Key Points
-
Heart failure is a heterogeneous, multifactorial and progressive syndrome that presents as a wide spectrum of phenotypes and is often accompanied by other comorbidities. It represents a major health problem owing to its high morbidity and mortality and substantial health-care costs.
-
The goals of treatment are to reduce mortality and morbidity, prevent heart disease or its progression, and manage comorbidities that cause and/or contribute to the pathogenesis of heart failure.
-
Despite considerable therapeutic advances, treatment of acute decompensated heart failure has not changed much over the past 25 years, as new drugs have failed to reduce morbidity and mortality rates compared to placebo. Furthermore, no treatment strategy has improved clinical outcomes in patients with heart failure with preserved left ventricular ejection fraction.
-
There is an unmet need for the development of new drugs with a favourable safety profile that can improve cardiac performance and clinical outcomes in patients, and prevent the progression of heart failure.
-
In recent years, new potential therapeutic targets that are involved in the pathogenesis of heart failure have been identified and several promising new drugs are currently under investigation, both in experimental models and in clinical trials in patients with heart failure.
-
However, the positive results that are obtained in preclinical and Phase II studies are not always confirmed in large Phase III trials. In this Review, we provide a detailed analysis of the possible explanations for this discrepancy.
-
The development of new effective and safe drugs for the treatment of heart failure should be based on a better understanding of the pathophysiological mechanisms that are directly implicated in the onset and/or maintenance of heart failure, robust preclinical data and well-designed clinical trials.
Abstract
Despite considerable therapeutic advances, heart failure remains a medical and socioeconomic problem. Thus, there is a compelling need for new drugs that could improve clinical outcomes. In recent years, new potential therapeutic targets that are involved in the pathogenesis of heart failure have been identified, and new drugs are currently under investigation. A repeated finding is that the positive results that have been observed in preclinical studies and Phase II trials are not always confirmed in Phase III studies. This Review analyses the new therapeutic targets (for example, ventricular remodelling, renin–angiotensin–aldosterone system activation, defects in Ca2+ cycling, and so on), the mechanism of action, efficacy and future perspectives of new drugs that are currently under development for the treatment of heart failure, and the possible explanations for the discrepancy between Phase II and Phase III trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dickstein, K. et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. J. Heart Fail. 10, 933–989 (2008). These are the guidelines for the diagnosis and treatment of acute and chronic heart failure.
Hunt, S. A. et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J. Am. Coll. Cardiol. 53, e1–e90 (2009).
Lloyd-Jones, D. et al. Heart disease and stroke statistics — 2010 update: a report from the American Heart Association. Circulation 121, e46–e215 (2010).
Fang, J., Mensah, G. A., Croft, J. B. & Keenan, N. L. Heart failure-related hospitalization in the US, 1979 to 2004. J. Am. Coll. Cardiol. 52, 428–434 (2008).
Gheorghiade, M. Acute heart failure syndromes. J. Am. Coll. Cardiol. 53, 557–573 (2009).
López-Sendón, J. et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur. Heart J. 25, 1454–1470 (2004).
López-Sendón, J. et al. Expert consensus document on β-adrenergic receptor blockers. Task Force on beta-blockers of the European Society of Cardiology. Eur. Heart J. 25, 1341–1362 (2004).
Felker, G. M. et al. Clinical trials of pharmacological therapies in acute heart failure syndromes: lessons learned and directions forward. Circ. Heart Fail. 3, 314–325 (2010). This study analyses why we have failed to develop new, safe and effective drugs for the treatment of acute heart failure syndromes (AHFSs), and identifies current barriers and potential solutions for moving the field forward.
Tamargo, J., Amorós, I., Barana, A., Caballero, R. & Delpón, E. New investigational drugs for the management of acute heart failure syndromes. Curr. Med. Chem. 17, 363–390 (2010).
Sata, Y. & Krum, H. The future of pharmacological therapy for heart failure. Circ. J. 74, 809–817 (2010).
Gheorghiade, M. et al. Clinical development of pharmacologic agents for acute heart failure syndromes: a proposal for a mechanistic translational phase. Am. Heart. J. 161, 224–232 (2011). This article recognizes that the lack of in-depth understanding of novel molecules before pivotal trials are performed is one of the reasons why the clinical benefits that are observed in Phase II trials are not reproduced in Phase III ADHF trials. This study also outlines the translational phase of research for the clinical development of therapies for AHFS as an important first step towards greater success in AHFS clinical trials.
