Abstract
Prophylactic indomethacin may decrease Severe Intraventricular Hemorrhage (SIVH). Our goal was to develop a predictive model for SIVH using parameters available by six hours of age. De-identified data for preterm infants born ≤ 34 weeks gestational age was abstracted from Vermont Oxford Network database. Using clinical variables available by 6 hrs of age the model was developed and validated. Statistical methods were used to evaluate the ability of the model to discriminate infants with and without SIVH and, to compare observed and predicted risk. The model achieved excellent discrimination as indicated by ROC curve of 0·85. A good agreement was noted between observed and predicted risk (HLtest: p = 0·22). Application of the model to patients receiving indomethacin suggests a benefit at the highest risk levels. We have developed a valid predictive model for predicting SIVH as well as shown that exposure to indomethacin decreases the incidence of SIVH overall.
Similar content being viewed by others
Introduction
Intraventricular Hemorrhage (IVH), especially severe IVH (SIVH) classified using Papile's classification as Grade III and IV, can lead to significant long term neuro-developmental impairment in preterm infants1,2,3,4. The incidence of severe IVH varies inter-institutionally from three to twelve percent, while for any IVH it ranges from ten to thirty percent5.
Low dose indomethacin therapy has been shown to decrease the occurrence of IVH and white matter injury in the preterm infant population as well as improve long-term neurodevelopmental outcomes6,7,8,9. Additional data have demonstrated that early low dose indomethacin aids in early closure of patent ductus arteriosus (PDA), with lower need for surgical ligation of symptomatic PDA and less white matter injury on neuro-imaging10,11,12. Despite these benefits it is not widely used because of the potential risks from impaired platelet function and decreased systemic blood flow, resulting in necrotizing enterocolitis, spontaneous intestinal perforation and transient renal impairment. The use of indomethacin for IVH prophylaxis varies widely amongst NICU's, from no use to limited use. When using gestational age (GA) as a cut off point for treatment, infants less than 26 weeks are most often treated. This excludes preterm infants born at higher gestational age but who may remain at risk for SIVH.
Our primary aim was to develop a predictive model using clinical parameters available within the first six hours of life that would help target indomethacin therapy to preterm infants at highest risk of SIVH, while minimizing exposure to infants at lower risk.
Results
Observations from the four participating hospitals were restricted to the 2917 infants with complete data and excluded patients with any indomethacin exposure, as this is a prophylactic treatment for IVH. Infants excluded from the analysis because of missing ultrasound (n = 253) were significantly more likely to be outborn (34.0% vs. 19.4%. p<0.001), had a slightly greater mean gestational age [29.9 wks (±3.2) vs. 28.9 wks (±2.4), p< 0.001] and had higher mean birth weight [1359.7 grams (±491.8) vs. 1163.7 (±299.1), p<0.001]. No significant differences between infants with missing and non-missing ultrasound data emerged for sex, Apgar score, steroid use and mode of delivery.
Results of the univariable analyses for the 2917 infants with complete data are given in Table 1. Infants in the SIVH group had lower mean gestational age, birth weight, 5-minute APGAR score, antenatal steroid administration rate, were frequently outborn and delivered via C-Section.
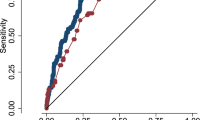
Training and evaluating the models: Table 2 shows the results of model training and validation. These models were developed on infants who did not receive any indomethacin exposure (n = 2917). The areas under the curve (AUC) for the four separate training datasets were consistent, ranging from 0·84 to 0·86. Regarding the external validation, the AUCs for test data were also relatively consistent, ranging from 0·82 to 0·88.
In view of the consistency of the AUCs above, a final model was generated using data from all centers combined. The AUC for the final model is 0·85 (95% CI: 0·81 –0·88). Figure 1 shows a box-plot of predicted probabilities for cases and non-cases derived from the final model. Model calibration appears satisfactory, as demonstrated by the Hosmer-Lemeshow goodness-of-fit test, (p = 0·22) and the graph of the observed vs. predicted deciles (Figure 2).
Using the model equation we also developed a calculator available online which estimates the individual infant's risk of developing SIVH, after providing the seven clinical predictor variables (Figure 3).
Snapshot of the online calculator which estimates the individual infant's risk of developing SIVH, after providing the seven clinical predictor variables.
Available online via open access at www.neoqic.org.
