Abstract
Several preclinical models have been recently developed for metabolic associated fatty liver disease (MAFLD) and associated hepatocellular carcinoma (HCC) but comprehensive analysis of the regulatory and transcriptional landscapes underlying disease in these models are still missing. We investigated the regulatory and transcriptional landscape in fatty livers and liver tumours from DIAMOND mice that faithfully mimic human HCC development in the context of MAFLD. RNA-sequencing and ChIP-sequencing revealed rewiring of the Wnt/β-catenin regulatory network in DIAMOND tumours, as manifested by chromatin remodelling and associated switching in the expression of the canonical TCF/LEF downstream effectors. We identified splicing as a major mechanism leading to constitutive oncogenic activation of β-catenin in a large subset of DIAMOND tumours, a mechanism that is independent on somatic mutations in the locus and that has not been previously shown. Similar splicing events were found in a fraction of human HCC and hepatoblastoma samples.
Similar content being viewed by others
Introduction
Liver cancer is among the leading causes for cancer-related death worldwide and the projection is that almost 1.5 million people will be affected by 20401. Importantly, many of the newly diagnosed cases in developed countries are associated with obesity, metabolic dysfunction and associated complications, including Metabolic Associated Fatty Liver Disease (MAFLD). Obesity is predicted to become the leading cause for liver cancer in western countries, and fatty-liver-associated cancer will thus contribute increasingly to liver-cancer mortality worldwide2,3.
Molecular classification of HCC broadly defines two subclasses: proliferative and non-proliferative HCC. The proliferative class is characterized by gene signatures of poor prognosis, often carries TP53 mutations and has a worse clinical outcome, while the non-proliferative class frequently carries β-catenin mutations and is associated with a better clinical outcome4. TP53 and CTNNB1 are also some of the most frequently mutated genes in HCC (each in ~ 30% of cases), only mutations in the TERT promoter are more frequent (~ 60%)4. Importantly, CTNNB1 mutations are most often found in the absence of Hepatitis B virus infection and have been associated with liver cancer in the context of metabolic syndrome5. These coding mutations disrupt the β-catenin degradation domain encoded in the exon 3 of CTNNB1, either via point mutations or larger in-frame deletions that remove key residues that upon phosphorylation target the protein for degradation5. This leads to stabilization of β-catenin in the cytosol and subsequent translocation into the nucleus6. In the nucleus, β-catenin interacts with LEF/TCF transcription factors to regulate gene expression. Recent reports have demonstrated that similar activation of β-catenin, either via engineered deletions or CRISPR targeting of Ctnnb1 exon 3 in the liver, is sufficient to generate tumours in mice7,8.
Recent evidence highlights how mutations in non-coding regulatory elements or disruptions of 3D chromatin architecture can lead to aberrant gene expression in cancer9,10,11,12,13. It is also well known that dysfunction of other regulatory mechanisms such as alternative splicing contribute importantly to cancer aetiology14 including HCC15,16,17. Still, a thorough understanding on the role of the regulatory genome or altered splicing patterns in the aetiology of MAFLD-associated HCC is lacking.
To better characterize the molecular underpinnings of liver cancer aetiology in the context of metabolic syndrome and fatty liver disease, various mouse models have been developed in recent years18,19,20,21,22. Among them, the DIAMOND mouse model mimics several aspects of MAFLD-driven liver cancer. This mouse model is generated upon feeding western diet (WD) to an isogenic strain isolated from an interbreeding between C57Bl/6J and Sv129/vImJ, and that in response develop MAFLD and liver tumours with an incidence of ~ 90%19.
Herein, we present an in-depth analysis of the transcriptional and regulatory landscape of DIAMOND liver tumours and fatty liver tissue, in comparison to healthy litter mate controls fed regular diet. The study describes extensive rewiring of the Wnt gene regulatory network in DIAMOND mice and demonstrates that alternative splicing is a key feature of MAFLD and HCC in this model. More specifically, we identified diet-induced aberrant splicing, independent on locus-specific somatic mutations, as one molecular driver underlying oncogenic β-catenin-signalling in a large subset of these mouse liver tumours. Similar aberrant splicing of β-catenin in human liver cancer samples was also observed, highlighting the potential importance also in the development of human liver cancer.
Results
DIAMOND mice develop HCC in the context of obesity and insulin resistance
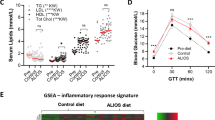
To investigate in depth the genome-wide transcriptional and epigenetic changes that occur during development of MAFLD and obesity-related hepatocellular carcinoma (HCC), we performed RNA-sequencing (RNA-seq) and Chromatin ImmunoPrecipitation-sequencing (ChIP-seq) of liver tumours (HCC) and liver control tissue (FL) from DIAMOND mice, as well as liver tissue from healthy control mice fed a regular diet (RD) (Fig. 1a). The feeding regimens were introduced at 8 weeks of age and mice were sacrificed at approximately one year after the start of the diet. In accordance with previous findings19, DIAMOND mice developed obesity, insulin resistance and dyslipidemia (Figs. S1 and S2). There were no significant changes in fasting blood glucose or plasma insulin levels at the start of the diet, nor after 42 weeks of diet (Fig. S1c). However, Insulin Tolerance Test (ITT) at T = 16 w as well as Glucose Tolerance (GTT) and Glucose Stimulated Insulin Secretion (GSIS) tests at T = 19 w demonstrated significant insulin resistance in the DIAMOND mice compared to the controls (Fig. S1d–f).
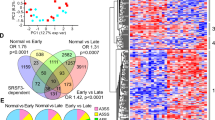
Gene expression changes associated with HCC development in DIAMOND mice. (a) Schematic workflow. (b) Photomicrograph of DIAMOND liver with tumour. Scale bar corresponds to 5 mm. (c) Venn diagram of differentially expressed genes (DEGs) in pairwise comparisons between RD, FL and HCC. (d) Upset plot of differentially expressed genes categorised as early, gradual, tumor-specific, fatty liver specific and switching. (e) Heatmap of gene expression levels for early, gradual, tumor-specific and fatty liver-specific genes. Enrichment of KEGG (f) and GO (g) terms for each of the defined categories.
