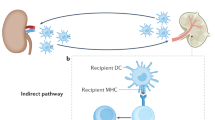
Key Points
-
The type and timing of an infection influences its impact on alloreactivity and transplantation outcome. Infections before transplantation can generate pathogen-specific memory T cells that cross-react with allogeneic MHC molecules through direct allorecognition — a process termed heterologous immunity. The high frequency of memory T cells generated through heterologous immunity is thought to serve as a major barrier to transplantation tolerance in humans.
-
Tissue damage and infections are common following transplantation, especially in the immediate post-transplant period, and an increased risk for infection persists as a result of lifelong pharmacological immunosuppression. Tissue damage releases damage-associated molecular patterns (DAMPs), whereas infections release pathogen-associated molecular patterns (PAMPs). Both sets of molecules can stimulate innate immunity and enhance alloreactivity.
-
Pattern-recognition receptor (PRR)-mediated signals can directly enhance antigen-presenting cell (APC) maturation, upregulate the expression of co-stimulatory ligands, increase antigen presentation and induce the production of pro-inflammatory cytokines. Cytokines can function in a localized manner by enhancing cognate APC–T cell interactions, but they can also have systemic effects that promote the activation and define the differentiation of bystander alloreactive T cells.
-
Some infections may have systemic immunosuppressive effects by triggering the release of glucocorticoids. This glucocorticoid release is crucial for limiting the pathogenicity of microorganism-specific immune responses, but it can also result in an increased susceptibility to secondary infections and a transient suppression of alloreactivity.
-
Tissue damage that arises during the brain or cardiac death of the transplant donor, from cold ischaemia (owing to the storage and transport of procured donor organs) followed by reperfusion in the recipient, from warm ischaemia and as a result of surgically induced injury can cause the release of DAMPs, which can stimulate inflammation and innate immunity. Furthermore, DAMPs released by infected cells can synergize with PAMPs to stimulate alloreactivity and promote rejection.
-
An increasing number of agonists and antagonists targeting PRRs are being developed for the treatment of cancer, autoimmunity and inflammatory diseases. These drugs may be useful in reducing ischaemia–reperfusion injury and the stimulatory effects of infection on alloreactivity. However, success in this approach depends on identifying pathways that block the stimulation of alloreactivity but leave protective immunity to infections intact.
Abstract
Investigations over the past two decades are revealing complexities in the regulation of the innate immune response, and how this response, in turn, controls adaptive immunity. Microbial exposure, infections and tissue damage that accompany solid-organ transplantation result in the release of pathogen- and damage-associated molecular patterns, as well as pathogen- or allograft-derived antigens. Here, we review these triggers of innate and adaptive immunity, and discuss emerging paradigms of the many ways in which infections and tissue damage might directly or indirectly affect alloreactivity and the outcome of transplanted allografts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fishman, J. A. & Issa, N. C. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect. Dis. Clin. North Am. 24, 273–283 (2010). A recent review describing the spectrum of infections that occur after solid-organ transplantation. This range reflects the impact of the use of increasingly potent immunosuppressive drugs and antimicrobial prophylaxis, as well as improved diagnosis.
Ahmed, E. B., Alegre, M. L. & Chong, A. S. Role of bacterial infections in allograft rejection. Expert Rev. Clin. Immunol. 4, 281–293 (2008).
Jamieson, A. M., Yu, S., Annicelli, C. H. & Medzhitov, R. Influenza virus-induced glucocorticoids compromise innate host defense against a secondary bacterial infection. Cell Host Microbe 7, 103–114 (2010). This study demonstrates that viral infections can trigger the release of endogenous glucocorticoids that protect against pro-inflammatory cytokine-mediated lethality but also result in systemic immunosuppression and compromised host defence against secondary bacterial infection.
Silverman, M. N., Pearce, B. D., Biron, C. A. & Miller, A. H. Immune modulation of the hypothalamic–pituitary–adrenal (HPA) axis during viral infection. Viral Immunol. 18, 41–78 (2005).
