Key Points
-
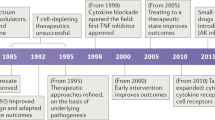
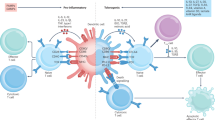
The main goal of antigen-specific immunotherapies (ASIs) in autoimmune diseases is to reprogramme or remove autoreactive cells and to induce immune tolerance to self-antigens
-
Depleting autoreactive T cells, B cells and plasma cells could be a future therapeutic strategy for rheumatic diseases and could lead to temporary improvement in disease
-
Awakening antigen-specific tolerance by inducing regulatory T (Treg) cells using antigen-specific tolerogenic peptides alone or coupled to cells or nanoparticles can reprogramme autoreactive cells, resulting in long-term tolerance
-
T cells can be engineered to specifically target and delete self-reactive B cells or to redirect the specificity of Treg cells towards self-antigens
-
The use of antigen-boosted tolerogenic dendritic cells and DNA-based vaccines are promising ASIs.
Abstract
The main goal of antigen-specific immunotherapy (ASI) in autoimmune and rheumatic diseases is to reprogramme or remove autoreactive cells and/or induce immune tolerance to self-antigens. Current therapies in these diseases either treat symptoms or slow down disease progression but are not yet curative or preventative — disease-specific treatments are urgently needed. In contrast to the nonspecific treatments in current use that induce generalized immune suppression, which is associated with several adverse effects including increased risk of infections, ASIs target a restricted subset of B cells or T cells, and thus do not compromise systemic immunity and host defence. This Review provides a summary of novel approaches for identifying autoepitopes and detecting and targeting autoreactive cells that might help in the development of ASIs. Promising approaches include the use of tolerizing peptides coupled to MHC constructs and/or nanocompounds, tolerizing dendritic cells and antigen-specific vaccines. Following studies in animal models of rheumatoid arthritis and systemic lupus erythematosus, several of these strategies have now entered clinical trials. However, to use these approaches in humans, several important limitations must first be addressed, such as; selecting the proper immunodominant autoantigen; identifying the optimal timing, dosing and route of administration; finding biomarkers for monitoring the therapy; and optimizing methodology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goodnow, C. C., Sprent, J., Fazekas de St Groth, B. & Vinuesa, C. G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435, 590–597 (2005).
Burns, J., Rosenzweig, A., Zweiman, B. & Lisak, R. P. Isolation of myelin basic protein-reactive T-cell lines from normal human blood. Cell. Immunol. 81, 435–440 (1983).
Matsumoto, Y. New approach to immunotherapy against organ-specific autoimmune diseases with T cell receptor and chemokine receptor DNA vaccines. Curr. Drug Targets Immune Endocr. Metabol. Disord. 5, 73–77 (2005).
Andersson, P. O. & Wadenvik, H. Chronic idiopathic thrombocytopenic purpura (ITP): molecular mechanisms and implications for therapy. Expert Rev. Mol. Med. 6, 1–17 (2004).
Schultze, J. L., Grabbe, S. & von Bergwelt-Baildon, M. S. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 25, 659–664 (2004).
Aletaha, D. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588 (2010).
Soos, L. et al. Clinical evaluation of anti-mutated citrullinated vimentin by ELISA in rheumatoid arthritis. J. Rheumatol. 34, 1658–1663 (2007).
Baeten, D. et al. Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium: relevance to antifilaggrin autoantibodies. Arthritis Rheum. 44, 2255–2262 (2001).
Szarka, E. et al. Recognition of new citrulline-containing peptide epitopes by autoantibodies produced in vivo and in vitro by B cells of rheumatoid arthritis patients. Immunology 141, 181–191 (2014).
Burkhardt, H. et al. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur. J. Immunol. 35, 1643–1652 (2005).
Cornillet, M. et al. The fibrin-derived citrullinated peptide beta60-74Cit(6)(0),(7)(2),(7)(4) bears the major ACPA epitope recognised by the rheumatoid arthritis-specific anticitrullinated fibrinogen autoantibodies and anti-CCP2 antibodies. Ann. Rheum. Dis. 73, 1246–1252 (2014).