Using the Vetscan 2 “mammalian liver profile” system to assess liver function at T = 38 w, we observed increased blood levels of various factors in DIAMOND mice compared to healthy controls, including alkaline phosphatase, aspartate transaminase, bile acids, albumin, urea nitrogen and cholesterol (Fig. S2a). Also, at the end-point liver triglyceride and cholesterol levels were significantly increased in the DIAMOND mice (Fig. S2b). These data indicated both significant liver damage and dyslipidemia in DIAMOND mice as compared to littermate controls. Only 25% of the control livers (2 out of 8) carried tumours, while ~ 89% (16 out of 18) of the DIAMOND livers had one or several tumours at the endpoint of the experiment (Fig. 1b). Taken together, these data corroborate previously published data19 on glucose and lipid homeostasis as well as tumour incidence in these mice, and demonstrate the robustness of the phenotypes reported for this model, also in our hands.
Transition to malignancy is associated with increased activation of Wnt-signalling
RNA-seq analysis demonstrated that 2727 genes were differentially expressed in HCC tumours compared to FL samples, and 3559 genes as compared to RD samples. Out of these, 1248 genes were differentially expressed in both comparisons (Fig. 1c). To better understand these transcriptional changes that take place in the development of MAFLD and associated HCC in these mice, and to identify gene expression changes that are likely associated with the malignant phenotype, we grouped the genes into 5 distinct categories based on their expression profiles (Fig. 1d,e): early (892), gradual (1396), tumour-specific (1248), fatty liver-specific (1911), and switching (23) genes. We defined early genes as genes where expression changes are evident already in FL tissue and maintained also in HCC. Gradual refers to gradual changes from RD to FL to HCC. Tumour-specific and fatty liver-specific were only differentially expressed in HCC or FL tissue, respectively. Switching genes were defined as dysregulated in opposite directions in FL and HCC compared to RD samples (i.e. up in FL, down in HCC, or down in FL, up in HCC). Next, we performed enrichment analysis of gene ontology terms (GO) and KEGG pathways for these 5 gene categories. The most enriched KEGG and GO terms for early genes were related to coagulation cascades and wound healing (Fig. 1f,g, Table S1). Although this was also enriched for gradual genes, the most highly enriched terms were related to metabolism, metabolic function, and cell cycle (Fig. 1f,g, Table S1). This was further reflected by enrichment of terms related to mitochondria, cellular respiration, and cell cycle in the GO cellular compartment (Table S1). Of note, although not among the top 5 most highly enriched KEGG terms, NAFLD (Non-Alcoholic Fatty Liver Disease, previous nomenclature for MAFLD) was also enriched (Table S1). Analysis of the tumour-specific genes revealed hepatocellular carcinoma, hippo- and Wnt-signalling pathways among the top three enriched KEGG terms, but also identified GO terms related to morphogenesis and developmental processes, including Wnt-signalling (Fig. 1f,g, Tables S1 and S2). This confirms DIAMOND mice as a relevant model for human HCC, especially in the context of aberrant Wnt-activation. Although there is a large group of genes that we defined as gradual, most dysregulated genes (1911 out of 2137) in non-tumorous fatty liver tissue compared to RD are fatty liver-specific. These FL-specific genes appear to be largely associated with fibrotic processes as they were enriched for terms related to extracellular matrix organization, collagen, and protein processing (Fig. 1f,g, Table S1). This is in concordance with the different morphology of the tumours and fatty liver tissue, where fibrosis was only present in the latter (Fig. S3). Lastly, the very few members among the switching genes were enriched for a diverse set of terms including tryptophan, xenobiotic and retinol metabolism, as well as steroid hormone biosynthesis (Table S1). Taken together, these analyses demonstrate extensive differences in the transcriptional landscape of RD livers compared to FL and HCC, but also between FL and HCC and highlight extensive transcriptional changes in the Wnt-signalling pathway as one main driver of tumourigenesis in this model.
Gradual isation of the chromatin landscape in DIAMOND liver tumours
Spatial and temporal specificity of gene expression rely, among others, on non-coding regulatory sequences in the genome such as enhancers. These can be located far (up to megabases) from their target genes in the linear genome and their regulatory activity is intimately linked to their epigenetic status23,24. To chart the gene regulatory changes that take place during HCC development, we performed ChIP-seq for the active enhancer (H3K27ac) and repressive (H3K27me3) chromatin marks in tumour (HCC, n = 3) and non-tumour (FL, n = 2) liver tissue from DIAMOND mice, as well as in healthy control livers (RD, n = 3). Genomic annotations of H3K27ac regions were similar in RD, FL and HCC; however, H3K27me3 peaks in RD livers were more frequently located close to transcriptional start sites (< 5 kb from TSS) as compared to FL and HCC peaks (Fig. 2a). Importantly, differential binding analysis revealed significant redistribution of active and repressive chromatin marks in the three conditions (Fig. 2b–d).
Tumour-associated active regulatory regions integrate transduction of Wnt-signalling. (a) Genomic distribution of H3K27ac and H3K27me3 chromatin marks in RD, FL and HCC tissue. (b) Upset plot of differentially binding regions for H3K27ac and H3K27me3 in pairwise comparisons between RD, FL and HCC, and categorised as early, gradual, tumor-specific, fatty liver specific and switching regions. (c) Heatmap and density plots depicting read distribution ± 10 kb around differential binding regions of H3k27ac and H3K27me3 for the defined categories. (d) Genomic distribution of H3K27ac (left) and H3K27me3 (right) for early, gradual, tumour-specific and fatty-liver specific regions. (e) Enrichment of GO terms for gradual and tumour-specific H3K27ac regions, and for gradual H3K27me3 regions. (f) Tcf/Lef motifs present in tumour-specific (marked by light blue box) and gradual regions (light green box) enriched for H3K27ac.
To better understand these regulatory changes that take place, and similar to the RNA-seq analysis, we defined differential regions as early, gradual, tumour-specific, fatty liver-specific, and switching (Fig. 2b,c, Tables S3 and S4). This revealed that most of the redistribution of H3K27ac occurred gradually. In fact, these changes made up ~ 48% of the total number of differential regions, while tumour-specific and fatty liver-specific regions constituted ~ 28% and 22% of the differential regions, respectively. Less than 2% of the peaks were associated with early or switching regions. In gradual and tumour-specific regions loss or gain of H3K27ac occurred with similar frequency (54/46% depletion/enrichment, and 51/49% depletion/enrichment, respectively) while fatty-liver specific regions were strongly biased towards enrichment (~ 85%). The by far most frequent change in repressive H3K27me3 was the gain of gradual regions (~ 93% of all differential binding events).
Among the differential regions, H3K27ac peaks were mostly located in introns and intergenic with a small proportion (< 13%) located to promoter regions (< 1 kb from TSS) (Fig. 2d). In contrast, differential H3K27me3-regions were more frequently located within 5 kb from the nearest gene (~ 50%, Fig. 2d).