Final model applied to the indomethacin group: We also wanted to examine the effect of any indomethacin exposure on the incidence of severe IVH. We reasoned that if indomethacin prevents severe IVH, the observed risk of severe IVH in the sample of patients who had indomethacin exposure would be less than the risk predicted by our model, because the model was generated using only patients with no indomethacin exposure. Thus, we hypothesized that using the predictive model in the indomethacin group would result in discernibly poor fit. To this end, we applied the prediction model to the group of patients (n = 944) who had any indomethacin exposure. Table 3 shows the distribution of the seven predictor factors by whether indomethacin was given or not. On average, those receiving indomethacin were born earlier, had lower birth weights, were slightly more likely to have received antenatal steroids, were more frequently transferred from another institution and were slightly more likely to be delivered by C-section.
Although those receiving indomethacin were more than twice as likely to have severe IVH (RR = 2·26, 95% CI: 1·80–2·82), applying the risk equation to those receiving indomethacin does suggest a beneficial effect. As anticipated because the initial model construction excluded patients with indomethacin exposure, the test of model fit indicates that the predictive model does not summarize well the IVH risk profile of those exposed to indomethacin (H-L goodness-of-fit: p < 0·001). Figure 4 shows the plot of the observed vs. predicted risk of severe IVH by risk decile. The total predicted risk among those receiving indomethacin is 14·9%, as compared to the actual risk of 12·6%. Comparing the predicted versus observed risk of severe IVH by risk decile demonstrated that the greatest level of discrepancy occurs in the highest risk decile. In this decile, the level of risk predicted by the model is 44·9%, whereas the observed risk is 23·4%. This difference may be attributable to the effect of indomethacin.
Discussion
In preterm infants, the occurrence of severe IVH is associated with poor neurodevelopmental outcomes, such as severe motor and cognitive delay, as well as cerebral palsy. About thirty-five to forty percent of infants with SIVH will suffer from neurocognitive delays20. The scoring systems such as Clinical Risk Index for Babies (CRIB) and Score for Neonatal Acute Physiology (SNAP, SNAP- II, SNAPPE- II) predict illness severity, mortality and long term outcomes21,22,23. However, these have the limitation of requiring post natal data beyond the first few hours of life and inability to predict individual patient risk. This precludes their use for the early intervention strategies that need to be initiated within the first hours of life. Likewise Heuchan et al. for the Australian and New Zealand Neonatal Network, developed a predictive model to allow for risk adjustments for variation of IVH occurrence in different NICUs in their network24. They also used antenatal and perinatal factors most commonly associated with IVH to create their model but acknowledged the limitation of applying it to individual infants. Thus, at this time, we are unaware of any predictive model that can calculate the risk of intraventricular hemorrhage for any given infant early in life for counseling purposes as well as to assist the clinician in targeting neuroprotective strategies based on individual risk.
Primarily, we have created a simple, clinician-friendly, validated predictive model utilizing perinatal clinical parameters that are available at birth for preterm infants born ≤34 weeks gestational age, especially, for the subset at the highest risk decile for SIVH. Although any IVH increases the risk of neurodevelopmental impairment, the higher grades are more likely to affect long term outcomes. Thus, this is the reason we chose to direct our model specifically towards SIVH. In our model we found that low gestational age, birth weight, five minute Apgar score and being outborn had a strong association with occurrence of SIVH, which is in agreement with the current literature. Interestingly in our dataset cesarean delivery was associated with higher incidence of severe IVH. This differs from some reports which have noted that cesarean delivery may either be protective25, or have no difference against IVH as compared to vaginal delivery26. This difference may be due to our focus on severe IVH as opposed to any IVH reported in the other studies. We did find that maternal antenatal steroid administration had a protective effect which also agrees with previously published reports. We did not find gender to be an important contributor, despite overall morbidity and mortality data suggesting that female gender appears to provide benefit27. Additional clinical values, such as maternal magnesium therapy, admission temperature and blood pressure, may also further improve the predictive accuracy of the model. Future iterations of the model will incorporate this data.
As compared to the model by Heuchen et al. that had an ROC area under the curve of 0·76, our predictive model showed better discrimination (AUC = 0·85), as well as substantial agreement between observed and predicted risk24. This provides reassurance regarding the generalizability of the model to other patient populations with different clinical practices.
The model may provide clinical utility, by identifying preterm infants at highest risk of developing severe intraventricular hemorrhage and may allow targeted prophylactic indomethacin treatment, while minimizing adverse effects by sparing exposure to those at lower risk. Clinicians wishing to apply decision-making algorithms to individual patients, as suggested by Sinclair, require an estimate of the patient's baseline risk29. Up to now, the estimates of baseline risk were crude, as well as subjective, at best. The predictive model described herein provides the clinician an objective and validated estimate of the baseline risk necessary to apply therapeutic decision tools. We caution, though, that the model should be used to support the clinician's interpretation and decision-making – not replace it.