Ruzek, M. C., Miller, A. H., Opal, S. M., Pearce, B. D. & Biron, C. A. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J. Exp. Med. 185, 1185–1192 (1997).
Nadeau, S. & Rivest, S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J. Neurosci. 23, 5536–5544 (2003).
Cannon, R. M. et al. BK viral disease in renal transplantation. Curr. Opin. Organ Transplant. 16, 576–579 (2011).
Sinnakirouchenan, R. & Holley, J. L. Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv. Chronic Kidney Dis. 18, 428–432 (2011).
Hadjiliadis, D. Special considerations for patients with cystic fibrosis undergoing lung transplantation. Chest 131, 1224–1231 (2007).
Yang, H. & Welsh, R. M. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J. Immunol. 136, 1186–1193 (1986).
Smith, C., Miles, J. J. & Khanna, R. Advances in direct T-cell alloreactivity: function, avidity, biophysics and structure. Am. J. Transplant. 12, 15–26 (2012).
Gras, S., Kjer-Nielsen, L., Chen, Z., Rossjohn, J. & McCluskey, J. The structural bases of direct T-cell allorecognition: implications for T-cell-mediated transplant rejection. Immunol. Cell Biol. 89, 388–395 (2011).
Seder, R. A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature Immunol. 4, 835–842 (2003).
Macedo, C. et al. Contribution of naive and memory T-cell populations to the human alloimmune response. Am. J. Transplant. 9, 2057–2066 (2009).
Heeger, P. S. et al. Pretransplant frequency of donor-specific, IFN-γ-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J. Immunol. 163, 2267–2275 (1999).
Valujskikh, A., Pantenburg, B. & Heeger, P. S. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am. J. Transplant. 2, 501–509 (2002).
Nadazdin, O. et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci. Transl. Med. 3, 86ra51 (2011).
Adams, A. B. et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J. Clin. Invest. 111, 1887–1895 (2003). A demonstration that virally induced alloreactive memory T cells are a potent barrier to the induction of tolerance in mice. This study proposed three concepts: memory T cell responses to viruses can cross-react with alloantigens; memory T cells are resistant to co-stimulation blockade; and memory T cells accumulate in humans.
Brehm, M. A. et al. Allografts stimulate cross-reactive virus-specific memory CD8 T cells with private specificity. Am. J. Transplant. 10, 1738–1748 (2010).
Pantenburg, B., Heinzel, F., Das, L., Heeger, P. S. & Valujskikh, A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J. Immunol. 169, 3686–3693 (2002).
Herman, A., Kappler, J. W., Marrack, P. & Pullen, A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu. Rev. Immunol. 9, 745–772 (1991).
Amir, A. L. et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood 115, 3146–3157 (2010).
Melenhorst, J. J. et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 116, 4700–4702 (2010).
Pearl, J. P. et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am. J. Transplant. 5, 465–474 (2005).
Wojciechowski, D. & Vincenti, F. Challenges and opportunities in targeting the costimulation pathway in solid organ transplantation. Semin. Immunol. 23, 157–164 (2011).
Kotsimbos, T. C. et al. Chlamydia pneumoniae serology in donors and recipients and the risk of bronchiolitis obliterans syndrome after lung transplantation. Transplantation 79, 269–275 (2005).
Husain, S. et al. Simkania negevensis in bronchoalveolar lavage of lung transplant recipients: a possible association with acute rejection. Transplantation 83, 138–143 (2007).
Botha, P. et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 85, 771–774 (2008).
Weigt, S. S. et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am. J. Transplant. 9, 1903–1911 (2009).
Abbott, K. C. et al. Late urinary tract infection after renal transplantation in the United States. Am. J. Kidney Dis. 44, 353–362 (2004).
