Abstract
Atherosclerosis and height loss are each reportedly associated with cardiovascular disease. However, no studies have found an association between atherosclerosis and height loss. A retrospective study of 2435 individuals aged 60–89 years who underwent annual health check-ups was conducted. Atherosclerosis was defined as carotid intima-media thickness (CIMT) ≥ 1.1 mm. Height loss was defined as being in the highest quintile of height decrease per year, as in our previous studies. Among study participants, 555 were diagnosed as having atherosclerosis. Independent of known cardiovascular risk factors, atherosclerosis was positively associated with height loss. The adjusted odds ratio (OR) was 1.46 (95% confidence interval, 1.15, 1.83). Essentially the same associations were observed for men and women. The adjusted OR (95% CI) was 1.43 (1.01, 2.04) for men and 1.46 (1.07, 1.99) for women. Among older individuals, atherosclerosis is associated with height loss. This result can help clarify the mechanism underlying the association between height loss and cardiovascular disease.
Similar content being viewed by others
Introduction
Height loss is positively associated with death due to cardiovascular disease1. A study of older men reported that height loss is independently associated with an increased risk of all-cause mortality and coronary heart disease2. Therefore, cardiovascular risk could be associated with height loss among older individuals.
Carotid intima-media thickness (CIMT), a widely used surrogate marker of atherosclerosis3, is reported to be positively associated with cardiovascular disease4.
Previously, levels of circulating CD34-positive cells, which play an important role in vascular repair5,6,7, were revealed to be inversely associated with height loss among men aged 60–69 years8. Since circulating CD34-positive cell count is inversely associated with all-cause and cardiovascular mortality9,10, insufficient endothelial repair related to lower CD34-positive cell count might cause height loss among older individuals.
However, CD34-positive cells also play an important role in the development of atherosclerosis; they differentiate into mature cells such as foam cells and macrophages11. Since macrophages12 and foam cells13 contribute to the development of pathological atherosclerosis, CD34-positive cells are necessary for the development of atherosclerosis as evaluated with CIMT14.
Such studies raise the question of whether height loss could be associated with lower circulating CD34-positive cell count8. Although height loss is reported to be associated with cardiovascular disease1,2 and atherosclerosis as evaluated with CIMT is a known risk factor for cardiovascular disease4, circulating CD34-positive cells are a known cause of atherosclerosis. Clarifying the association between atherosclerosis as evaluated with CIMT and height loss could help clarify the mechanism of height loss among older individuals. To investigate the association between atherosclerosis and height loss among older individuals, a prospective study of older Japanese individuals aged 60–89 years who underwent annual health check-ups was conducted.
Material and methods
The methods used in the present study, including CIMT measurement, have been described elsewhere15.
Study population
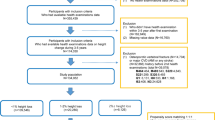
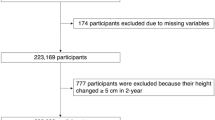
The study population comprised 2947 residents of Goto city aged 60–89 years who underwent an annual medical check-up during 2014–2016, which was considered the baseline evaluation. Goto city, which is located in western Japan, is surrounded by rural communities. Participants without data on blood pressure (n = 2) or drinking and smoking (n = 7) were excluded. We also excluded 501 participants who did not undergo an annual health check-up during the follow-up period (2015–2019). To avoid the influence of metastatic fractures, participants with an annual decrease in height > 5 cm per year (n = 2) were also excluded. The remaining 2,435 individuals, aged 72.3 years ± 7.0 years (range 60–89 years), were enrolled in the study. The mean follow-up period was 3.3 ± 1.2 years. There were no significant differences in the characteristics of individuals who were included or excluded.
To ensure that participants understood the objective of the study, written consent forms in Japanese were made available. Informed consent was obtained from all study participants. All study procedures were in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments. Ethics approval was obtained from the Ethics Committee for Human Research of Nagasaki University. The study was also approved by the Ethics Committee of the Nagasaki University Graduate School of Biomedical Sciences (project registration number: 14051404-15).
Data collection and laboratory measurements
Trained interviewers obtained information on clinical characteristics. An automatic body composition analyzer (BF-220; Tanita, Tokyo, Japan) was used to calculate body mass index (BMI, kg/m2) after measuring height and weight. Blood pressure in the right arm was measured after at least 5 min of rest in a sitting position with a blood pressure measuring device (HEM-907; Omron, Kyoto, Japan) and recorded by a trained observer.