Schwenzer, A. et al. Identification of an immunodominant peptide from citrullinated tenascin-C as a major target for autoantibodies in rheumatoid arthritis. Ann. Rheum. Dis. 75, 1876–1883 (2016).
Pruijn, G. J. Citrullination and carbamylation in the pathophysiology of rheumatoid arthritis. Front. Immunol. 6, 192 (2015).
Tiffin, N., Adeyemo, A. & Okpechi, I. A diverse array of genetic factors contribute to the pathogenesis of systemic lupus erythematosus. Orphanet J. Rare Dis. 8, 2 (2013).
Sun, X. Y., Shi, J., Han, L., Su, Y. & Li, Z. G. Anti-histones antibodies in systemic lupus erythematosus: prevalence and frequency in neuropsychiatric lupus. J. Clin. Lab. Anal. 22, 271–277 (2008).
Konya, C., Paz, Z. & Tsokos, G. C. The role of T cells in systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 26, 493–501 (2014).
Noack, M. & Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 13, 668–677 (2014).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Kerkman, P. F. et al. Identification and characterisation of citrullinated antigen-specific B cells in peripheral blood of patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1170–1176 (2015).
Pozsgay, J. et al. in vitro eradication of citrullinated protein specific B-lymphocytes of rheumatoid arthritis patients by targeted bifunctional nanoparticles. Arthritis Res. Ther. 18, 15 (2016).
Gaynor, B. et al. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc. Natl Acad. Sci. USA 94, 1955–1960 (1997).
Putterman, C. & Diamond, B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J. Exp. Med. 188, 29–38 (1998).
Newman, J., Rice, J. S., Wang, C., Harris, S. L. & Diamond, B. Identification of an antigen-specific B cell population. J. Immunol. Methods 272, 177–187 (2003).
Jacobi, A. M., Zhang, J., Mackay, M., Aranow, C. & Diamond, B. Phenotypic characterization of autoreactive B cells — checkpoints of B cell tolerance in patients with systemic lupus erythematosus. PLoS ONE 4, e5776 (2009).
van Schooten, W. C., Strang, G. & Palathumpat, V. Biological properties of dendritic cells: implications to their use in the treatment of cancer. Mol. Med. Today 3, 254–260 (1997).
Mihaylova, N. et al. Selective silencing of disease-associated B-lymphocytes by chimeric molecules targeting their Fc gamma IIb receptor. Int. Immunol. 20, 165–175 (2008).
Jeltsch-David, H. & Muller, S. Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model. Autoimmun. Rev. 13, 963–973 (2014).
Horton, H. M. et al. Antibody-mediated coengagement of FcgammaRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J. Immunol. 186, 4223–4233 (2011).
Szili, D. et al. Suppression of innate and adaptive B cell activation pathways by antibody coengagement of FcgammaRIIb and CD19. MAbs 6, 991–999 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02725515 (2017).
Cohen, M. D. & Keystone, E. Rituximab for rheumatoid arthritis. Rheumatol. Ther. 2, 99–111 (2015).
Hoffman, W., Lakkis, F. G. & Chalasani, G. B. Cells, antibodies, and more. Clin. J. Am. Soc. Nephrol. 11, 137–154 (2016).
Manz, R., Assenmacher, M., Pfluger, E., Miltenyi, S. & Radbruch, A. Analysis and sorting of live cells according to secreted molecules, relocated to a cell-surface affinity matrix. Proc. Natl Acad. Sci. USA 92, 1921–1925 (1995).
Taddeo, A. et al. Selection and depletion of plasma cells based on the specificity of the secreted antibody. Eur. J. Immunol. 45, 317–319 (2015).
Hiepe, F. & Radbruch, A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat. Rev. Nephrol. 12, 232–240 (2016).
Caraux, A. et al. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologica 95, 1016–1020 (2010).
Suurmond, J. & Diamond, B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 125, 2194–2202 (2015).
Iking-Konert, C. et al. First clinical trials of a new heteropolymer technology agent in normal healthy volunteers and patients with systemic lupus erythematosus: safety and proof of principle of the antigen-heteropolymer ETI-104. Ann. Rheum. Dis. 63, 1104–1112 (2004).