The majority of IVH occurs either prenatally or within the first seventy-two hours of birth, with a small percentage of IVH occurring later. Since multiple perinatal factors from pre-natal to post-natal are associated with IVH, there may be multiple opportunities to prevent SIVH at different times. Preventing or delaying preterm birth, delivery at a tertiary care perinatal center, maternal antenatal steroids and maternal magnesium sulfate for neuroprotection are some prenatal interventions. Decreasing the severity of respiratory disease by judicious use of surfactant therapy and prophylactic use of indomethacin therapy within first six hours of birth are examples of postnatal interventions. Low-dose indomethacin therapy may reduce the risk of severe IVH by 35%, as shown in the TIPP trial as well as additional studies6,9. Additionally, follow-up studies demonstrated no adverse outcomes at 36 months and at school age infants with IVH had worse outcomes than no IVH7,8,30. Of note though the long term follow-up studies did not adjust for other morbidities such as late onset sepsis, NEC, or BPD which may affect long term neurodevelopmental outcomes in the absence of severe IVH. Additionally Miller et al have shown in their prospective observational study that prophylactic indomethacin exposure was independently associated with a lower risk of white matter injury or brain abnormality, even when adjusted for other clinical parameters12. A recent Cochrane meta-analysis also noted significant positive effects of prophylactic indomethacin therapy, including decreased incidence of periventricular leukomalacia or PDA and need for surgical ligation10. Although there are concerns related to the vasoconstrictive mechanism of indomethacin, none of the studies documented a higher incidence of necrotizing enterocolitis or renal complications. Despite this there has not been wide acceptance of this potentially beneficial therapy. Clyman et al have suggested that indomethacin prophylaxis may be a reasonable choice in intensive care nurseries where IVH and PDA are frequent management problems28.
Further support for the validity of the model is seen when the model is applied to infants receiving indomethacin. Assuming that any exposure to indomethacin would confer some protection against SIVH, we would expect our model to over-predict the risk of SIVH among exposed infants and thus, fit poorly. This is in fact what we found. Overall, the model predicted 14·9% incident SIVHs among indomethacin-exposed infants, compared to the actual incidence of 12·6%.
It is clear, however, that the model requires further development and validation. While the overall prediction characteristics of the model are good, there were some risk deciles where observed and expected estimates differed. One limitation of the data was that we were unable to distinguish early (≤72 hrs) vs. later onset of the SIVH. Although the majority of lesions occur early, the small proportion of late onset lesions may compromise model fit, since other clinical or patient factors may mediate their occurrence. Also, additional clinical values, such as maternal magnesium therapy, admission temperature and blood pressure, may further improve the predictive accuracy of the model. Future iterations of the model will incorporate this data.
Although we also wanted to determine the risk of developing gastrointestinal pathology secondary to indomethacin exposure, we did not have sufficient data available for calculating a hazard ratio – this analysis will be addressed in future work. However, it is reassuring to note that in the Cochrane review, there was no increase in the risk of necrotizing enterocolitis or renal complications10.
In conclusion we have developed a predictive model for calculating the risk for developing SIVH for individual VLBW infants using seven clinical variables readily available at birth, using an open access online calculator. This model may be useful for parental counseling and as an aid in clinical decision-making regarding IVH prophylaxis.
Methods
Study design and participants
This was a retrospective cohort analysis of preterm infants ≤34 weeks gestational age at birth admitted to four participating Level III Massachusetts Neonatal Intensive Care Units, belonging to the Neonatal Quality Improvement Collaborative of Massachusetts (NeoQIC). The data was abstracted from January 2000 - December 2010 for three of the participating NICUs and from 2006 to 2010 for one NICU. The study utilized de-identified patient level data as reported to the Vermont Oxford Network (VON) Database.
IRB approval was obtained by each participating NICU from their respective IRB prior to starting the study. Data sharing agreements were also approved by the participating institutions. Parental consent was not deemed necessary since the analysis used a retrospective de-identified cohort.
Study Population
All infants born at ≤34 weeks gestational age admitted to the four NICUs with available data during the study period were eligible for the study. Infants had their first head ultrasound (HUS) usually within or close to 72 hr of life and then serially per each unit's protocol. Regarding early deaths, in general, infants who received an ultrasound and had the data on the clinical predictors were included in the model. Thus, any infant who died prior to receiving or otherwise did not receive an ultrasound was excluded from the study (i.e., they did not have a determination of the outcome). Infants who received an ultrasound and died at a later date were included in the model. Additionally, all infants < 23 weeks gestational age, or who had congenital or chromosomal anomalies were excluded from model creation and validation, as were patients with any postnatal indomethacin exposure. However, data from patients with indomethacin exposure were subsequently used to deduce the impact of indomethacin exposure on the observed versus the predicted SIVH incidence.