Wang, T. et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am. J. Transplant. 10, 1524–1533 (2010). This study demonstrated that an acute infection with L. monocytogenes in tolerant mouse recipients can precipitate the acute rejection of allografts. This effect was dependent on IL-6 and IFNβ, indicating the potential instability of transplantation tolerance that is dependent on peripheral mechanisms.
Ahmed, E. B., Wang, T., Daniels, M., Alegre, M. L. & Chong, A. S. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am. J. Transplant. 11, 936–946 (2011). A demonstration that different bacterial infections have distinct effects on alloreactivity. The production of IL-6 during staphylococcal infection prevented tolerance mediated by CD154-specific antibodies, whereas L. monocytogenes infections produced the same outcome by triggering the release of IFNβ. Interestingly, P. aeruginosa had no effect on alloreactivity in this model.
Renaud, C. & Campbell, A. P. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr. Opin. Infect. Dis. 24, 333–343 (2011).
Kuo, E. et al. Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann. Thorac. Surg. 82, 1043–1050 (2006).
Baron, C., Forconi, C. & Lebranchu, Y. Revisiting the effects of CMV on long-term transplant outcome. Curr. Opin. Organ Transplant. 15, 492–498 (2010).
Carlquist, J. F. et al. Accelerated rejection of murine cardiac allografts by murine cytomegalovirus-infected recipients. Lack of haplotype specificity. J. Clin. Invest. 91, 2602–2608 (1993).
Cook, C. H. et al. Disruption of murine cardiac allograft acceptance by latent cytomegalovirus. Am. J. Transplant. 9, 42–53 (2009). This study demonstrated that in this experimental setting transplantation into an environment of latent CMV infection permits the reactivation of the virus, which then infects the graft and promotes intragraft responses that prevent long-term allograft acceptance.
Welsh, R. M. et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J. Virol. 74, 2210–2218 (2000).
Williams, M. A. et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J. Immunol. 167, 4987–4995 (2001).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Mills, K. H. TLR-dependent T cell activation in autoimmunity. Nature Rev. Immunol. 11, 807–822 (2011).
Alegre, M. L. et al. The multiple facets of Toll-like receptors in transplantation biology. Transplantation 86, 1–9 (2008).
Palmer, S. M. et al. The role of innate immunity in acute allograft rejection after lung transplantation. Am. J. Respir. Crit. Care Med. 168, 628–632 (2003).
Palmer, S. M. et al. Donor polymorphisms in Toll-like receptor-4 influence the development of rejection after renal transplantation. Clin. Transplant. 20, 30–36 (2006).
Kastelijn, E. A. et al. Polymorphisms in innate immunity genes associated with development of bronchiolitis obliterans after lung transplantation. J. Heart Lung Transplant. 29, 665–671 (2010).
Palmer, S. M. et al. Genetic regulation of rejection and survival following human lung transplantation by the innate immune receptor CD14. Am. J. Transplant. 7, 693–699 (2007).
Chen, L. et al. TLR engagement prevents transplantation tolerance. Am. J. Transplant. 6, 2282–2291 (2006).
Goldstein, D. R., Tesar, B. M., Akira, S. & Lakkis, F. G. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 111, 1571–1578 (2003).
Tesar, B. M., Zhang, J., Li, Q. & Goldstein, D. R. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a Toll-like receptor signal adaptor protein. Am. J. Transplant. 4, 1429–1439 (2004).
Walker, W. E. et al. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J. Immunol. 177, 5307–5316 (2006).
McKay, D., Shigeoka, A., Rubinstein, M., Surh, C. & Sprent, J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur. J. Immunol. 36, 1994–2002 (2006).
Zielinski, C. E. et al. Dissecting the human immunologic memory for pathogens. Immunol. Rev. 240, 40–51 (2011).
Aytekin, C. et al. Bacille Calmette-Guerin lymphadenitis and recurrent oral candidiasis in an infant with a new mutation leading to interleukin-12 receptor β-1 deficiency. J. Investig. Allergol. Clin. Immunol. 21, 401–404 (2011).