Fasting blood samples were collected in EDTA-2K and siliconized tubes. Serum high-density lipoprotein cholesterol (HDLc), serum triglycerides (TG), hemoglobin A1c (HbA1C), and serum creatinine were measured using standard laboratory procedures at SRL, Inc. (Tokyo, Japan). The glomerular filtration rate (GFR) was estimated using an established method recently proposed by a working group of the Japanese Chronic Kidney Disease Initiative16.
CIMT was measured with ultrasonography of the left and right common carotid arteries by an experienced vascular technician using LOGIQ Book XP with a 10-MHz transducer (GE Healthcare, Milwaukee, WI, USA). The maximum CIMT values for the left and right common carotid arteries were calculated using digital edge-detection software (Intimascope; MediaCross, Tokyo, Japan) and a previously described protocol17. Intimascope is a software developed to minimize measurement errors in CIMT measurement. This software makes it possible to automatically recognize the edges of the internal and external membranes of blood vessels and automatically determine distances at a sub-pixel level (estimated to be 0.01 mm) using a polynomial measurement formula18. Baseline subclinical atherosclerosis was diagnosed as CIMT ≥ 1.1 mm, as in previous studies14,19 because < 1.1 mm was reported as normal CIMT in a previous study20.
Height loss was defined as being in the sex-specific highest quintile of height decrease per year (2.004 mm/year for men and 2.416 mm/year for women), as in our previous studies21,22.
Statistical analysis
The characteristics of the study population are presented as means ± standard deviation (SD) or n (%), except for TG. Since TG values had a skewed distribution, logarithmic transformation was performed and the median (interquartile range) was presented.
Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to determine the association between atherosclerosis and height loss. Three different models were used to calculate ORs and 95% CIs. The first model only included adjustment for age and sex (Model 1). Model 2 also included further adjustment for height at baseline. Finally, Model 3 further adjusted for other potential confounding factors: systolic blood pressure (mmHg), BMI (kg/m2), drinking status (non-drinker, often drinker, or daily drinker), smoking status (never smoker, former smoker, or current smoker), HDLc (mg/dL), TG (mg/dL), HbA1c (%), and GFR (mL/min/1.73 m2). We also performed sex-specific analyses.
All statistical analyses were performed with SAS for Windows version 9.4(SAS Inc., Cary, NC). P-values < 0.05 were regarded as statistically significant.
Results
Characteristics of the study population in relation to atherosclerosis
Among the present study population, 555 participants were diagnosed as having atherosclerosis at baseline. Table 1 shows the clinical characteristics of the study population by atherosclerosis status at baseline. Compared with participants without atherosclerosis, there was a significantly higher prevalence of men among participants with atherosclerosis. Participants with atherosclerosis were significantly older and had higher BMI, systolic blood pressure, and HbA1c and significantly lower diastolic blood pressure, HDLc, and GFR.
Association between atherosclerosis and height loss
Table 2 shows the ORs and 95% CIs for height loss by atherosclerosis status among all participants. Atherosclerosis was significantly positively associated with height loss. Even after adjustment for height at baseline (Model 2) and known cardiovascular risk factors (Model 3), this association remained significant.
Table 3 shows sex-specific associations between atherosclerosis and height loss. Independent of known confounding factors, atherosclerosis was positively associated with height loss among both men and women.
Sensitivity analysis
To assess sensitivity, we performed the main analyses with height loss defined as being in the highest quartile of height decrease per year, as in our previous study21,22. We obtained essentially the same results. The OR (95% CI) for height loss and atherosclerosis was 1.34 (1.08, 1.67) for Model 1, 1.34 (1.08, 1.67) for Model 2, and 1.31 (1.05, 1.62) for Model 3.
Discussion
The main finding of the present prospective study is that among older participants, atherosclerosis is positively associated with height loss. This association was observed among both men and women.
A previous retrospective study of Japanese individuals aged 40–74 years revealed an independent positive association between hypertension and height loss among men23. Hypertension, which is a well-known cardiovascular risk factor in Asia24, is closely associated with atherosclerosis as evaluated with CIMT25. CIMT, a widely used surrogate marker of atherosclerosis3, is positively associated with cardiovascular disease4. Therefore, higher cardiovascular risk should be associated with height loss among older individuals. In addition, atherosclerosis as evaluated with CIMT could be positively associated with height loss among older individuals.
Early endothelial dysfunction predicts progression of CIMT26. Because progression of CIMT is a result of aggressive endothelial repair27, endothelial dysfunction might be underlying the process of height loss.
Insufficient endothelial repair also causes endothelial dysfunction. Since CD34-positive cells play an important role in vascular repair5,6,7, a shortage of CD34-positive cells might lead to insufficient endothelial repair28. Circulating CD34-positive cell count, which has been reported to be inversely associated with all-cause and cardiovascular mortality9,10, has also been reported to be inversely associated with height loss8.