Cornacoff, J. B. et al. Primate erythrocyte-immune complex-clearing mechanism. J. Clin. Invest. 71, 236–247 (1983).
[No authors listed]. Abetimus: abetimus sodium, LJP 394. BioDrugs 17, 212–215 (2003).
Horowitz, D. M. & Furie, R. A. Abetimus sodium: a medication for the prevention of lupus nephritis flares. Expert Opin. Pharmacother. 10, 1501–1507 (2009).
Cardiel, M. H. et al. Abetimus sodium for renal flare in systemic lupus erythematosus: results of a randomized, controlled phase III trial. Arthritis Rheum. 58, 2470–2480 (2008).
Enouz, S., Carrie, L., Merkler, D., Bevan, M. J. & Zehn, D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J. Exp. Med. 209, 1769–1779 (2012).
Kyewski, B. & Derbinski, J. Self-representation in the thymus: an extended view. Nat. Rev. Immunol. 4, 688–698 (2004).
Sewell, A. K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 12, 669–677 (2012).
Eisenbarth, G. S. & Kotzin, B. L. Enumerating autoreactive T cells in peripheral blood: a big step in diabetes prediction. J. Clin. Invest. 111, 179–181 (2003).
Trudeau, J. D. et al. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J. Clin. Invest. 111, 217–223 (2003).
Tisch, R. & McDevitt, H. Insulin-dependent diabetes mellitus. Cell 85, 291–297 (1996).
Anderson, B., Park, B. J., Verdaguer, J., Amrani, A. & Santamaria, P. Prevalent CD8+ T cell response against one peptide/MHC complex in autoimmune diabetes. Proc. Natl Acad. Sci. USA 96, 9311–9316 (1999).
Vincent, B. G. et al. Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J. Immunol. 184, 4196–4204 (2010).
Bischof, F. et al. Analysis of autoreactive CD4 T cells in experimental autoimmune encephalomyelitis after primary and secondary challenge using MHC class II tetramers. J. Immunol. 172, 2878–2884 (2004).
Kobezda, T., Ghassemi-Nejad, S., Mikecz, K., Glant, T. T. & Szekanecz, Z. Of mice and men: how animal models advance our understanding of T-cell function in RA. Nat. Rev. Rheumatol. 10, 160–170 (2014).
Gertel, S., Serre, G., Shoenfeld, Y. & Amital, H. Immune tolerance induction with multiepitope peptide derived from citrullinated autoantigens attenuates arthritis manifestations in adjuvant arthritis rats. J. Immunol. 194, 5674–5680 (2015).
Wehrens, E. J., Prakken, B. J. & van Wijk, F. T cells out of control — impaired immune regulation in the inflamed joint. Nat. Rev. Rheumatol. 9, 34–42 (2013).
Miyara, M., Ito, Y. & Sakaguchi, S. TREG-cell therapies for autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 10, 543–551 (2014).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011).
Desreumaux, P. et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology 143, 1207–1217.e1–2 (2012).
Ahmed, M. S. & Bae, Y. S. Dendritic cell-based immunotherapy for rheumatoid arthritis: from bench to bedside. Immune Netw. 16, 44–51 (2016).
Bluestone, J. A. et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl Med. 7, 315ra189 (2015).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02772679 (2017).
Marcos, M. A. et al. Coelomic and bone marrow-derived B cells. Developmental constraints versus antigen-specific selection. Ann. NY Acad. Sci. 651, 433–442 (1992).
Krall, W. J. & Braun, J. in vivo retroviral marking of antigen-specific B lymphocytes. Semin. Immunol. 4, 19–28 (1992).
Venigalla, R. K. et al. Reduced CD4+,CD25- T cell sensitivity to the suppressive function of CD4+,CD25high, CD127-/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 58, 2120–2130 (2008).
Briner, T. J., Kuo, M. C., Keating, K. M., Rogers, B. L. & Greenstein, J. L. Peripheral T-cell tolerance induced in naive and primed mice by subcutaneous injection of peptides from the major cat allergen Fel d I. Proc. Natl Acad. Sci. USA 90, 7608–7612 (1993).
Kaliyaperumal, A., Michaels, M. A. & Datta, S. K. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides: tolerance spreading impairs pathogenic function of autoimmune T and B cells. J. Immunol. 162, 5775–5783 (1999).
