higher allocation of resources to growth as an adult may come at a cost to current reproduction, which may also require significant inputs of resources. However, this same allocation to growth may decrease adult mortality, increasing the chance that an adult will reproduce in the future.
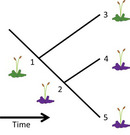
Trade-offs are complicated by associations among the genes responsible for them, as well as by patterns in resource allocation (de Jong 1993). For example, two traits X and Y may occur in the same combinations more often than expected by chance due to past evolution or the same or nearby genes controlling their expression, resulting in linkage between the traits. Here, natural selection favoring a particular value for trait X will also inevitably cause Y to be positively but indirectly selected. Such patterns can also arise if individuals in a population vary systematically in their allocation patterns to each trait, causing correlations among life history trait values (Figure 2). Finally, some trade-offs can favor the evolution of complex life histories. For example, the trade-off between parental care of self v. care of offspring is thought to have favored the evolution of larval stages in many kinds of organisms, including amphibians and insects. Because the diet of a larva is typically different from that of an adult of the same species, the production of larvae reduces the possibility of food competition between parent and offspring.
Environmental Variation
Life histories evolve in response to the environment (i.e., extrinsic factors) as well as to internal constraints. The environment is continually changing, giving natural selection a "moving target". The Red Queen hypothesis states that the tendency for natural selection to change over time, due to continual change in the environment, creates a situation in which no organism is perfectly adapted and all species continually evolve (van Valen 1973). The hypothesis' name is a reference to the character in Lewis Carroll's book, Through the Looking-Glass, who tells Alice that it takes all her energy to run just to stay in the same place.
The degree and pattern of environmental variation can favor specific life history strategies. Environmental variability can be temporal or spatial and deterministic, stochastic, or predictable. Deterministic environmental variability is a shift in an environmental factor away from the previous mean and is not thought to commonly result in the evolution of specific life histories. For example, current climate change is a deterministic change toward a warmer climate, and its main impact on species is likely to be to cause changes in geographic distribution. Stochastic environmental variability is random variation in an environmental factor and does not involve long-term changes in the mean value of the factor. Predictable environmental variability is typically cyclical, as are diurnal or seasonal patterns in sunlight or temperature. Strong levels of environmental variation can create stressful conditions for the organisms that experience them. Many of the adaptations that allow organisms to deal with or escape these kinds of variability have created the diversity of life histories currently observed in nature.
Random Variation

Stochastic environmental variation may favor the evolution of bet-hedging traits. These traits involve an apparent drop in fitness in the short-term in order to maximize fitness in the long-term. Fitness is a geometric property, meaning that it is multiplicative rather than additive across generations. Its geometric nature means that mean fitness is more strongly influenced by harsh periods than favorable periods, and so increased variability in fitness causes mean fitness to drop across generations. Bet-hedging traits counteract the influence of harsh periods on fitness by stabilizing the variation in fitness over time. By stabilizing fitness over the long-term, bet-hedging traits may be favored over other traits that appear to convey greater fitness in the short-term. Vegetative dormancy in herbaceous plants may be one such trait because although it involves the foregoing of reproduction and sprouting in a particular year, it may also prevent mortality from increasing to substantial levels and make future reproduction more likely (Shefferson 2009; Figure 4).
Predictable Environmental Variation
Cyclical variation in the environment causes natural selection to favor different strategies at different times. Phenotypic plasticity, in which one genotype produces different phenotypes under different environmental conditions, is a common result (Schlichting 1986). Phenotypic plasticity can include gradual change in a trait or dramatic and discrete change from time to time. As an example of dramatic change, plants often flower according to a mechanism of discrete phenotypic plasticity, in which they sense seasonal changes in red:far-red light ratios, and use them to sprout at the proper time of the year. As an example of gradual change, within a growing season, plant growth will change to compensate for expected patterns in precipitation and temperature, as well as available nutrients.
Historical Contingency
References and Recommended Reading
Charnov, E. L.
& Schaffer, W. M. Life-history consequences of natural selection: Cole's
result revisited. American Naturalist
107, 791–793 (1973).
Cole, L. C. The population consequences of life history phenomena. Quarterly Review of Biology 29, 103–137 (1954).
De Jong, G. Covariances between traits deriving from successive allocations of a resource. Functional Ecology 7, 75–83 (1993).
Hamilton, W. D. The moulding of senescence by natural selection. Journal of Theoretical Biology 12, 12–45 (1966).
Law, R. Optimal life histories under age-specific predation. The American Naturalist 114, 399–417 (1979).
Roach, D. A. Evolutionary senescence in plants. Genetica 91, 53–64 (1993).
Roff, D. A. Life History Evolution. Sunderland, MA: Sinauer Associates, Inc., 2002.
Schlichting, C. D. The evolution of phenotypic plasticity in plants. Annual Review of Ecology and Systematics 17, 667–693 (1986).
Shefferson, R. P. The evolutionary ecology of vegetative dormancy in mature herbaceous perennial plants. Journal of Ecology 97, 1000–1009 (2009).
Stearns, S. C. The Evolution of Life Histories. Oxford, UK: Oxford University Press, 1992.
Van Valen, L. A new evolutionary law. Evolutionary Theory 1, 1–30 (1973).


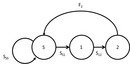
 Figure 1
Figure 1