Allen, L. A., Hernandez, A. F., O'Connor, C. M. & Felker, G. M. End points for clinical trials in acute heart failure syndromes. J. Am. Coll. Cardiol. 53, 2248–2258 (2009). This review summarizes the variety of end points used in completed and ongoing AHFS studies, and suggests steps for greater standardization of end points across AHFS trials.
Ouzounian, M., Lee, D. S. & Liu, P. P. Diastolic heart failure: mechanisms and controversies. Nature Clin. Pract. Cardiovasc. Med. 5, 375–386 (2008).
Borlaug, B. A. & Paulus, W. J. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur. Heart J. 32, 670–679 (2011). This review analyses the recent advances in our understanding of the diastolic and non-diastolic mechanisms underlying HFPEF and discusses some novel therapeutic strategies that are currently under investigation.
Mebazaa, A. et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 297, 1883–1891 (2007).
Konstam, M. A. et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet 374, 1840–1848 (2009).
Swedberg, K. et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376, 875–885 (2010). This was the first demonstration of clinical efficacy in a large morbi–mortality trial with ivabradine, which is a novel selective bradycardic drug that does not have direct haemodynamic effects.
Felker, G. M. & Maisel, A. S. A global rank end point for clinical trials in acute heart failure. Circ. Heart Fail. 3, 643–646 (2010).
Felker, G. M., O'Connor, C. M. & Braunwald, E. Heart failure clinical research network investigators. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ. Heart Fail. 2, 56–62 (2009).
Iyengar, S. & Abraham, W. T. Diuretics for the treatment of acute decompensated heart failure. Heart Fail. Rev. 12, 125–130 (2007).
Felker, M. G. et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 364, 797–805 (2011). In patients with ADHF, there are no substantial differences in the global assessment of symptoms or short-term impact on renal function when furosemide is administered at a high or low dose or via intermittent bolus or continuous infusions.
Herout, P. M. et al. Impact of worsening renal function during hospital admission on resource utilization in patients with heart failure. Am. J. Cardiol. 106, 1139–1145 (2010).
Bock, J. S. & Gottlieb, S. S. Cardiorenal syndrome: new perspectives. Circulation 121, 2592–2560 (2010).
Vallon, V., Miracle, C. & Thomson, S. Adenosine and kidney function: potential implications in patients with heart failure. Eur. J. Heart Fail. 10, 176–187 (2008).
Shah, R. H. & Frishman, W. H. Adenosine 1 receptor antagonism: a new therapeutic approach for the treatment of decompensated heart failure. Cardiol. Rev. 17, 125–131 (2009).
Cotter, G. et al. The PROTECT pilot study: a randomized, placebo controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J. Card. Fail. 14, 631–640 (2008).
Massie, B. M. et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N. Engl. J. Med. 363, 1419–1428 (2010).
Cleland, J. G., Coletta, A. P. & Clark, A. L. Clinical trials update from the European Society of Cardiology Heart Failure Meeting 2010: TRIDENT 1, BENEFICIAL, CUPID, RFA-HF, MUSIC, DUEL, handheld BNP, phrenic nerve stimulation, CHAMPION and CABG with CRT study. Eur. J. Heart Fail. 12, 883–888 (2010).
Fonarow, G. C. et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 50, 768–777 (2007).
Metra, M. et al. Vasodilators in the treatment of acute heart failure: what we know, what we don't. Heart Fail. Rev. 14, 299–307 (2009).
Potter, L. R., Abbey-Hosch, S. & Dickey, D. M. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 27, 47–72 (2006).
Mitrovic, V., Hernandez, A. F., Meyer, M. & Gheorghiade, M. Role of guanylate cyclase modulators in decompensated heart failure. Heart Fail. Rev. 14, 309–319 (2009).
Sackner-Bernstein, J. D., Kowalski, M., Fox, M. & Aaronson, K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA 293, 1900–1905 (2005).
Hernandez, A. F. et al. Acute study of clinical effectiveness of nesiritide in decompensated heart failure trial (ASCEND-HF). Circulation 122, Abstract 21828 22–27 (2010).
McKie, P. M., Sangaralingham, S. J. & Burnett, J. C. Jr. CD-NP: an innovative designer natriuretic peptide activator of particulate guanylyl cyclase receptors for cardiorenal disease. Curr. Heart Fail. Rep. 7, 93–99 (2010).