Thus, our study shows a gradual remodelling of the gene regulatory landscape in obesity-related HCC in this model. This data suggests that the chromatin environment formed in the liver under conditions of overnutrition is an important factor contributing to a pro-tumourigenic niche that can trigger HCC.
Tumour-associated active regulatory regions integrate transduction of Wnt-signalling
To further investigate how redistribution of chromatin marks related to the molecular underpinnings of tumourigenesis, we performed GO analysis for the genes closest to the differential regions for both chromatin marks (Tables S3 and S4). The most highly enriched GO terms for genes in close proximity to tumour-specific H3K27ac regions were associated to Wnt-signalling (Fig. 2e). Genes near gradual regions were also associated to Wnt-signalling, albeit to a lesser extent (Tables S3 and S5). The gradual regions were most significantly associated with genes related to development and developmental processes (Fig. 2e). For H3K27me3, a large majority of the differential regions were gradual regions, and similarly to the gradual H3K27ac regions, these were associated to genes involved in developmental processes, and in particular axonogenesis and synapse organisation (Fig. 2e, Table S4). This is interesting in view of recent reports on nerve degeneration in MAFLD25 and the emerging data on the role of the neuron-tumour crosstalk in malignancies in general26. Importantly, also for these gradual regions, Wnt-related GO terms were enriched (Tables S4 and S6).
Next, we investigated the putative transcription factor binding motifs in differentially binding regions, assessing enriched and depleted regions separately. Importantly, the most significantly enriched transcription factor motif in tumour-specific H3K27ac regions was LEF1, while TCF7 and TCF7L1 motifs were also highly enriched (Fig. 2f). These together with TCF7L2 are the classical effectors of the canonical Wnt-pathway. Motifs for all four of these were also present in gradually enriched regions (Fig. 2f). In contrast, TCF/LEF motifs were not enriched in H3K27ac-depleted regions in tumours nor in gradually depleted H3K27ac regions. Also, of the differential H3K27me3 peaks, only the fatty liver-specifically enriched regions were associated with the canonical Wnt-signalling via enrichment of TCF7L2 motifs. The gradually repressed regions, which constituted the lion part of differential H3K27me3 binding, were most highly enriched for IRF8 binding motifs. These motifs were also present in the gradually depleted and tumour-specific depleted H3K27ac. This is interesting, as IRF8 has been proposed to suppress HCC progression27 and in the context of leukemia, depletion of IRF8 in combination with activated β-catenin enhances malignant gene expression profile28. Taken together, these data indicate that canonical Wnt-signalling is the major direct activator of aberrant gene expression program in DIAMOND tumours.
Rewiring of the Wnt/β-catenin gene regulatory network accompanies DIAMOND liver tumourigenesis
Given the enrichment of Wnt/β-catenin GO terms and TCF/LEF transcription factor binding motifs in tumour-associated DEGs and active chromatin, respectively, we explored in detail the RNA-seq gene expression data for individual core components of the canonical Wnt/β-catenin-pathway. A tumour-specific upregulation of Lef1 and Tcf7 genes was observed, as well as downregulation of Tcf7l1, while Tcf7l2 expression was unaltered (Fig. 3a). Upregulation of Lef1 expression was accompanied by redistribution of chromatin marks, including gain of active marks in regions both proximal and distal to the gene promoter (Fig. 3b). Furthermore, Ctnnb1 expression was increased in tumours compared to both FL and RD livers and among the components of the β-catenin destruction complex, Axin1 and the known β-catenin target Axin2 was significantly upregulated, while Apc, Gsk3a and Gsk3b were not differentially expressed. Increased Axin2 expression was also associated with tumour-specific H3K27ac enrichment in several regions surrounding the gene. In line with increased activation of Wnt-signalling, the downstream targets Lgr5 and Myc were also significantly upregulated. Thus, DIAMOND tumourigenesis is not only accompanied by upregulation of Wnt-signalling and associated chromatin remodelling, but also by a rewiring in the downstream effectors, which is likely to also affect the set of downstream target genes and contribute to a malignant gene regulatory program.
Rewiring of the Wnt-Ctnnb1 gene regulatory network accompanies DIAMOND liver tumourigenesis. (a) Normalized read counts for core components of the canonical Wnt-Ctnnb1-pathway in RD, FL and HCC tissue. A clear switch in the expression of TF effectors can be seen in tumour tissue, where Lef1 and Tcf7 are upregulated while Tcf7l1 levels are downregulated. Individual data points, mean ± SD are indicated. *p.adj. < 0.05, **p.adj. < 0.01, ***p.adj. < 0.001, ****p.adj. < 0.0001, ns = not significant. (b) Redistribution of H3K27ac in the Lef1 and Axin2 loci. Grey bars indicate H3K27ac regions that are gained specifically in tumours.
Western diet induces distinct alternative splicing patterns in DIAMOND livers and links aberrant splicing in tumours to Wnt-signalling
Apart from the genome-wide regulatory and gene expression changes that occur in liver tumours in DIAMOND mice, we also investigated the general splicing patterns in the HCC tumours, FL and RD control liver tissue. Although it is known that cancer cells often display aberrant splicing, only few studies29,30,31,32 have addressed the role of splicing in the context of MAFLD. Interestingly, our analysis revealed a significant global increase in the number of alternative splice events (ASE), not only in HCC, but also in FL tissue (Fig. 4a). This quantitative difference reflects an increased number of ASEs per spliced gene as well as a higher number of genes that are subjected to alternative splicing (Fig. 4b,c), meaning that it is not merely a consequence of differences in transcript abundance. Of note, 60 genes involved in mRNA-splicing (out of 296, GO: 000398, “mRNA splicing, via spliceosome”) are also differentially expressed in our RNA-seq data. Unsupervised clustering based on these genes accurately group the samples into RD, FL, and HCC, where the FL cluster is more similar to RD than to tumours (Fig. 4d). Interestingly, analysis of published RNA-seq data from human patients also demonstrates that quantitative differences in ASEs are associated with human MAFLD (Fig. 4e).
Changes in alternative splicing and altered expression of splice factors accompanies FL and HCC in DIAMOND mice. (a) Total number of ASE (Alternative Splice Events) per sample for RD livers, FL and HCC tissue in DIAMOND mice. (b) Frequency of ASE per gene and sample, and (c) total number of genes with ASE per sample, for same mice as in (a). Individual data points, mean ± SD are indicated. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t-test) (d) Unsupervised clustering of RD, FL and HCC tissue based on 60 differentially expressed splice factors. Scale from red to blue indicates fold change over average read count for each row. (e) Number of alternative splice events in human MAFLD livers compared to controls. Early and moderate indicate severity of MAFLD. Individual data points, mean ± SD are indicated. ****p < 0.0001, ns = not significant (Student’s t-test) (f) Average number of differential ASE in pairwise comparisons between RD, FL and HCC. Legend indicates ASEs leading to changes in ncRNA or UTRs, ORF disruptions due to exon exclusion (EXC), ORF disruptions due to intron inclusion (INC) or alternative protein isoforms.