However, active arterial wall thickening (CIMT increase ≥ 0.01 mm/year) requires CD34-positive cells14. Thus, characteristics associated with a lower chance of CIMT progression being linked with a higher risk of height loss seems like a contradiction. This contradiction might be explained by the reduction in circulating CD34-positive cells due to consumption.
The development of atherosclerosis (CIMT ≥ 1.1 mm), which is the result of aggressive endothelial repair, is inversely associated with active arterial wall thickening14,19. Since aggressive endothelial repair induces a shortage of CD34-positive cells due to consumption, participants with established atherosclerosis (CIMT ≥ 1.1 mm) could have lower levels of circulating CD34-positive cells.
Therefore, although circulating CD34-positive cell count is inversely associated with height loss8, established atherosclerosis could be positively associated with height loss among older individuals. However, the pathological mechanism underlying the association between atherosclerosis and height loss has not yet been clarified.
Intervertebral disc degeneration and compression vertebrae fractures related to osteoporosis are known causes of height loss among older individuals. Disc degeneration is associated with atherosclerosis of the abdominal aorta29,30. Oxidative stress activates intervertebral disc degeneration31, progression of osteoporosis32, and development of atherosclerosis33. Since CIMT is associated with calcification of the abdominal aorta34, oxidative stress might be underlying the association between height loss and atherosclerosis as evaluated with CIMT.
However, in the present study, men and women had essentially the same associations between height loss and atherosclerosis. Since the prevalence of osteoporosis is much higher among women than among men35, the influence of compression vertebrae fractures related to osteoporosis on height loss might be limited.
Anemia is frequently diagnosed in older individuals and it is multifactorial36. Clonal hematopoiesis of indeterminate potential (CHIP) is an important explainable factor that induces anemia among elderly individuals. CHIP, which is common in the normal aging population37, is associated with an increased risk of anemia based on hemoglobin level38. CHIP is also associated with a pro-inflammatory state that has been linked to atherosclerosis39 and arteriosclerotic disease40. Chronic inflammation induces the progression of atherosclerosis41. Aging is a process that reduces hematopoiesis42 and increases inflammation43. In addition, there is a reduction in the number of circulating hematopoietic stem cells known as CD34-positive cells in elderly individuals as compared to younger individuals44. Since there is an inverse association between hemoglobin level and height loss21 and between circulating CD34-positive cell count and height loss8, the process of aging might have a strong influence on the association between atherosclerosis and height loss.
Furthermore, aging is known to increase oxidative stress45. Low CD34-positive cell production related to aging amplifies the association between oxidative stress and hypertension25. Hypertension is an independent risk factor for height loss23. Therefore, such associations also explain why aging should have a strong influence on the association between atherosclerosis and height loss. In fact, participants with height loss were significantly older than participants without height loss, as shown in our previous study46.
One clinical implication of the present study is that atherosclerosis as evaluated with CIMT could be an efficient tool for estimating the risk of height loss among older individuals. Since height loss among older men might increase the risk of all-cause mortality and coronary heart disease2, the present findings also help clarify the potential mechanism underlying the association between height loss and cardiovascular disease among older individuals.
The potential limitations of this study warrant consideration. Vertebral fractures associated with osteoporosis and intervertebral disc degeneration might play an important role in height loss among adults, but those data were not available to us, as in our previous studies8,21,22,23. Further investigation with data on those diseases is necessary. An efficient cutoff point to define height loss has not been established. In the present study, we defined height loss as being in the highest quintile of height decrease per year. However, our sensitivity analysis based on quartiles of height decrease per year showed essentially the same associations. Oxidative stress and hypoxia might play important roles in the present associations. However, we had no data to evaluate oxidative stress and hypoxia. Further epidemiological investigations with data on levels of hypoxia inducing factor, superoxide dismutase, and 8-hydroxydeoxyguanosine are required. Diurnal changes in height47 might influence the present results. However, CIMT does not have diurnal changes. Thus, diurnal changes in height might weaken the present results even though we found a significant positive association between atherosclerosis and height loss. Since height loss starting in middle age is an independent risk factor for cardiovascular mortality in old age1, there is a possibility that the participants included in the analysis represent a healthy survivor cohort. This type of selection bias might weaken the association between height loss and atherosclerosis because higher CIMT is an independent risk factor for cardiovascular disease4. However, significant associations between atherosclerosis and height loss were observed in the present study.
Conclusion
In conclusion, among older Japanese individuals, atherosclerosis as evaluated with CIMT was positively associated with height loss. Although further investigation is necessary, the present findings are helpful for estimating the risk of height loss and clarifying the mechanism that might be underlying the association between height loss and cardiovascular disease.