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Lopez, C. B. & Hermesh, T. Systemic responses during local viral infections: type I IFNs sound the alarm. Curr. Opin. Immunol. 23, 495–499 (2011).
Chan, S. E., Schwartz, J. M. & Rosen, H. R. Treatment of hepatitis C in solid organ transplantation. Drugs 64, 489–498 (2004).
Thornley, T. B. et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J. Immunol. 179, 6620–6629 (2007).
Wang, T. et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J. Immunol. 180, 5991–5999 (2008).
Takeuchi, O., Hoshino, K. & Akira, S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165, 5392–5396 (2000).
Chen, L. et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J. Immunol. 182, 6217–6225 (2009).
Perona-Wright, G., Mohrs, K. & Mohrs, M. Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nature Immunol. 11, 520–526 (2010).
Weigt, S. S. et al. CXCR3 chemokine ligands during respiratory viral infections predict lung allograft dysfunction. Am. J. Transplant. 12, 477–484 (2012).
Belperio, J. A. et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J. Immunol. 169, 1037–1049 (2002).
Jackson, J. A. et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am. J. Transplant. 11, 2228–2234 (2011). This study demonstrated that BK polyomavirus infection leads to elevated levels of IFNγ-induced CXCL9 and CXCL10 expression and increased allograft rejection, suggesting that the same chemokines recruit both antiviral T cells and alloreactive T cells into the graft.
Ho, J. et al. Validation of urinary CXCL10 as a marker of borderline, subclinical, and clinical tubulitis. Transplantation 92, 878–882 (2011).
Gaipl, U. S. et al. Clearance deficiency and systemic lupus erythematosus (SLE). J. Autoimmun. 28, 114–121 (2007).
Blander, J. M. & Medzhitov, R. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304, 1014–1018 (2004).
Blander, J. M. & Medzhitov, R. On regulation of phagosome maturation and antigen presentation. Nature Immunol. 7, 1029–1035 (2006).
Blander, J. M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).
Torchinsky, M. B., Garaude, J., Martin, A. P. & Blander, J. M. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 458, 78–82 (2009).
Burlingham, W. J. et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J. Clin. Invest. 117, 3498–3506 (2007).
Nath, D. S., Basha, H. I. & Mohanakumar, T. Antihuman leukocyte antigen antibody-induced autoimmunity: role in chronic rejection. Curr. Opin. Organ. Transplant. 15, 16–20 (2010).
Yapici, U. et al. Interleukin-17 positive cells accumulate in renal allografts during acute rejection and are independent predictors of worse graft outcome. Transpl. Int. 24, 1008–1017 (2011).
Iwasaki, A. & Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010).
Jonker, M. et al. The autoimmune response to vimentin after renal transplantation in nonhuman primates is immunosuppression dependent. Transplantation 80, 385–393 (2005).
Saini, D. et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J. Heart Lung Transplant. 30, 624–631 (2011).
Newell, K. A. et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J. Clin. Invest. 120, 1836–1847 (2010).
Sanchez-Fueyo, A. Identification of tolerant recipients following liver transplantation. Int. Immunopharmacol. 10, 1501–1504 (2010).
Sagoo, P. et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J. Clin. Invest. 120, 1848–1861 (2011).
Pasare, C. & Medzhitov, R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299, 1033–1036 (2003).
Razonable, R. R. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J. Gastroenterol. 14, 4849–4860 (2008).
Erdbruegger, U. et al. Impact of CMV infection on acute rejection and long-term renal allograft function: a systematic analysis in patients with protocol biopsies and indicated biopsies. Nephrol. Dial. Transplant. 27, 435–443 (2011).
Goh, F. G. & Midwood, K. S. Intrinsic danger: activation of Toll-like receptors in rheumatoid arthritis. Rheumatology 51, 7–23 (2012).