Kilic, A. et al. A novel chimeric natriuretic peptide reduces cardiomyocyte hypertrophy through the NHE-1–calcineurin pathway. Cardiovasc. Res. 88, 434–442 (2010).
Packer, M. et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the omapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE). Circulation 106, 920–926 (2002).
Ruilope, L. M. et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375, 1255–1266 (2010).
Venkatasubramaniam, S., Newby, D. E. & Lang, N. N. Urocortins in heart failure. Biochem. Pharmacol. 80, 289–296 (2010).
Davis, M. E. et al. Urocortin 2 infusion in human heart failure. Eur. Heart J. 28, 2589–2597 (2007).
Tamargo, J., Duarte, J., Caballero, R. & Delpón, E. Cinaciguat, a soluble guanylate cyclase activator for the potential treatment of acute heart failure. Curr. Opin. Investig. Drugs 11, 1039–1047 (2010).
Lapp, H. et al. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation 119, 2781–2788 (2009).
Teichman, S. L., Unemori, E., Teerlink, J. R., Cotter, G. & Metra, M. Relaxin: review of biology and potential role in treating heart failure. Curr. Heart Fail. Rep. 7, 75–82 (2010).
Teerlink, J. R. et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding Phase IIb study. Lancet 373, 1429–1439 (2009).
Nishikimi, T. et al. Effects of long-term intravenous administration of adrenomedullin (AM) plus hANP therapy in acute decompensated heart failure: a pilot study. Circ. J. 73, 892–898 (2009).
Zannad, F. et al. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 364, 11–21 (2011). This trial showed that eplerenone reduces the risk of death and the risk of hospitalization compared to placebo in patients with systolic heart failure and mild symptoms of heart failure. These results expanded the benefit of aldosterone antagonists to a much broader patient population, unless they were contraindicated (for example, in severe renal dysfunction or hyperkalaemia).
Funder, J. W. Reconsidering the roles of the mineralocorticoid receptor. Hypertension 53, 286–290 (2009).
Jensen, C., Herold, P. & Brunner, H. R. Aliskiren: the first renin inhibitor for clinical treatment. Nature Rev. Drug Discov. 7, 399–410 (2008).
Funke-Kaiser, H., Zollmann, F. S., Schefe, J. H. & Unger, T. Signal transduction of the (pro)renin receptor as a novel therapeutic target for preventing end-organ damage. Hypertens. Res. 33, 98–104 (2009).
Latini, R. et al. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur. Heart. J. 25, 292–299 (2004).
McMurray, J. J. et al. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ. Heart Fail. 1, 17–24 (2008).
Solomon, S. D. et al. Effect of the direct renin inhibitor aliskiren on left ventricular remodelling following myocardial infarction with systolic dysfunction. Eur. Heart J. 32, 1227–1234 (2011).
Yokokawa, F. & Maibaum, J. Recent advances in the discovery of non-peptidic direct renin inhibitors as antihypertensives: new patent applications in years 2000–2008. Expert. Opin. Ther. 18, 581–602 (2008).
Unger, T. & Dahlöf, B. Compound 21, the first orally active, selective agonist of the angiotensin type 2 receptor (AT2): implications for AT2 receptor research and therapeutic potential. J. Renin Angiotensin Aldosterone Syst. 11, 75–77 (2010).
Sica, D. A. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Failure. Rev. 10, 23–29 (2005).
Jansen, P. M., van den Meiracker, A. H. & Jan Danser, A. H. Aldosterone synthase inhibitors: pharmacological and clinical aspects. Curr. Opin. Investig. Drugs 10, 319–326 (2009).
Mulder, P. et al. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: a comparison with spironolactone. Eur. Heart J. 29, 2171–2179 (2008).
Amar, L. et al. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension 56, 831–838 (2010).
Mann, D. L. & Bristow, M. R. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111, 2837–2849 (2005).
Landmesser, U., Wollert, K. C. & Drexler, H. Potential novel pharmacological therapies for myocardial remodelling. Cardiovasc. Res. 81, 519–527 (2009). This review summarizes the molecular mechanisms that lead to left ventricular remodelling and dysfunction and analyses old and novel therapeutic strategies for preventing or reversing adverse left ventricular remodelling.
Porter, K. E. & Turner, N. A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 123, 255–278 (2009).