Data availability
According to ethical guidelines in Japan, we cannot provide individual data due to participant privacy considerations. In addition, the informed consent obtained does not include a provision for publicly sharing data. Qualified researchers may apply to access a minimal dataset by contacting Prof. Takahiro Maeda, Principal Investigator, Department of General Medicine, Nagasaki University, Nagasaki, Japan at tamaeda@nagasaki-u.ac.jp or the Office of Data Management at ritouken@vc.fctv-net.jp. Information about data requests is also available online at: https://www.mh.nagasaki-u.ac.jp/soshin/ (accessed on 25th August 2023) and http://www.med.nagasaki-u.ac.jp/cm/ (accessed on 28th August 2023).
References
Masunari, N. et al. Height loss starting in middle age predicts increased mortality in the elderly. J. Bone Miner. Res. 27(1), 138–145 (2012).
Wannamethee, S. G., Shaper, A. G., Lennon, L., & Whincup, P. H. Height loss in older men: Associations with total mortality and incidence of cardiovascular disease. Arch. Intern. Med. 166(22), 2546–2552 (2006).
Nezu, T., Hosomi, N., Aoki, S. & Matsumoto, M. Carotid intima-media thickness for atherosclerosis. J. Atheroscler. Thromb. 23(1), 18–31 (2016).
Naqvi, T. Z. & Lee, M. S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc. Imaging 7(10), 1025–1038 (2014).
Shi, Q. et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 92(2), 362–367 (1998).
Daub, K. et al. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 20(14), 2559–2561 (2006).
Stellos, K. et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 117(2), 206–215 (2008).
Shimizu, Y. et al. Association between circulating CD34-positive cell count and height loss among older men. Sci. Rep. 12(1), 7175 (2022).
Muggeridge, D., Dodd, J. & Ross, M. D. CD34(+) progenitors are predictive of mortality and are associated with physical activity in cardiovascular disease patients. Atherosclerosis 333, 108–115 (2021).
Mandraffino, G. et al. Circulating progenitor cells and the elderly: A seven-year observational study. Exp. Gerontol. 47(5), 394–400 (2012).
Stellos, K. et al. Platelet aggregates-induced human CD34+ progenitor cell proliferation and differentiation to macrophages and foam cells is mediated by stromal cell derived factor 1 in vitro. Semin. Thromb. Hemost. 36(2), 139–145 (2010).
Tabas, I. & Bornfeldt, K. E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 118(4), 653–667 (2016).
Maguire, E. M., Pearce, S. W. A. & Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vascul. Pharmacol. 112, 54–71 (2019).
Shimizu, Y. et al. Circulating CD34+ cells and active arterial wall thickening among elderly men: A prospective study. Sci. Rep. 10(1), 4656 (2020).
Shimizu, Y. & Maeda, T. Influence of height on endothelial maintenance activity: A narrative review. Environ. Health Prev. Med. 26(1), 19 (2021).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53(6), 982–992 (2009).
Hara, T. et al. Evaluation of clinical markers of atherosclerosis in young and elderly Japanese adults. Clin. Chem. Lab. Med. 44(7), 824–829 (2006).
Yanase, T. et al. Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software, intimascope. Am. J. Hypertens. 19(12), 1206–1212 (2006).
Shimizu, Y. et al. Normal anti-thyroid peroxidase antibody (TPO-Ab) titers and active arterial wall thickening among euthyroid individuals: A prospective study. J. Clin. Med. 11(3), 521 (2022).
Kawamori, R. et al. Prevalence of carotid atherosclerosis in diabetic patients. Ultrasound high-resolution B-mode imaging on carotid arteries. Diabetes Care 15(10), 1290–1294 (1992).
Shimizu, Y., Hayakawa, H., Takada, M., Okada, T. & Kiyama, M. Hemoglobin and adult height loss among Japanese workers: A retrospective study. PLoS ONE 16(8), e0256281 (2021).
Shimizu, Y. et al. HbA1c and height loss among Japanese workers: A retrospective study. PLoS ONE 18(10), e0291465 (2023).
Shimizu, Y. et al. Association between height and hypertension: A retrospective study. BioMed 2(3), 303–309 (2022).
Zhang, Y. et al. Cardiovascular risk assessment tools in Asia. J. Clin. Hypertens. (Greenwich) 24(4), 369–377 (2022).
Shimizu, Y. et al. Gamma-glutamyl transpeptidase (γ-GTP) has an ambivalent association with hypertension and atherosclerosis among elderly Japanese men: A cross-sectional study. Environ. Health Prev. Med. 24(1), 69 (2019).