Shigeoka, A. A. et al. Nod1 and Nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. J. Immunol. 184, 2297–2304 (2010).
Shigeoka, A. A. et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J. Immunol. 178, 6252–6258 (2007).
Zhai, Y. et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 173, 7115–7119 (2004).
Shigeoka, A. A. et al. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J. Immunol. 185, 6277–6285 (2010).
Krüger, B. et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl Acad. Sci. USA 106, 3390–3395 (2009).
Terasaki, P. I., Cecka, J. M., Gjertson, D. W. & Takemoto, S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 333, 333–336 (1995).
Matsuoka, N. et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J. Clin. Invest. 120, 735–743 (2010). A demonstration that HMGB1 has a crucial role in the initial events that occur during the early loss of transplanted islets. In the absence of HMGB1 or RAGE, but not TLR4, this early islet graft loss was prevented.
Tesar, B. M. et al. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 6, 2622–2635 (2006).
Shen, H. et al. Haptoglobin activates innate immunity to enhance acute transplant rejection in mice. J. Clin. Invest. 122, 383–387 (2012).
Goldstein, D. R. The identity of innate immune activators in organ transplantation: origins from within or exterior to the host? Am. J. Transplant. 7, 1692–1694 (2007).
Hreggvidsdottir, H. S. et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J. Leukoc. Biol. 86, 655–662 (2009).
Badell, I. R. et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J. Clin. Invest. 120, 4520–4531 (2010).
Kitchens, W. H. et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8+ memory T cells. Am. J. Transplant. 12, 69–80 (2012).
Weaver, T. A. et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature Med. 15, 746–749 (2009).
Kitchens, W. H., Larsen, C. P. & Ford, M. L. Integrin antagonists for transplant immunosuppression: panacea or peril? Immunotherapy 3, 305–307 (2011).
Hennessy, E. J., Parker, A. E. & O'Neill, L. A. Targeting Toll-like receptors: emerging therapeutics? Nature Rev. Drug Discov. 9, 293–307 (2010).
Fukuzawa, N., Petro, M., Baldwin, W. M., Gudkov, A. V. & Fairchild, R. L. A TLR5 agonist inhibits acute renal ischemic failure. J. Immunol. 187, 3831–3839 (2011).
Navarro-Millan, I., Singh, J. A. & Curtis, J. R. Systematic review of tocilizumab for rheumatoid arthritis: a new biologic agent targeting the interleukin-6 receptor. Clin. Ther. 34, 788–802 (2012).
Drobyski, W. R. et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol. Blood Marrow Transplant. 17, 1862–1868 (2011).
Ferrer, I. R. et al. Cutting edge: rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J. Immunol. 185, 2004–2008 (2010). This study demonstrated that the immunosuppressive drug rapamycin augments the magnitude of CD8+ T cell responses to L. monocytogenes infection but suppresses the response to skin grafts, thereby highlighting a fundamental difference between responses to infectious agents and allografts that can potentially be exploited in the clinic.
Araki, K., Ellebedy, A. H. & Ahmed, R. TOR in the immune system. Curr. Opin. Cell Biol. 23, 707–715 (2011).
Geddes, K., Magalhaes, J. G. & Girardin, S. E. Unleashing the therapeutic potential of NOD-like receptors. Nature Rev. Drug Discov. 8, 465–479 (2009).
Sander, L. E. et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474, 385–389 (2011).
Maslowski, K. M. & Mackay, C. R. Diet, gut microbiota and immune responses. Nature Immunol. 12, 5–9 (2011).
Round, J. L., O'Connell, R. M. & Mazmanian, S. K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 34, J220–J225 (2010).
Wu, L. et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science 309, 774–777 (2005).
Oh, P. L. et al. Characterization of the ileal microbiota in rejecting and nonrejecting recipients of small bowel transplants. Am. J. Transplant. 12, 753–762 (2011).