Brown, R. D., Ambler, S. K., Mitchell, M. D. & Long C. S. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu. Rev. Pharmacol. Toxicol. 45, 657–687 (2005).
Spinale, F. G. & Wilbur, N. M. Matrix metalloproteinase therapy in heart failure. Curr. Treat. Options Cardiovasc. Med. 11, 339–346 (2009).
Hudson, M. P. et al. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J. Am. Coll. Cardiol. 48, 15–20 (2006).
Singh, R. B., Dandekar, S. P., Elimban, V., Gupta, S. K. & Dhalla, N. S. Role of proteases inthe pathophysiology of cardiac disease. Mol. Cell. Biochem. 263, 241–256 (2004).
Fildes, J. E., Shaw, S. M., Yonan, N. & Williams, S. G. The immune system and chronic heart failure: is the heart in control? J. Am. Coll. Cardiol. 53, 1013–1020 (2009).
Ikonomidis, I. et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 117, 2662–2669 (2008).
Abbate, A. et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am. J. Cardiol. 105, 1371–1377 (2010).
Timmers, L. et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ. Res. 102, 257–264 (2008).
Kelly, D. J. et al. Tranilast attenuates diastolic dysfunction and structural injury in experimental diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 293, H2860–H2869 (2007).
Tsai, E. J. & Kass, D. A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 122, 216–238 (2009).
Wollert, K. C. & Drexler, H. Regulation of cardiac remodeling by nitric oxide: focus on cardiac myocyte hypertrophy and apoptosis. Heart Fail. Rev. 7, 317–325 (2002).
Forfia, P. R. et al. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J. Am. Coll. Cardiol. 49, 1079–1088 (2007).
Guazzi, M. Clinical use of phosphodiesterase-5 inhibitors in chronic heart failure. Circ. Heart Fail. 1, 272–280 (2008).
Guazzi, M. et al. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ. Heart Fail. 4, 8–17 (2011).
Qin, F., Simeone, M. & Patel, R. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic. Biol. Med. 43, 271–281 (2007).
Saliaris, A. P. et al. Chronic allopurinol administration ameliorates maladaptive alterations in Ca2+ cycling proteins and β-adrenergic hyporesponsiveness in heart failure. Am. J. Physiol. Heart Circ. Physiol. 292, H1328–H1335 (2007).
Hare, J. M. et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 51, 2301–2309 (2008).
Palaniyandi, S. S., Sun, L., Ferreira, J. C. & Mochly-Rosen, D. Protein kinase C in heart failure: a therapeutic target? Cardiovasc. Res. 82, 229–239 (2009).
Backs, J. & Olson, E. N. Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 98, 15–24 (2006).
Bossuyt, J. et al. Ca2+/calmodulin-dependent protein kinase IIδ and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ. Res. 102, 695–702 (2008).
Zhang, R. et al. Calmodulin kinase II inhibition protects against structural heart disease. Nature Med. 11, 409–417 (2005).
Sossalla, S. et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ. Res. 107, 1150–1161 (2010).
Willemsen, S. et al. Effects of alagebrium, an advanced glycation end-product breaker, in patients with chronic heart failure: study design and baseline characteristics of the BENEFICIAL trial. Eur. J. Heart Fail. 12, 294–300 (2010).
Wehrens, X. H., Lehnart, S. E. & Marks, A. R. Intracellular calcium release and cardiac disease. Annu. Rev. Physiol. 67, 69–98 (2005).
Ikeda, Y., Hoshijima, M. & Chien, K. R. Toward biologically targeted therapy of calcium cycling defects in heart failure. Physiology 23, 6–16 (2008).
Belevych, A. et al. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys. J. 93, 4083–4092 (2007).
Lompré, A. M. et al. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 121, 822–830 (2010).
Blayney, L. M. & Lai, F. A. Ryanodine receptor-mediated arrhythmias and sudden cardiac death. Pharmacol. Ther. 123, 151–177 (2009).
Poller, W., Hajjar, R., Schultheiss, H. P. & Fechner, H. Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure. Cardiovasc. Res. 86, 353–364 (2010).
Jin, D. et al. Abstract 3911: a novel small molecule approach for treating mechano-electrical dysfunction in heart failure. Circulation 120, S885 (2009).
Ai, X., Curran, J. W., Shannon, T. R., Bers, D. M. & Pogwizd, S. M. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 97, 1314–1322 (2005).