To further dissect the observed changes in splicing patterns for our samples, a differential ASEs analysis was performed. Although the total number of ASEs were similar in FL and HCC tissues, the number of differential ASEs compared to RD was greater in HCC than in FL, indicating qualitative differences in the actual splicing events. Still, the proportion of different types of ASEs were similar for the differential ASEs in all comparisons (Fig. 4f). In both FL and HCC differential ASEs were enriched for genes related to splicing and mRNA processing as compared to RD. Strikingly, also genes associated with Wnt-signalling and the β-catenin-TCF-complex were enriched (Tables S7 and S8).
Our data shows that western diet in this model induces extensive gene regulatory changes at the level of splicing that may be an important part of the general disease aetiology. It also suggests that rewiring of the Wnt gene regulatory network in this context involves both transcriptional and co-transcriptional mechanisms, specifically connecting aberrant splicing to Wnt-signalling.
Constitutive activation of β-catenin by alternative splicing contributes to Wnt/β-catenin -driven transcriptional activation in DIAMOND tumours
The enrichment of Wnt-signalling among the biological processes identified in our differential ASE analysis prompted us to further investigate specific genes in this pathway in more detail. The Ctnnb1 gene was of particular interest due to its high frequency of mutations in liver cancer and its crucial role as the key effector in canonical Wnt-signalling. Strikingly, we identified a Ctnnb1 transcript isoform excluding exon 3 in 60% (3 out of 5) of the sequenced DIAMOND tumours, where 25% of the Ctnnb1 reads mapped to this region displayed exon 3 skipping (Fig. 5a). Further RT-PCR analysis followed by Sanger sequencing revealed that in total 8 out of 18 (~ 44%) tumours presented Ctnnb1 transcripts with exon 3 exclusion (Fig. S4a,b). Exclusion of exon 3 was not detected in RD or FL tissue. No somatic mutations were detected in the Ctnnb1 exon 3 region in 6 out of these 8 tumours displaying exon 3 exclusion (Fig. S4c). This is relevant since it suggests that aberrant splicing underlies Ctnnb1 exon 3 exclusion in this model, and in contrast to previous reports, it is not dependent on genomic mutations (e.g. alterations of regulatory splice sequences) in the locus, but it is rather induced by other mechanisms such as alterations in the splicing machinery.
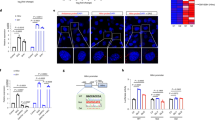
WD induces exclusion of Ctnnb1 in DIAMOND tumours. (a) Exon-inclusion ratio (percent spliced-in, PSI) for Ctnnb1 exon 3 in RD, FL and HCC RNA-seq samples. Individual data points and vast-tools estimated PSI are indicated. **p < 0.01, ns = not significant. (b) Schematic of exon 3 inclusion and exclusion transcripts and site of BaseScope probe annealing. (c) Photomicrograph of representative sections from RD (upper left), FL (upper right), and HCC Ctnnb1 exon 3 inclusion (lower left) and exclusion (lower right) tumours. Blue dots show normal exon 3 inclusion transcripts, red dots show exon 3 exclusion. Scalebar equals to 50 µm. (d) Ctnnb1 exon 3 exclusion calculated as % of red dots (Ex2-4probe) over total number of dots (Ex2-4 probe + Ex 2–3 probe). INC denotes exon 3 inclusion tumours and EXC denotes exon 3 exclusion tumours. Individual data points, mean ± SD are indicated. *p < 0.05, **p < 0.01 (Student’s t-test) (e) Frequency of aberrant splicing in CTNNB1 exon 3 in human cell lines and primary tumours for HCC and hepatoblastoma. Legend indicates exclusion of exon 3 , deletion of exon 3-exon 4, coding mutations in exon 3, and Ctnnb1 wildtype transcripts.
To corroborate this finding and characterize the spatial and quantitative expression of this Ctnnb1 splice variant at the single cell level, we performed BaseScope assays on paraffin sections from HCC as well as FL and RD control livers (Fig. 5b–d). To this end, one probe spanning the junction of exon 2-exon 3 and one probe for the aberrant exon 2-exon 4 junction was used (Fig. 5b). As expected, the exon 2-3 probe was detected in all tissues while no signal from the exon 2-4 probe was detected in RD control tissues, FL or tumours previously identified by RNA-seq as not having the exon 3 exclusion transcript. In contrast, in tumours characterized by sequencing as exon 3 exclusion tumours, an average of 12% of the total number of Ctnnb1 transcripts detected by BaseScope did not include exon 3, corroborating on tissue sections the results from the splicing analysis (Fig. 5d). These transcripts were detected in most of the Ctnnb1-expressing cells throughout the tissue and co-expressed with the normal exon 3 inclusion transcripts.
In addition, analysis of published RNA-seq datasets for human primary liver tumours and cell lines showed that > 1% (6 out of 471 primary tumours, 1 out of 57 HCC cell lines) of HCC patients carried Ctnnb1 transcripts excluding exon 3 (Fig. 5e). Importantly, to reduce the contribution of somatic mutations to aberrant exon 3 exclusion, transcripts were only considered in this analysis if the entire exon was excluded and the whole exons 2 and 4 were present, which still does not exclude potential somatic mutations deleting only the whole of exon 3, and that has been reported in hepatoblastoma and HCC. We detected exon 3 coding mutations in ~ 17% of HCC samples and genomic deletion of a large part of exon 3 together with exon 4 in ~ 9% of samples (Fig. 5e). In none of these cases did we detect a combination of exon 3 exclusion and other types of rearrangements in this exon. This was also evident in hepatoblastoma, where exclusion of exon 3 occurred in > 22% of the cases (10 out of 44 tumours, 2 out of 8 cell lines), mutations in exon 3 in > 31% (14 out of 44 tumours, 4 out of 8 cell lines), and exon 3-exon 4 deletion only in one cell line (HepG2 cells). Importantly, combined analysis of RNA-seq and whole genome sequencing data demonstrated that in MHCC97 cells, the exclusion of exon 3 is not accompanied by genomic mutations in the locus. This data suggests that similar to what we observed in DIAMOND mice, splicing of the Ctnnb1 exon 3 can be disrupted in humans even in the absence of any changes in the DNA sequence. It is important to note that the frequency of such events is yet to be determined as this requires the combined analysis of DNA and RNA sequencing data from multiple samples. Still, our data highlights a previously unappreciated direct role of aberrant splicing as a potential molecular driver underlying oncogenic β-catenin signalling in specific cellular contexts, such as in the case of specific diets or overnutrition.