Acknowledgements
We apologize to the many authors whose papers could not be cited or fully discussed owing to space limitations. We thank S. Bhorade, M. Josephson, C. Nagler, M. Miller, K. Pursell and J. Williams for their critical reading of the manuscript. This work was supported in part by US National Institute of Allergy and Infectious Diseases (NIAID) grant R01 AI071080 to M.-L.A., and by Roche Organ Transplantation Research Foundation grant 979162997 and NIAID grant R01 AI072630 to A.S.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Minor histocompatibility antigens
-
In the context of solid-organ transplantation, minor histocompatibility antigens are derived from polymorphic alleles of genes that differ between the donor and recipient. These minor antigens are presented as peptides on recipient MHC molecules to recipient T cells, which then cause the rejection of the grafted organ.
- Ischaemia–reperfusion injury
-
Cellular damage caused by the return of blood flow to a tissue after a period of inadequate blood supply. The absence of oxygen and nutrients causes cellular damage and the restoration of blood flow results in inflammation.
- Donor-specific transfusion
-
(DST). A treatment for inducing transplant tolerance that involves the infusion into a transplant recipient of spleen cells from a donor who also provides the organ or tissue.
- MHC tetramers
-
Fluorescently labelled tetravalent complexes of MHC class I or class II molecules complexed with antigenic peptides. They can be used to identify antigen-specific T cells by flow cytometry.
- Severe combined immunodeficiency mice
-
(SCID mice). Mice with a defect in DNA recombination that results in an absence of B and T cell development. Such mice are incompetent at rejecting tissue grafts from allogeneic and xenogeneic sources.
- Superantigen
-
A microbial protein that activates all T cells expressing a particular set of T cell receptor (TCR) Vβ chains by crosslinking the TCR to a particular MHC molecule regardless of the peptide presented.
- Graft-versus-host disease
-
(GVHD). Tissue damage in a recipient of allogeneic tissue resulting from the activity of donor lymphocytes that recognize the tissues of the recipient as foreign. GVHD varies markedly in extent, but it can be life-threatening in severe cases. Damage to the liver, skin and gut mucosa are common clinical manifestations.
- Bronchiolitis obliterans syndrome
-
(BOS). A fibroproliferative process of the small airways that results in multifocal bronchiolar obliterations and is presumed to reflect chronic Iung allograft rejection. BOS is the major factor that limits the survival of lung transplant recipients.
- Glucocorticoids
-
A group of compounds that belong to the corticosteroid family. These compounds can be either naturally produced (hormones) or synthetic. They affect metabolism and have anti-inflammatory and immunosuppressive effects. Many synthetic glucocorticoids (for example, dexamethasone) are used in clinical medicine as anti-inflammatory drugs.
- Haptoglobin
-
A plasma protein that can bind to free haemoglobin in the bloodstream.
- Endotoxemia
-
Endotoxemia is caused by the presence in the blood of lipopolysaccharide (endotoxin), which is derived from Gram-negative bacteria. It results in systemic activation of the inflammatory response, the development of shock, multi-organ failure and death. Models of endotoxemia are used in experimental settings to induce systemic inflammation, but they do not necessarily mimic human sepsis.
Rights and permissions
About this article
Cite this article
Chong, A., Alegre, ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol 12, 459–471 (2012). https://doi.org/10.1038/nri3215
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3215
This article is cited by
-
Unlocking the secrets: the power of methylation-based cfDNA detection of tissue damage in organ systems
Clinical Epigenetics (2023)
-
Sterile inflammation in thoracic transplantation
Cellular and Molecular Life Sciences (2021)
-
Epidemiology, risk factors, and clinical impact of early post-transplant infection in older kidney transplant recipients: the Korean organ transplantation registry study
BMC Geriatrics (2020)
-
Evidence for the important role of inflammation in xenotransplantation
Journal of Inflammation (2019)
-
Advances in islet encapsulation technologies
Nature Reviews Drug Discovery (2017)