Yano, M. et al. Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation 112, 3633–3643 (2005).
Mackrill, J. J. Ryanodine receptor calcium channels and their partners as drug targets. Biochem. Pharmacol. 79, 1535–1543 (2010).
Toischer, K. et al. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res. Cardiol. 105, 279–287 (2010).
Couvreur, N. et al. Chronic heart rate reduction with ivabradine improves systolic function of the reperfused heart through a dual mechanism involving a direct mechanical effect and a long-term increase in FKBP12/12.6 expression. Eur. Heart J. 31, 1529–1537 (2010).
Lemmens, K., Segers, V. F., Demolder, M. & De Keulenaer, G. W. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J. Biol. Chem. 281, 19469–19477 (2006).
Gao, R. et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J. Am. Coll. Cardiol. 55, 1907–1914 (2010).
Ashrafian, H., Frenneaux, M. P. & Opie, L. H. Metabolic mechanisms in heart failure. Circulation 116, 434–448 (2007).
Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S. & Stanley, W. C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 (2010).
Ussher, J. R. & Lopaschuk, G. D. Targeting malonyl CoA inhibition of mitochondrial fatty acid uptake as an approach to treat cardiac ischemia/reperfusion. Basic Res. Cardiol. 104, 203–210 (2009).
Inzucchi, S. E. & McGuire, D. K. New drugs for the treatment of diabetes: part II: incretin-based therapy and beyond. Circulation 117, 574–584 (2008).
Ban, K. et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350 (2008).
Sokos, G. G., Nikolaidis, L. A., Mankad, S., Elahi, D. & Shannon, R. P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J. Card. Fail. 12, 694–699 (2006).
Read, P. A., Khan, F. Z., Heck, P. M., Hoole, S. P. & Dutka, D. P. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ. Cardiovasc. Imaging 3, 195–201 (2010).
Witteles, R. M. & Fowler, M. B. Insulin-resistant cardiomyopathy: clinical evidence, mechanisms, and treatment options. J. Am. Coll. Cardiol. 51, 93–102 (2008).
Eurich, D. T. et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ 335, 497–501 (2007). This meta-analysis showed that metformin is the only antidiabetic agent that is not associated with measurable harm in patients with diabetes and heart failure, but it is associated with reduced mortality.
MacDonald, M. R. et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the UK. General Practice Research database. Diabetes Care 33, 1213–1218 (2010).
Bertrand, L. et al. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am. J. Physiol. Heart Circ. Physiol. 291, H239–H250 (2006).
Ren, J., Dominguez, L. J., Sowers, J. R. & Davidoff, A. J. Metformin but not glyburide prevents high glucose-induced abnormalities in relaxation and intracellular Ca2+ transients in adult rat ventricular myocytes. Diabetes 48, 2059–2065 (1999).
Sacca, L. Heart failure as a multiple hormonal deficiency syndrome. Circ. Heart Fail. 2, 151–156 (2009).
Pingitore, A. et al. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J. Clin. Endocrinol. Metab. 93, 1351–1358 (2008).
Soukoulis, V. et al. Micronutrient deficiencies an unmet need in heart failure. J. Am. Coll. Cardiol. 54, 1660–1673 (2009).
Tavazzi, L. et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372, 1223–1230 (2008).
Lavie, C. J., Milani, R. V., Mehra, M. R. & Ventura, H. O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 54, 585–594 (2009).
Pocok, S. J. et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur. Heart J. 27, 65–75 (2007).
Massion, P. B. & Balligand, J. L. Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): lessons from genetically modified mice. J. Physiol. 546, 63–75 (2003).
Kamp, O. et al. Effect of the long-term administration of nebivolol on clinical symptoms, exercise capacity and left ventricular function in patients with heart failure and preserved left ventricular ejection fraction: background, aims and design of the ELANDD study. Clin. Res. Cardiol. 99, 75–82 (2010).
Silberman, G. A. et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation 121, 519–528 (2010).
Wohlfart, P. et al. Antiatherosclerotic effects of small-molecular-weight compounds enhancing endothelial nitric-oxide synthase (eNOS) expression and preventing eNOS uncoupling. J. Pharmacol. Exp. Ther. 325, 370–379 (2008).
Phan, T. T. et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J. Am. Coll. Cardiol. 54, 402–409 (2009).