Discussion
In this study, we analysed the transcriptional and regulatory landscape in DIAMOND mice and found increased activation together with rewiring of the Wnt gene regulatory network (Fig. 6a). This rewiring includes a switch in the expression of TCF/LEF effectors, where tumours show upregulation of Lef1 and Tcf7, and downregulation of Tcf7l1. This switch may be triggered by the increased β-catenin levels/activity, as similar observations were made when modulating active levels of β-catenin in mammalian nephron progenitor cells33. The change in Wnt effectors is likely an important driver of tumour-specific gene expression, as TCF/LEF binding motifs are enriched in tumour-specific active chromatin, and given that Lef1/Tcf7 are mostly transcriptional activators while Tcf7l1 is known to have mainly repressive functions.
Rewiring of the Wnt regulatory network and genome-wide alteration of alternative splicing in DIAMOND HCC tumours. (a) Scheme illustrating the regulatory remodelling and transcriptional changes in RD, FL and HCC. These changes include gradual redistribution of chromatin marks around TCF/Lef binding motifs, changes in expression of Wnt-related genes including TCF/Lef expression levels, as well as increased alternative splicing of Wnt-related genes. (b) Schematic representation of the genome wide alternative splicing events in DIAMOND livers. Alternative splicing events are more abundant in both FL and HCC compared to RD, but a significant amount of the splicing events are specific to each of these groups, resulting in differential alternative transcripts.
Numerous studies have highlighted the importance of Wnt/β-catenin-signalling in HCC tumourigenesis and mutations in components of this pathway have been described as key events for liver tumourigenesis in specific contexts34. It is well known that Ctnnb1 exon 3 is a hotspot for oncogenic coding mutations5,6,35 that lead to its constitutive activation due to disabling of phosphorylation sites required for cytoplasmic degradation of the protein. Recent studies have also exploited this feature and showed that in vivo CRISPR-targeting of exon 3 in livers is sufficient to induce Wnt-dependent HCC7,8. However, to the best of our knowledge, it has not been previously reported that alternative splicing, independent of somatic mutations in the locus, can cause exclusion of Ctnnb1 exon 3 and consequent constitutive activation of the protein. In this study, we demonstrate that western diet in DIAMOND mice induces such exclusion of Ctnnb1 exon 3 in a large subset of the tumours, and that this is not accompanied by mutations in the corresponding genomic region. Whether a similar connection between metabolic input and exon 3 splicing exists in human patients remains to be investigated. Still, combining publicly available whole-genome and transcriptome sequencing data, we demonstrated that MHCC97 cells present Ctnnb1 exon 3 exclusion independently of somatic mutations in the locus, thus providing a proof-of concept for the presence of this phenomenon also in human HCC.
Notably, all sequenced DIAMOND tumours show a consistent upregulation of Ctnnb1 and Lef1, while Tcf7l2 is not differentially regulated. This expression pattern of these genes is similar to human HCC subtype G636,37 and contrasts somewhat with tumours generated via targeted genomic deletions of Ctnnb1 exon 37 that give rise to two distinct tumour types with either all three genes upregulated or all three genes unchanged. This indicates that metabolic input may be an important modulator of the transcriptional response to aberrant Wnt-activation that should be considered in the clinical setting.
Importantly, changes in splicing patterns are also induced in FL tissue in DIAMOND mice (Fig. 6b), in line with recent reports on altered splicing in MAFLD30,31,32,38. In combination with the recent data showing that induction of β-catenin activity is sufficient to trigger liver tumourigenesis in mice7, this suggests that altered splicing is a key event that promotes formation of a cellular environment that facilitates malignant transformation in this model.
Changes in the expression of genes encoding specific splice factors have been reported to drive tumourigenesis or predispose to malignancy in HCC15, 16, 39. Since the Wnt-signalling pathway is overrepresented among the differential ASE in both fatty liver tissue and tumour tissue in DIAMOND mice, it raises the question of whether this is related to metabolic dysregulation of specific splice factors important for this pathway or whether it reflects a more stochastic process where enrichment of Wnt-signalling is a consequence of selection at the cellular level.
Taken together, we show that western diet induces genome-wide chromatin remodelling and gene expression changes, as well as altered splicing patterns in DIAMOND livers (Fig. 6). These genome-wide changes contribute to the rewiring of the Wnt/β-catenin gene regulatory network that plays a pivotal role in HCC development. In particular, western diet in this model induces aberrant splicing that leads to the exclusion of Ctnnb1 exon 3 in a subset of DIAMOND tumours. This demonstrates a new splice-dependent mechanism underlying oncogenic activation of β-catenin that is independent on somatic mutations in the locus.
Given the connection between diet and alternative splicing, new questions arise on how splicing patterns and expression of specific splice factors relate to not only caloric intake but also macronutrient composition, and how different types of diet may induce or alleviate such aberrations in different genetic backgrounds.
Methods
Animals and diets
All experiments were performed in compliance with national and institutional laws and are reported in accordance with the ARRIVE guidelines. The study was approved by the Regional Ethics Committee at the Court of Appeal of Northern Norrland (Ethical approval ID A39-2018). Male B6/129 mice were purchased from Sanyal Biotechnology and housed at 12:12 h light/dark cycle in a temperature/humidity controlled (22 °C and 50% humidity) room and ad libitum feeding. These experiments are only done in males since females are not commercially available i.e. the company controls the breeding scheme to assure the maintenance of the DIAMOND genetic background. Diet intervention was performed as previously described19. At 8 weeks of age, DIAMOND mice (n = 20) were fed western diet (high-fat, high carbohydrate, Harlan TD.88137) supplemented with fructose/glucose water (23.1 g/L d-fructose + 18.9 g/L d-glucose) while littermate controls (n = 10) were fed a standard chow diet (Harlan TD.7012) with normal water. After ~ 52 weeks mice were sacrificed. Food intake and body weight were measured weekly. The researchers were aware of which experimental groups the animals belonged to.