Halcox, J. P. et al. Endothelial function predicts progression of carotid intima-media thickness. Circulation 119(7), 1005–1012 (2009).
Shimizu, Y. et al. Cardio-ankle vascular index and circulating CD34-positive cell levels as indicators of endothelial repair activity in older Japanese men. Geriatr. Gerontol. Int. 19(6), 557–562 (2019).
Shimizu, Y. Mechanism underlying vascular remodeling in relation to circulating CD34-positive cells among older Japanese men. Sci. Rep. 12(1), 21823 (2022).
Kauppila, L. I., Penttilä, A., Karhunen, P. J., Lalu, K. & Hannikainen, P. Lumber disc degeneration and atherosclerosis of the abdominal aorta. Spine (Phila Pa 1976) 19(8), 923–929 (1994).
Kauppila, L. I. Atherosclerosis and disc degeneration/low-back pain—A systematic review. Eur. J. Vasc. Endovasc. Surg. 37(6), 661–670 (2009).
Cao, G. et al. The role of oxidative stress in intervertebral disk degeneration. Oxid. Med. Cell Longev. 2022, 2166817 (2022).
Kimball, J. S., Johnson, J. P. & Carlson, D. A. Oxidative stress and osteoporosis. J. Bone Joint Surg. Am. 103(15), 1451–1461 (2021).
Förstermann, U., Xia, N. & Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120(4), 713–735 (2017).
Wong, N. D. et al. Abdominal aortic calcium and multi-site atherosclerosis: The multiethnic study of atherosclerosis. Atherosclerosis 214(2), 436–441 (2011).
Yoshimura, N. et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J. Bone Miner. Metab. 27(5), 620–628 (2009).
Stauder, R., Valent, P. & Theurl, I. Anemia at older age: etiologies, clinical implications, and management. Blood 131(5), 505–514 (2018).
Groarke, E. M. & Young, N. S. Aging and hematopoiesis. Clin. Geriatr. Med. 35(3), 285–293 (2019).
van Zeventer, I. A. et al. Mutational spectrum and dynamics of clonal hematopoiesis in anemia of older individuals. Blood 135(14), 1161–1170 (2020).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377(2), 111–121 (2017).
Marnell, C. S., Bick, A. & Natarajan, P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J. Mol. Cell Cardiol. 161, 98–105 (2021).
Engström, G., Melander, O. & Hedblad, B. Carotid intima-media thickness, systemic inflammation, and incidence of heart failure hospitalizations. Arterioscler. Thromb. Vasc. Biol. 29(10), 1691–1695 (2009).
Zhao, J., Ghimire, A. & Liesveld, J. Marrow failure and aging: The role of “Inflammaging”. Best Pract. Res. Clin. Haematol. 34(2), 101283 (2021).
Jenny, N. S. Inflammation in aging: Cause, effect, or both?. Discov. Med. 13(73), 451–460 (2012).
Moazzami, K. et al. The association between baseline circulating progenitor cells and vascular function: The role of aging and risk factors. Vascular Medicine. 27(6), 532–541 (2022).
Hajam, Y. A. et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 11(3), 552 (2022).
Shimizu, Y. et al. Association between smoking and height loss in Japanese workers: A retrospective study. PLoS ONE 19(2), e0298121 (2024).
Healey, E. L., Burden, A. M., McEwan, I. M. & Fowler, N. E. Diurnal variation in stature: Do those with chronic low-back pain differ from asymptomatic controls?. Clin. Biomech. (Bristol, Avon) 26(4), 331–336 (2011).
Acknowledgements
We are grateful to staff from Goto City Hall for their outstanding support.
Funding
This study was supported by Grants-in-Aids for Scientific Research from Japan Society for the Promotion of Science (No.21H02575, No.22K06421). This study was also supported by The Japan Agency for Medical Research and Development [22rea522104h0001] (https://www.amed.go.jp/en/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.S. designed the study and performed the statistical analyses, interpreted the data, and drafted the manuscript or revised it. K.A., H.Y., S.Y.K. (Shin-Ya Kawashiri), Y.N. (Yuko Noguchi) and Y.N. (Yasuhiro Nagata) assisted with the design of the study, were involved in data collection, and checked the manuscript. Y.H., and S.N. checked the manuscript. T.M. participated in the study concept and checked the manuscript. T.M. was the general coordinator and also designed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimizu, Y., Arima, K., Yamanashi, H. et al. Association between atherosclerosis and height loss among older individuals. Sci Rep 14, 7776 (2024). https://doi.org/10.1038/s41598-024-57620-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57620-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.