Zaza, A., Belardinelli, L. & Shryock, J. C. Pathophysiology and pharmacology of the cardiac “late sodium current.” Pharmacol. Ther. 119, 326–339 (2008).
Rastogi, S. et al. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am. J. Physiol. Heart Circ. Physiol. 295, H2149–H2155 (2008).
Finley, J. J., Konstam, M. A. & Udelson, J. E. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation 118, 410–421 (2008).
Gheorghiade, M. et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA 297, 1332–1343 (2007).
Konstam, M. A. et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 297, 1319–1331 (2007).
Anand, I. S. Anemia and chronic heart failure implications and treatment options. J. Am. Coll. Cardiol. 52, 501–511 (2008).
Okonko, O. D. et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. J. Am. Coll. Cardiol. 51, 103–112 (2008).
Anker, S. D. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New Engl. J. Med. 361, 2436–2448 (2009).
Timmer, S. A., De Boer, K., Knaapen, P., Götte, M. J. & Van Rossum, A. C. The potential role of erythropoietin in chronic heart failure: from the correction of anemia to improved perfusion and reduced apoptosis? J. Card. Fail. 15, 353–361 (2009).
Ngo, K. et al. Erythropoiesis-stimulating agents for anaemia in chronic heart failure patients. Cochrane Database Syst. Rev. 20 Jan 2010 (doi:10.1002/14651858.CD007613.pub2).
Jin, B., Luo, X., Lin, H., Li, J. & Shi, H. A meta-analysis of erythropoiesis-stimulating agents in anaemic patients with chronic heart failure. Eur. J. Heart Fail. 12, 249–253 (2010).
Van der Meer, P., Groenveld, H., Januzzi, J. & Van Veldhuisen, D. J. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart 95, 1309–1314 (2009).
Singh, A. K. et al. Correction of anemia with epopoetin alfa in chronic kidney disease. N. Engl. J. Med. 355, 2085–2098 (2006).
Bennett, C. L. et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 299, 914–924 (2008).
McMurray, J. J. et al. Design of the reduction of events with darbopoetin alfa in heart failure (RED-HF): a Phase III, anemia correction, morbidity–mortality trial. Eur. Heart J. 11, 795–801 (2009).
Tamargo, J. et al. Investigational positive inotropic agents for acute heart failure. Cardiovasc. Hematol. Disord. Drug Targets 9, 193–205 (2009).
Teerlink, J. R. et al. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart Fail. Rev. 14, 243–253 (2009).
Goldhaber, J. & Hamilton, J. A. Role of inotropic agents in the treatment of heart failure. Circulation 121, 1655–1660 (2010). In this article, the authors reviewed the most appropriate use of inotropic agents in four patients with heart failure.
De Luca, L., Colucci, W. S., Nieminen, M. S., Massie, B. M. & Gheorghiade, M. Evidence-based use of levosimendan in different clinical settings. Eur. Heart J. 27, 1908–1920 (2006).
Cleland, J. G., Freemantle, N., Coletta, A P. & Clark, A. L. Clinical trials update from the American Heart Association: REPAIRAMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur. J. Heart. Fail. 8, 105–110 (2006).
Gheorghiade, M. et al. Hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure. J. Am. Coll. Cardiol. 51, 2276–2285 (2008).
Shah, S. J. et al. Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: results from the hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure (HORIZON-HF) trial. Am. Heart. J. 157, 1035–1041 (2009).
Teerlink, J. R. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail. Rev. 14, 289–298 (2009).
Tamargo, J., López-Farré, A., Caballero, R. & Delpón, E. Omecamtiv mecarbil. Drugs Future 34, 950–961 (2009).
Japp, A. G. et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 52, 908–913 (2008).
Japp, A. G. et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 121, 1818–1827 (2010).
Acknowledgements
This work was supported by grants from the Carlos III Health Institute (PI080665 and ISCIII-RETIC RD06/0009-FEDER) and the Spanish Society of Cardiology. We would like to thank E. Delpón for her critical reading and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
José Lopez-Sendón declares an association with the following pharmaceutical companies: Servier, Pfizer, Amgen and Novartis.
Supplementary information
Supplementary Information (Table S1)
Major large-scale randomized clinical trials comparing the effects of new developing drugs in patients with heart failure (PDF 457 kb)
Related links
Related links
FURTHER INFORMATION
Glossary
- Left ventricular ejection fraction
-
(LVEF). The percentage of blood that is pumped out from the left ventricles of the heart with each heart beat.