Metabolic measurements, body composition and histology
For Insulin Tolerance Test, non-starved mice were injected intraperitoneally with insulin (Actrapid Penfill) (1 U/kg body weight). Intraperitoneal Glucose Tolerance Test (GTT) combined with Glucose Stimulated Insulin Secretion (GSIS) were performed on 6 h fasted and sedated mice (Hypnorm (Veta Pharma)/Midazolam (Hamlenmice)) following i.p. administration of glucose (SIGMA #G7021) (1 g/kg body weight). Blood glucose and plasma insulin levels were analysed from blood samples taken at indicated time points for both procedures using a Glucometer (Ultra 2, One Touch) and the ultra-sensitive mouse insulin ELISA kit (Chrystal Chem Inc. #90080), respectively.
Blood profiling of liver function was performed using the Mammalian Liver Profile rotor on a VetScan VS2 (Abaxis, Inc.). Liver TG and cholesterol were measured using Serum Triglyceride Determination Kit (Sigma-Aldrich #TR0100) and Cholesterol / Cholesteryl Ester Quantitation Kit (MBL #JM-K603-100) according to manufacturer’s recommendations. Body composition of live mice was measured using EchoMRI (EchoMRI LLC; EchoMRI 3-in-1). Liver and tumour histology was assessed using haematoxylin and eosin (H&E) and picrosirius red in paraffin-embedded sections using standard procedures.
RNA-seq
Library preparation
Approximately 30 mg of frozen liver and tumour tissues were used for total RNA extraction using the RNeasy Plus Universal minikit (Qiagen, #73404). Integrity and quality of RNA were determined on the Bioanalyzer (Agilent, #5067-1511). 1 ug of total RNA was used for library preparation, firstly the RNA was enriched for mRNA using the NEBNext Poly(A) mRNA Magnetic Isolation Module (NEB, E7490) followed by the NEBNext Ultra II RNA Library Prep Kit for Illumina (NEB, E7770). The quality and concentration of libraries were assessed with Bioanalyzer (Agilent, #5067-4626) and Qubit (Thermofisher, #Q33230).
150 bp paired-end sequences were obtained using the NovaSeq 6000 Illumina Sequencer, with a coverage of ~ 37 million reads per library. Quality control of fast files was done with FastQC.
Gene expression analysis
Raw reads were aligned to the mouse genome (mm10, UCSC) using STAR (options: –outSAMtype BAM SortedByCoordinate –seedSearchStartLmax 12 –outFilterScoreMinOverLread 0.3 –alignSJoverhangMin 15 –outFilterMismatchNmax 33 –outFilterMatchNminOverLread 0 –outFilterType BySJout –outSAMattributes NH HI AS NM MD –outSAMstrandField intronMotif –quantMode GeneCounts)40.
Genes with a minimum of 10 reads along all the samples were kept for further analysis. Normalisation and differential expression analysis were performed using DESeq2. Genes were considered differentially expressed with p-value < 0.01 and FDR < 0.01 (False Discovery Rate). The differentially expressed genes were grouped into early, gradual, tumour-specific, fatty liver-specific, and switching genes. Early genes were defined as genes differentially expressed in both HCC and FL compared to RD. Gradual genes included differentially expressed genes in FL vs RD but that are also increasingly dysregulated in HCC vs FL, as well as genes differentially expressed in HCC vs RD but not compared to FL. Tumour-specific genes were differentially expressed in tumour samples compared to both RD and FL while fatty liver-specific genes were differentially expressed in FL samples compared to RD, HCC or both. Lastly, a small group of switching-genes were identified that were differentially up or downregulated in FL samples compared to RD, and the opposite in HCC samples. Gene Ontology (GO) and KEGG enrichment analysis for each cluster was performed using clusterProfiler (p-value < 0.01 and FDR < 0.01)41. For pairwise comparisons of gene expression of selected genes in the Wnt-signalling pathway, normalized counts and the adjusted p-value obtained with DESeq2 was used.
Alternative splicing analysis
PSI values were calculated using vast-tools with default parameters42. An event was considered an ASE in a sample if its PSI value is between 10 and 90% and the read coverage was sufficient (minimum of “VLOW” coverage score or higher in vast-tools). A t-test was performed to compare the total number of events, total number of genes with ASE or the ratio of ASE/gene per group. RNA-seq datasets of human MAFLD samples (control: n = 10 and MAFLD: n = 206) were obtained from GSE13525143. Statistical significance on comparisons of Ctnnb1 exon 3 exclusion between the groups was calculated with vast-tool default parameters.
An event was considered differentially spliced if the absolute estimated dPSI was higher than 10% and higher than the minimum value of dPSI at a 99% confidence based on vast-tools diff software42.
ChIP-seq
Library preparation
For ChIP-seq experiments approximately 200 mg of fresh liver and tumour tissue was cut into smaller pieces and fixed for 15 min in 1 ml fixing solution (1% formaldehyde, 50 mM HEPES–KOH, 100 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM EGTA) at RT with gentle mixing. Cross-linking was stopped by adding 1/20 volume of 2.5 M glycine for 5 min at RT. Samples were then washed 2 × 5 min at 4 °C with cold 1 × PBS containing protease inhibitor cocktail (Roche, #4693132001), snap frozen and stored at − 80 °C until awaiting further processing. Liver and tumour tissues were pulverised before lysis on cell lysis buffer at 4 °C for 5–10 min. Nuclei were pelleted and resuspended in 1 × PBS and the volume corresponding to 50–70 mg of tissue was used for chromatin extraction by adding nuclei lysis buffer at 4 °C for 5 min. Chromatin was sonicated on a Covaris E220 device (PIP: 105, Duty factor: 2, Cycles/burst: 200, Duration: 5 min). ChIP was performed with anti-H3K27ac (Abcam, ab4729) and anti-H3K27me3 (Abcam, ab192985) antibodies. ChIP-seq libraries were prepared with the NEBNext Ultra II DNA Library Prep Kit for Illumina (NEB, E7645) and quality and concentration were assessed with the Bioanalyzer (Agilent, #5067-4626) and Qubit (Thermofisher, #Q33230).
150 bp paired-end sequences were obtained using the NovaSeq 6000 Illumina Sequencer, with coverage of ~ 52 million reads per library. Quality control of fast files was done with FastQC.
Data analysis
Raw reads were mapped to the mouse genome (mm10, UCSC) using bowtie2 (options: –threads 4—very-sensitive)44. Samtools was used to convert SAM files into BAM files. Enriched peaks were called using MACS2 (options: callpeak –gsize 1.87e9 –broad –broad-cutoff 0.05 –f BAMPE)45. DiffBind package was used for differential binding analysis46,47. Peaks were annotated using ChIPseeker48 and Gene Ontology (GO) and KEGG enrichment analysis for each cluster was performed using clusterProfiler (p-value < 0.01 and FDR < 0.01)41. Motif enrichment analysis for each subset of regions was done using HOMER with default settings49.