Kang, H. K., Michaels, M. A., Berner, B. R. & Datta, S. K. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J. Immunol. 174, 3247–3255 (2005).
Zhang, L. et al. Major pathogenic steps in human lupus can be effectively suppressed by nucleosomal histone peptide epitope-induced regulatory immunity. Clin. Immunol. 149, 365–378 (2013).
Wooley, P. H. The usefulness and the limitations of animal models in identifying targets for therapy in arthritis. Best Pract. Res. Clin. Rheumatol. 18, 47–58 (2004).
Leavenworth, J. W., Tang, X., Kim, H. J., Wang, X. & Cantor, H. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J. Clin. Invest. 123, 1382–1389 (2013).
Sthoeger, Z., Zinger, H., Sharabi, A., Asher, I. & Mozes, E. The tolerogenic peptide, hCDR1, down-regulates the expression of interferon-alpha in murine and human systemic lupus erythematosus. PLoS ONE 8, e60394 (2013).
Sharabi, A., Zinger, H., Zborowsky, M., Sthoeger, Z. M. & Mozes, E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-beta. Proc. Natl Acad. Sci. USA 103, 8810–8815 (2006).
Vanderlugt, C. L. & Miller, S. D. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2, 85–95 (2002).
Gertel, S., Shoenfeld, Y. & Amital, H. Tolerogenic citrullinated peptide for arthritis. Oncotarget 6, 19344–19345 (2015).
Turley, D. M. & Miller, S. D. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J. Immunol. 178, 2212–2220 (2007).
Sauter, B. et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 (2000).
Getts, D. R. et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J. Immunol. 187, 2405–2417 (2011).
Kundig, T. M. et al. On T cell memory: arguments for antigen dependence. Immunol. Rev. 150, 63–90 (1996).
Sayegh, M. H. & Turka, L. A. T cell costimulatory pathways: promising novel targets for immunosuppression and tolerance induction. J. Am. Soc. Nephrol. 6, 1143–1150 (1995).
Capini, C. et al. Antigen-specific suppression of inflammatory arthritis using liposomes. J. Immunol. 182, 3556–3565 (2009).
Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016).
Maldonado, R. A. et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl Acad. Sci. USA 112, E156–E165 (2015).
Hunter, Z. et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 8, 2148–2160 (2014).
Getts, D. R. et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 30, 1217–1224 (2012).
McCarthy, D. P. et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine 13, 191–200 (2017).
Anderson, R. P. & Jabri, B. Vaccine against autoimmune disease: antigen-specific immunotherapy. Curr. Opin. Immunol. 25, 410–417 (2013).
Marion, T. N. & Postlethwaite, A. E. Chance, genetics, and the heterogeneity of disease and pathogenesis in systemic lupus erythematosus. Semin. Immunopathol. 36, 495–517 (2014).
Garren, H. et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann. Neurol. 63, 611–620 (2008).
Roep, B. O. et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl Med. 5, 191ra82 (2013).
Ho, P. P. et al. Tolerizing DNA vaccines for autoimmune arthritis. Autoimmunity 39, 675–682 (2006).
Gottlieb, P., Utz, P. J., Robinson, W. & Steinman, L. Clinical optimization of antigen specific modulation of type 1 diabetes with the plasmid DNA platform. Clin. Immunol. 149, 297–306 (2013).
James, E. A. et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 66, 1712–1722 (2014).
Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 342, 1432–1433 (2013).
Fransson, M. et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflamm. 9, 112 (2012).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016).
Hilkens, C. M. & Isaacs, J. D. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin. Exp. Immunol. 172, 148–157 (2013).
Raker, V. K., Domogalla, M. P. & Steinbrink, K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front. Immunol. 6, 569 (2015).
Benham, H. et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci. Transl Med. 7, 290ra87 (2015).
Bin Joo, Y. et al. Phase 1 study of immunotherapy using autoantigen-loaded dendritic cells in patients with anti-citrullinated peptide antigen positive rheumatoid arthritis [abstract 946]. Arthritis Rheumatol. 66, S420–S421 (2014).
Bell, G. M. et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 76, 227–234 (2017).