- Inotropes
-
Drugs that increase (positive inotropes) or decrease (negative inotropes) myocardial contractility.
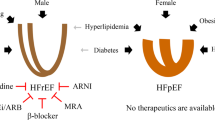
- Heart failure with preserved ejection fraction
-
(HFPEF). Patients who have heart failure but with a normal left ventricular ejection fraction; that is, above 40–50% (there is no consensus for the cut-off).
- Neurohumoral profile
-
A decrease in cardiac pump function that is associated with a neurohumoral response, which is characterized by the activation of the renin–angiotensin–aldosterone and sympathetic nervous systems and elevated circulating levels of natriuretic peptides, vasopressin, endothelin-1, urotensins, adrenomedullin and nitric oxide.
- Loop diuretics
-
Diurectics that inhibit the Na+/K+/2Cl− co-transporter in the luminal membrane of the thick ascending limb of the loop of Henle. They are the most powerful diuretics available.
- Ischaemic pre-conditioning
-
An increased tolerance to ischaemia and reperfusion that is induced by a previous sublethal period of ischaemia.
- Left ventricular filling pressure
-
The pressure that builds up in the left ventricle as it is being filled with blood.
- Myocardial oxygen demands
-
(MVO2). This relates to the amount of oxygen consumed per minute that is required to regenerate the ATP that is used by membrane transport mechanisms and in the excitation–contraction process. MVO2 is expressed as ml O2 per min per 100g of cardiac tissue.
- Lusitropic effects
-
The effects of a drug on the properties of ventricular relaxation.
- NT-proBNP
-
Amino terminal proBNP (NT-proBNP) is a cleavage product of the precursor brain natriuretic peptide (BNP).
- Neprilysin
-
A zinc-dependent metalloproteinase that degrades several small secreted peptides such as natriuretic peptides, angiotensin II and endothelin-1.
- Peripheral hypoperfusion
-
The decreased perfusion of organs (mainly skin, kidney and brain) as a result of a decrease in the pumping function of the heart, which results in cold, clammy skin, oliguria or lethargy.
- Crossbridge cycle
-
The entire process by which the myosin heads interact with the actin filament.
- dP/dtmax
-
The maximum rate of left ventricular systolic pressure development — an indirect index of cardiac contractility.
- Left ventricular assist devices
-
Surgically implanted mechanical pumps that take blood from the left ventricle and pump it into the aorta.
- Intestinal L cells
-
Cells that are found throughout the lining of the gastrointestinal tract and contain regulatory peptide hormones and/or biogenic amines.
- Global and regional left ventricular performance
-
Changes in global left ventricular or regional (segmental) left ventricular contraction and function, respectively.
- Functional class
-
A classification of the severity of heart failure that is based on the symptoms and exercise capacity of the patient (known as the New York Heart Association functional classification).
- Preload reduction
-
The preload is the pressure and volume of blood that is present in the left ventricle at the end of diastole. The major drugs that reduce the preload in heart failure are loop diuretics and nitrates, which dilate the veins to reduce the amount of blood returning to the heart via the venous system.
- Precipitant factors
-
Factors that may not be the underlying cause of the illness, but cause or contribute to the occurrence of heart failure or to hospitalization.
- Titin springs
-
A large part of the titin molecule (a giant protein that is located in the sarcomere) that functions as a spring and develops passive stiffness when sarcomeres are stretched.
Rights and permissions
About this article
Cite this article
Tamargo, J., López-Sendón, J. Novel therapeutic targets for the treatment of heart failure. Nat Rev Drug Discov 10, 536–555 (2011). https://doi.org/10.1038/nrd3431
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3431
This article is cited by
-
Isorhamnetin protects against cardiac hypertrophy through blocking PI3K–AKT pathway
Molecular and Cellular Biochemistry (2017)
-
Central nervous system circuits modified in heart failure: pathophysiology and therapeutic implications
Heart Failure Reviews (2014)
-
GRK2 blockade with βARKct is essential for cardiac β2-adrenergic receptor signaling towards increased contractility
Cell Communication and Signaling (2013)
-
Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome
Journal of Molecular Medicine (2013)
-
Selective PDE5A inhibition with sildenafil rescues left ventricular dysfunction, inflammatory immune response and cardiac remodeling in angiotensin II-induced heart failure in vivo
Basic Research in Cardiology (2012)