Ctnnb1 exon 3 exclusion in datasets from human subjects
Previously published human HCC and hepatoblastoma datasets were obtained from NCBI GEO or BioProject database with accession numbers: GSE9709850, GSE19356751, GSE18473352, GSE6548553, PRJNA86701154, PRJNA870935 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA870935), PRJEB2189955, GSE18340656, GSE8351857, and GSE13303958. Whole-genome sequencing and RNA-seq data for the MHCC97 HCC line was obtained from PRJNA588754 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA588754).
Raw reads were aligned to the human genome (hg38, UCSC) using STAR (options: –outSAMtype BAM SortedByCoordinate –seedSearchStartLmax 12 –outFilterScoreMinOverLread 0.3 –alignSJoverhangMin 15 –outFilterMismatchNmax 33 –outFilterMatchNminOverLread 0 –outFilterType BySJout –outSAMattributes NH HI AS NM MD –outSAMstrandField intronMotif –quantMode GeneCounts)40. Bam files were visualised in IGV to find samples with Ctnnb1 exon skipping or mutations.
RT-PCR and genomic PCR
Ctnnb1 exon 3 skipping was confirmed in several tumour samples by extracting total RNA from 5 to 20 mg of tissue using the RNeasy Plus Universal minikit (Qiagen, #73404). cDNA was synthesized using the RevertAid protocol (ThermoScientific, EP0451).
Genomic DNA was extracted by incubating 5 mg of tissue in 50 mM NaOH for 25 min at 95 °C and then 1/10th of 1 M Tris–HCl pH 8. Primers flanking the exon 3 were used for PCR amplification on both the cDNA and genomic DNA to amplify the region (Ctnnb1 exon 2 Fw: GGTACCTGAAGCTCAGCGCA and Ctnnb1 exon 4 Rv: CTGGTCCTCATCGTTTAGCA).
Gel bands were purified using the NucleoSpin Gel and PCR Clean-up kit (Macherey–Nagel, N. 740609.50) and sequenced by Sanger sequencing.
BaseScope RNA in situ hybridisation (ISH)
We employed the BaseScope RNA ISH assay (ACD, cat. no 323871) to detect the Ctnnb1 exon 3 skipped isoform. In brief, we utilised custom-designed probes from Advanced Cell Diagnostic (ACD) (Newark, CA, USA): (1) a mouse-specific probe (1ZZ) (BA-Mm-Ctnnb1-1zz-st-C1) that targets the exon 2-exon 3 junction to detect the normal Ctnnb1 and (2) a mouse-specific probe (1zz) (BA-Mm-Ctnnb1-E2E4-C2) that targets the exon 2-exon 4 junction to detect the novel Ctnnb1 exon 3 skipped isoform. Each batch run of the assay included an RD, an FL, an HCC exon 3 included and an HCC exon 3 excluded sample (as determined by RT-PCR).
Slides were scanned on a Zeiss Axioscan and quantification was done manually assisted by the Qupath software. The number of dots per field was determined in a total of 10 fields per sample. Data was represented as % of exon 3 exclusion which was calculated as percentual ratio of dots from exon 2-4 probe vs total (exon 2-4/(exon 2-4 + exon 2-3 dots). Statistical significance was assessed using a two-tailed Student’s t-test.
Statistical analysis
Prism 9 (GraphPad Software, LLC) was used for statistical analysis of body composition and metabolic parameters. Statistical methods, sample size and number of replicates for all analysis are indicated in figure legends and in the methods section for each experiment. Normality and log-normality tests were performed to select the appropriate statistical methods for analysis. RNA-seq and ChIP-seq data were analysed using specific R-packages (see separate section).
Data availability
All generated datasets for RNA-seq and ChIP-seq are available through the Gene Expression Omnibus repository under the accession number GSE250577. Additional data that support the finding of this study are available on reasonable request from the corresponding author.
References
International Agency for Research on Cancer. GLOBOCAN 2018. IARC. https://gco.iarc.fr/tomorrow/en
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018).
Younossi, Z. et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 17, 748-755.e3 (2019).
Villanueva, A. Hepatocellular carcinoma. New Engl. J. Med. 380, 1450–1462 (2019).
Rebouissou, S. et al. Genotype-phenotype correlation of CTNNB1 mutations reveals different β-catenin activity associated with liver tumor progression. Hepatology 64, 2047–2061 (2016).
Coste, A. D. L. et al. Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc. Natl. Acad. Sci. 95, 8847–8851 (1998).
Loesch, R. et al. Deleting the β-catenin degradation domain in mouse hepatocytes drives hepatocellular carcinoma or hepatoblastoma-like tumor growth. J. Hepatol. 77, 424–435 (2022).
Mou, H. et al. CRISPR-induced exon skipping of β-catenin reveals tumorigenic mutants driving distinct subtypes of liver cancer. J. Pathol. 259, 415–427 (2023).
Hnisz, D. et al. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science 351, 1454–1458 (2016).
Mansour, M. R. et al. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science 346, 1373–1377 (2014).
Bahr, C. et al. A Myc enhancer cluster regulates normal and leukaemic haematopoietic stem cell hierarchies. Nature 553, 515–520 (2018).
Weischenfeldt, J. et al. Pan-cancer analysis of somatic copy-number alterations implicates IRS4 and IGF2 in enhancer hijacking. Nat. Genet. 49, 65–74 (2017).
Xu, J. et al. Subtype-specific 3D genome alteration in acute myeloid leukaemia. Nature 611, 387–398 (2022).
Bradley, R. K. & Anczuków, O. RNA splicing dysregulation and the hallmarks of cancer. Nat. Rev. Cancer 23, 135–155. https://doi.org/10.1038/s41568-022-00541-7 (2023).
Yu, L. et al. MTR4 drives liver tumorigenesis by promoting cancer metabolic switch through alternative splicing. Nat. Commun. 11, 708 (2020).
Chang, C. et al. The aberrant upregulation of exon 10-inclusive SREK1 through SRSF10 acts as an oncogenic driver in human hepatocellular carcinoma. Nat. Commun. 13, 1363 (2022).
Lee, S. E., Alcedo, K. P., Kim, H. J. & Snider, N. T. Alternative splicing in hepatocellular carcinoma. Cell. Mol. Gastroenterol. Hepatol. 10, 699–712 (2020).
Nakagawa, H. et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343 (2014).
Asgharpour, A. et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 65, 579–588 (2016).
Wolf, M. J. et al. Metabolic activation of lntrahepatic CD8(+) T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564 (2014).
Møllerhøj, M. B. et al. Hepatoprotective effects of semaglutide, lanifibranor and dietary intervention in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH. Clin. Transl. Sci. 15, 1167–1186 (2022).