Bach, J. F., Koutouzov, S. & van Endert, P. M. Are there unique autoantigens triggering autoimmune diseases? Immunol. Rev. 164, 139–155 (1998).
Lorenz, H. M., Herrmann, M. & Kalden, J. R. The pathogenesis of autoimmune diseases. Scand. J. Clin. Lab. Invest. Suppl. 235, 16–26 (2001).
Miyara, M. et al. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 10, 744–755 (2011).
Sakaguchi, S., Miyara, M., Costantino, C. M. & Hafler, D. A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 (2010).
Odegard, J. M., Nepom, G. T. & Wambre, E. Biomarkers for antigen immunotherapy in allergy and type 1 diabetes. Clin. Immunol. 161, 44–50 (2015).
Sinha, S. et al. A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J. Neurosci. 27, 12531–12539 (2007).
Offner, H., Sinha, S., Burrows, G. G., Ferro, A. J. & Vandenbark, A. A. RTL therapy for multiple sclerosis: a phase I clinical study. J. Neuroimmunol. 231, 7–14 (2011).
Carambia, A. et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J. Hepatol. 62, 1349–1356 (2015).
Ho, P. P., Fontoura, P., Ruiz, P. J., Steinman, L. & Garren, H. An immunomodulatory GpG oligonucleotide for the treatment of autoimmunity via the innate and adaptive immune systems. J. Immunol. 171, 4920–4926 (2003).
Ho, P. P. et al. A suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J. Immunol. 175, 6226–6234 (2005).
Bresson, D. et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs . J. Clin. Invest. 116, 1371–1381 (2006).
Sarikonda, G. et al. Transient B-cell depletion with anti-CD20 in combination with proinsulin DNA vaccine or oral insulin: immunologic effects and efficacy in NOD mice. PLoS ONE 8, e54712 (2013).
Acknowledgements
The work of the authors was supported by the Hungarian National Science Fund (NKFI OTKA NK 104846) (G.S.) and by the European Union and the State of Hungary co-financed by the European Social Fund in the framework of TAMOP-4.2.4.A/2-11/1-2012-0001 'National Excellence Program' (Z.S.).
Author information
Authors and Affiliations
Contributions
All authors wrote the manuscript. Z.S. and J.P. researched the data for the article. Z.S. and G.S. undertook review and/or editing of the manuscript before submission and provided substantial contributions to discussions of its content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Tetramer
-
An oligomer comprised of four monomers; in the case of MHC–peptide tetramers, four copies of biotinylated MHC–peptide molecules are bound to streptavidin.
- Nanoparticles
-
Microscopic particles sized 1–100 nm.
- Mimetope
-
A protein or peptide that resembles the conformation or structure of an antigen, resulting in cross-reactivity.
- Heteropolymer
-
A compound formed from multiple subunits that are not all the same.
- Autoepitope
-
A part of the self-protein that is recognized by autoantibodies or autoreactive B cells or T cells.
- Anergy
-
Lymphocyte unresponsiveness.
- Epitope spreading
-
Diversification of epitope specificity, whereby during an autoimmune response additional new epitopes of the same antigen are recognized.
- Shared epitope
-
A common sequence of amino acids at residues 70–74 of HLA-DRB1, found in alleles associated with rheumatoid arthritis.
Rights and permissions
About this article
Cite this article
Pozsgay, J., Szekanecz, Z. & Sármay, G. Antigen-specific immunotherapies in rheumatic diseases. Nat Rev Rheumatol 13, 525–537 (2017). https://doi.org/10.1038/nrrheum.2017.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.107
This article is cited by
-
Transformed Salmonella typhimurium SL7207/pcDNA-CCOL2A1 as an orally administered DNA vaccine
AMB Express (2024)
-
Genetics and epigenetics of primary Sjögren syndrome: implications for future therapies
Nature Reviews Rheumatology (2023)
-
A fructosylated peptide derived from a collagen II T cell epitope for long-term treatment of arthritis (FIA-CIA) in mice
Scientific Reports (2021)
-
Engineering precision nanoparticles for drug delivery
Nature Reviews Drug Discovery (2021)
-
Biologia Futura: Emerging antigen-specific therapies for autoimmune diseases
Biologia Futura (2021)