The primary goal of the study was to create and validate a predictive model to assess an individual infant's risk for IVH based on clinical variables available within the first six hours of life. The second goal was to compare the observed versus predicted risk between the any indomethacin exposed group versus the non-exposed group.
Data Collection
The four participating NICUs currently report de-identified patient level data to the VON database. Each site provided the requisite fields, as available, for the study period. The VON database clearly defines the clinical variables reported to the database13. Data collection included the following variables: completed gestational age at the time of birth as documented by early fetal US and maternal LMP, birth weight, gender, maternal antenatal steroid administration, mode of delivery, location of delivery (inborn versus outborn), Apgar score at 5 minutes, admission temperature (available for only a subset of infants), any indomethacin therapy and grades of IVH. IVH was classified as Grades I to IV as defined by the modified Papile's classification for IVH, determined by cranial ultrasound. Two out of the four centers used indomethacin for IVH prophylaxis and all centers used either indomethacin or ibuprofen for treatment of patent ductus arteriosus (PDA). The above mentioned variables were chosen as they are readily available by 6 hours of life when indomethacin is given for prophylaxis. The biggest confounder would be blood pressure data at this time but this is not available through the VON database.
Statistical analysis
Preliminary analysis
The distributions of all variables were examined. Continuous data are reported with means and standard deviations. Categorical data are reported with frequency distributions. For univariable analyses of predictor variables and severe IVH, independent sample t-tests and Pearson's chi-square were used, as appropriate.
Developing and Validating the Predictive Models: For model development and validation, the sample was restricted to those patients who did not receive indomethacin (n = 2917). Multivariable logistic models were developed using seven clinical predictors: gestational age, birth weight, gender, any maternal antenatal steroid use, mode of delivery, location of delivery (inborn vs. outborn) and Apgar score at five minutes. Since admission temperature was available for only a subset of infants it was not used in the analysis. For ordinal and continuous predictors, we first examined whether departure from linearity in the logit was present. This was accomplished using statistical and graphical methods described by Hosmer and Lemeshow14 and Vittinghof15. Based on these evaluations, these predictors satisfied linearity assumptions and, as such, were analyzed in their original scales of measurement. In developing the model, we also assessed whether clinically relevant interactions were present among the seven variables. No significant interactions among predictors were found; thus, none were included in the predictive model.
Training models were developed using data from three centers, while validation was conducted on the excluded center. This procedure was run for each unique combination of sites for a total of four training/validation models. The approach not only allowed us to conduct an external validation of the predictive model, but also enabled us to examine the stability of the validation across the four models.
Model discrimination (for training, validation and the final predictive model) was determined by examining the area under the curve (AUC) of the receiver operating characteristic curve (ROC curve). Associated 95% confidence intervals were computed using standard errors as defined by DeLong, DeLong and Clarke-Pearson16,17,18,19.
For the final model, the Hosmer and Lemeshow goodness-of-fit test (H-L test) was used to assess how well the model summarized the data (i.e., model calibration). For the H-L test, the null hypothesis states that the model adequately summarizes the data. As such, a non-significant test suggests adequate model fit.
References
Schmidt, B. et al. Impact of bronchopulmonary dysplasia, brain injury and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 289, 1124–1129 (2003).
Laptook, A., O'Shea, T., Shankaran, S. & Bhaskar, B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 115, 673–680 (2005).
Vohr, B. et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 105, 1216–1226 (2000).
Vohr, B., Garcia Coll, C., Flanagan, P. & Oh, W. Effects of intraventricular hemorrhage and socioeconomic status on perceptual, cognitive and neurologic status of low birth weight infants at 5 years of age. J Pediatr. 121, 280–285 (1992).
Volpe, J. Intracranial hemorrhage: Germinal matrix- intraventricular hemorrhage of the premature infant. Neurology of the Newborn. 4th edition. Saunders; Philadelphia. 428–493 (2001).
Ment, L. et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: A multicenter randomized trial. J Pediatr. 124, 951–955 (1994).
Ment, L. et al. Neurodevelopmental outcome at 36 months' corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics. 98(4 Pt 1), 714–718 (1996).
Ment, L. et al. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 105(3 Pt 1), 485–491 (2000).