Green, C. D. et al. A new preclinical model of western diet-induced progression of non-alcoholic steatohepatitis to hepatocellular carcinoma. Faseb J. 36, e22372 (2022).
Remeseiro, S., Hörnblad, A. & Spitz, F. Gene regulation during development in the light of topologically associating domains. Wiley Interdiscip. Rev. Dev. Biol. 5, 169–185 (2016).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Adori, C. et al. Disorganization and degeneration of liver sympathetic innervations in nonalcoholic fatty liver disease revealed by 3D imaging. Sci. Adv. 7, eabg5733 (2021).
Zahalka, A. H. & Frenette, P. S. Nerves in cancer. Nat. Rev. Cancer 20, 143–157 (2020).
Wu, H. et al. Hepatic interferon regulatory factor 8 expression suppresses hepatocellular carcinoma progression and enhances the response to anti–programmed cell death protein-1 therapy. Hepatology https://doi.org/10.1002/hep.32316 (2022).
Scheller, M. et al. Cross talk between Wnt/β-catenin and Irf8 in leukemia progression and drug resistance. J. Exp. Med. 210, 2239–2256 (2013).
Wu, P., Zhang, M. & Webster, N. J. G. Alternative RNA splicing in fatty liver disease. Front. Endocrinol. 12, 613213 (2021).
Jobbins, A. M. et al. Dysregulated RNA polyadenylation contributes to metabolic impairment in non-alcoholic fatty liver disease. Nucleic Acids Res. 50, 3379–3393 (2022).
Li, Y. et al. DRAK2 aggravates nonalcoholic fatty liver disease progression through SRSF6-associated RNA alternative splicing. Cell Metab. 33, 2004-2020.e9 (2021).
Paterson, H. A. B. et al. Liver RBFOX2 regulates cholesterol homeostasis via Scarb1 alternative splicing in mice. Nat. Metab. 4, 1812–1829 (2022).
Guo, Q. et al. A β-catenin-driven switch in TCF/LEF transcription factor binding to DNA target sites promotes commitment of mammalian nephron progenitor cells. Elife 10, e64444 (2021).
Xu, C. et al. β-Catenin signaling in hepatocellular carcinoma. J. Clin. Invest. 132, e154515 (2022).
Monga, S. P. β-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 148, 1294–1310 (2015).
Boyault, S. et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 45, 42–52 (2007).
Calderaro, J. et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 67, 727–738 (2017).
Kumar, D. et al. Degradation of splicing factor SRSF3 contributes to progressive liver disease. J. Clin. Invest. 129, 4477–4491 (2019).
Sen, S., Langiewicz, M., Jumaa, H. & Webster, N. J. G. Deletion of serine/arginine-rich splicing factor 3 in hepatocytes predisposes to hepatocellular carcinoma in mice. Hepatology 61, 171–183 (2015).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 16, 284–287 (2012).
Tapial, J. et al. An atlas of alternative splicing profiles and functional associations reveals new regulatory programs and genes that simultaneously express multiple major isoforms. Genome Res. 27, 1759–1768 (2017).
Govaere, O. et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med 12, eaba4448 (2020).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Stark, R. & Brown, G. DiffBind: differential binding analysis of ChIP-Seq peak data. Bioconductor. (2011).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Yu, G., Wang, L.-G. & He, Q.-Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Qiu, Z. et al. A pharmacogenomic landscape in human liver cancers. Cancer Cell 36, 179-193.e11 (2019).
Repáraz, D. et al. Neoantigens as potential vaccines in hepatocellular carcinoma. J Immunother Cancer 10, e003978 (2022).
Liu, Y. et al. RNA sequencing analysis of hepatocellular carcinoma identified oxidative phosphorylation as a major pathologic feature. Hepatol. Commun. 6, 2170–2181 (2022).
Dong, H. et al. Identification of HBV-MLL4 integration and its molecular basis in Chinese hepatocellular carcinoma. PLOS ONE 10, e0123175 (2015).
Chang, Y.-S. et al. Integrated genomic analyses of hepatocellular carcinoma. Hepatol. Int. 1–15 (2022). https://doi.org/10.1007/s12072-022-10455-z
Losic, B. et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 11, 291 (2020).
Kurebayashi, Y. et al. Immunovascular classification of HCC reflects reciprocal interaction between immune and angiogenic tumor microenvironments. Hepatology 75, 1139–1153 (2022).
Crippa, S. et al. Mutant CTNNB1 and histological heterogeneity define metabolic subtypes of hepatoblastoma. EMBO Mol. Med. 9, 1589–1604 (2017).
Carrillo-Reixach, J. et al. Epigenetic footprint enables molecular risk stratification of hepatoblastoma with clinical implications. J. Hepatol. 73, 328–341 (2020).
Acknowledgements
We thank Ingela Lundberg for animal care and members of the Edlund laboratory for technical assistance on metabolic experiments. We also acknowledge the facilities and technical assistance of Umeå Center for Comparative Biology (UCCB). The computations were enabled by resources in Project SNIC 2021/23-439, 2321/22-541, 2022/23-376 and 2022/22-671 provided by the Swedish National Infrastructure for Computing (SNIC) at UPPMAX, partially funded by the Swedish Research Council through Grant Agreement No. 2018-05973. Finally, we acknowledge past and present members of the Hörnblad and Remeseiro laboratories for helpful discussions.
Funding
Open access funding provided by Umea University. Funding was provided by Kempestiftelserna (Grant Nos. SMK-1863 and JCK-2149), The Cancer Research Foundation Norrland (Grant Nos. AMP 18-940 and AMP 21-1043 ), and Lion's Cancer Research Foundation in Northern Sweden (LP 20-2232 and LP 22-2313).
Author information
Authors and Affiliations
Contributions
A.L.-P. designed, performed, and analysed all the NGS experiments and contributed to metabolic experiments as well as to the writing and editing of the manuscript. S.R. co-supervised some bioinformatics analysis and contributed to interpretation of data and editing of the manuscript. A.H. designed and directed the research, performed some of the experiments, secured funding, analysed and interpreted the data, and wrote the manuscript with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Pérez, A., Remeseiro, S. & Hörnblad, A. Diet-induced rewiring of the Wnt gene regulatory network connects aberrant splicing to fatty liver and liver cancer in DIAMOND mice. Sci Rep 13, 18666 (2023). https://doi.org/10.1038/s41598-023-45614-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45614-1
This article is cited by
-
Omics-based construction of regulatory variants can be applied to help decipher pig liver-related traits
Communications Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.