Yanowitz, T., Baker, R. & Brozanski, B. Prophylactic Indomethacin Reduces Grades III and IV Intraventricular Hemorrhages when Compared to Early Indomethacin Treatment of a Patent Ductus Arteriosus. Journal of Perinatol. 23, 317–322 (2003).
Fowlie, P. & Davis, P. Cochrane Review: Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Evid.-Based Child Health. 5, 416–471 (2010).
Vohr, B. et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 111(4 Pt 1), e340–346 (2003).
Miller, S. et al. Prolonged Indomethacin Exposure Is Associated With Decreased White Matter Injury Detected With Magnetic Resonance Imaging in Premature Newborns at 24 to 28 Weeks' Gestation at Birth. Pediatrics. 117, 1626–1631 (2006).
Vermont Oxford Network Database: Manual of Operations For Infants Born in 2010, Release 14.0.
Hosmer, D. & Lemeshow, S. Applied Logistic Regression. 2nd edition. New York: John, & Sons, eds., Inc. (2000).
Vittinghoff, E., Glidden, D., Shiboski, S. & McCulloch, C. Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated Measures Models. New York: Springer. (2005).
Altman, D. & Royston, P. What do we mean by validating a prognostic model? Stat Med. 19, 453–473 (2000).
DeLong, E., DeLong, D. & Clarke-Pearson, D. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 44, 837–845 (1988).
Harrell, F., Lee, K. & Mark, D. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy and measuring and reducing errors. Stat Med. 15, 361–387 (1996).
Cook, N. Use and Misuse of the Receiver Operating Characteristic Curve in Risk Prediction. Circulation. 115, 928–935 (2007).
Broitman, E. et al. for the National Institute of Child Health and Human Development Neonatal Research Network. Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. J Pediatr. 151, 500–505 (2007).
The International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet. 342, 193–198 (1993).
Richardson, D., Gray, J., McCormick, M., Workman, K. & Goldman, D. Score for neonatal acute physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 91, 617–623 (1993).
Richardson, D., Corcoran, J., Escobar, G. & Lee, S. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 138, 92–100 (2001).
Heuchan, A., Evans, N., Henderson Smart, D. & Simpson, J. on behalf of the Australian and New Zealand Neonatal Network. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995–97. Arch Dis Child Fetal Neonatal Ed. 86, F86–F90 (2002).
Dani, C. et al. Method of delivery and intraventricular haemorrhage in extremely preterm infant. J Matern Fetal Neonatal Med. 23, 1419–1423 (2010).
Riskin, A. et al. Delivery mode and severe intraventricular hemorrhage in single, very low birth weight, vertex infants. Obstet Gynecol. 112, 21–28 (2008).
Mohamed, M. & Aly, H. Male gender is associated with intraventricular hemorrhage. Pediatrics. 125, e333–339 (2010).
Clyman, R., Saha, S., Jobe, A. & Oh, W. Indomethacin Prophylaxis for Preterm Infants: the Impact of Two Multicentered Randomized Controlled Trials on Clinical Practice. J Pediatr. 150, 46–50 (2007).
Sinclair, J. Weighing risks and benefits in treating the individual patient. Clin Perinatol. 30(2), 251–268 (2003).
Schmidt, B. et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 344, 1966–1972 (2001).
Acknowledgements
We acknowledge the support of NeoQIC of Massachusetts for providing a forum to discuss potential collaborative projects and allowing the SIVH calculator to be hosted on the NeoQIC website for open access, (www.neoqic.org). We also acknowledge Mr. Rajeev K. Meharwal for creating the final version of the online calculator.
Author information
Authors and Affiliations
Contributions
S.G., R.S. and P.V. designed the protocol and analyzed the data. S.G., R.S., P.V., F.B., J.C. and E.M. assisted in data interpretation and manuscript preparation. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Role of funding sources: This is an unfunded study.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Singh, R., Gorstein, S., Bednarek, F. et al. A Predictive Model for SIVH risk in Preterm Infants and Targeted Indomethacin Therapy for Prevention. Sci Rep 3, 2539 (2013). https://doi.org/10.1038/srep02539
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02539
This article is cited by
-
Association between patent ductus arteriosus flow and home oxygen therapy in extremely preterm infants
Pediatric Research (2024)
-
A simple scoring system for prediction of IVH in very-low-birth-weight infants
Pediatric Research (2023)
-
Model for severe intracranial hemorrhage and role of early indomethacin in extreme preterm infants
Pediatric Research (2022)
-
A predictive clinical model for moderate to severe intraventricular hemorrhage in very low birth weight infants
Journal of Perinatology (2022)
-
Intraventricular hemorrhage and white matter injury: is persistent cerebral desaturation a missing link?
Pediatric Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.