Abstract
Bacterial and viral infections are serious public health issue. Therefore, this study aimed to evaluate the antibacterial, antibiofilm and antiviral potential of the Brazilian Red Propolis (BRP) crude hydroalcoholic extract, fractions, and isolated compounds, as well as their in vivo toxicity. The antibacterial activity was evaluated by determining the Minimum Inhibitory Concentration and the antibiofilm activity by determining the Minimum Inhibitory Concentration of Biofilm (MICB50). The viable bacteria count (Log10 UFC/mL) was also obtained. The antiviral assays were performed by infecting BHK-21 cells with Chikungunya (CHIKV) nanoluc. The toxicity of the BRP was evaluated in the Caenorhabditis elegans animal model. The MIC values for the crude hydroalcoholic extract sample ranged from 3.12 to 100 μg/mL, while fractions and isolated compounds the MIC values ranged from 1.56 to 400 μg/mL.The BRP crude hydroalcoholic extract, oblongifolin B, and gutiferone E presented MICB50 values ranging from 1.56 to 100 μg/mL against monospecies and multispecies biofilms. Neovestitol and vestitol inhibited CHIKV infection by 93.5 and 96.7%, respectively. The tests to evaluate toxicity in C. elegans demonstrated that the BRP was not toxic below the concentrations 750 μg/mL. The results constitute an alternative approach for treating various infectious diseases.
Similar content being viewed by others
Introduction
Despite important advances in the health area, infectious diseases have constituted a serious public health issue over time1. One example is periodontitis, an inflammatory disease that affects tooth‐supporting apparatus and which is caused by microorganisms present in dysbiosis plaque biofilms2. According to Mehrotra and Singh3, about 2.6% of African Americans, 5% of Africans, 0.2% of Asians, 1% of North Americans, and 0.3% of South Americans have been diagnosed with periodontitis in its most severe form. Periodontal treatment is essential not only for dental parameters, but also to avoid other pathological conditions such as adverse reactions in pregnancy, cardiovascular and respiratory diseases, cancer, lupus, rheumatoid arthritis, diabetes mellitus, and chronic kidney disease4. Even if the illness can be treated with antibiotics, the infection can be aggravated in patients lacking treatment or in the presence of resistant periodontopathogenic bacteria5.
Viral diseases also burden the global health system due to lack of vaccines and approved antivirals to combat important human viruses, including the Chikungunya Fever, caused by the Chikungunya virus (CHIKV)6. CHIKV was identified in 2014 and has become hyperendemic in Brazil7, This virus causes dengue-like symptoms such as fever, fatigue, arthralgia, and polyarthralgia8. By April 2022, 28,291 suspected cases of Chikungunya Fever had been registered and five deaths had been confirmed in Brazil; another eight deaths are under investigation9.
According to the World Health Organization, a considerable part of the worldwide population still depends on traditional medicine and employs natural products to treat several diseases10. Developing countries mainly use such products. In this scenario, Brazil is a valuable source of natural products given that it possesses diverse fauna and flora11. Brazilian Red Propolis (BRP), a resinous material produced by Apis mellifera bees through the collection of the exudates of two plant species: Dalbergia ecastaphyllum12,13 and Symphonia globulifera14 has excellent potential for developing new medicines. BRP is currently one of the most produced and commercialized types of Brazilian propolis. It is mainly found in the Brazilian mangroves of the Northeast, especially in Alagoas and Bahia states15.
BRP is composed of 50% resin, 30% wax, 10% essential oils, 5% pollen, and 5% other compounds, including secondary metabolites like flavonoids, isoflavonoids, cinnamic acid derivatives, esters, polyprenylated benzophenones, and some terpenes, which are considered the main biologically active constituents of this type of propolis16. The molecules isolated from BRP do not occur in any other type of propolis, which makes them rare and unique natural products17. Variations of this composition have been observed between locations. Some studies revealed that compounds such as formononetin and isoliquiritigenin are the most abundant in samples of Alagoas18. Instead, in “Canavieiras” sample, vestitol, neovestitol, medicarpin, and polyprenylated benzophenones have been identified as the main compounds and17. In this sense, BRP has been reported to possess antibacterial15,18,19,20 antiparasitic21,22,23,24,25,26,27, and antiviral activities28.
Considering the lack of treatment options for periodontitis and CHIKV infection, we have hypothesized that BRP and its isolated compounds are a promising candidate for treating these diseases. To the best of our knowledge, there are no data on the BRP antiviral action against CHIKV, and few studies have reported on its antibacterial action against periodontopathogenic bacteria13,18,28,29,30. The use of BRP as a therapeutic option could reduce the use of antibiotics in periodontitis cases and become a novel antiviral strategy against CHIKV28.
This study aimed to evaluate the in vitro antibacterial, antibiofilm, and antiviral potential of the BRP crude hydroalcoholic extract, fractions, and isolated compounds, as well as their toxicity in an in vivo model.
Results
BRP crude extract characterization
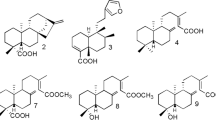
The chromatographic analysis revealed the presence of isoflavanes (vestitol, neovestitol, 7-O-methylvestitol), pterocaparns (medicarpin), and polyprenylated acylphloroglucinols (a mixture of guttiferone E/xanthochymol, and oblongifolin B) (Fig. 1), as main compounds of the BRP. The chromatographic profile of the fractions revealed the prominent presence of polyprenylated acylphloroglucinols on the hexane fraction, whereas the dichloromethane, ethyl acetate, and n-butanol fractions were composed mainly of isoflavanes (see Supplementary Figure S1).
Minimum inhibitory concentration of the BRP crude hydroalcoholic extract, fractions, and isolated compounds
Tables 1 and 2 show the MIC results for the crude hydroalcoholic extract, fractions and isolated compounds against periodontal bacteria included in the study. The MIC values for the crude hydroalcoholic extract sample ranged from 3.12 to 100 μg/mL, for the dichloromethane fraction from 1.56 to 200 μg/mL, ethyl acetate from 12.5 to 400 μg/mL, hexane from 3.12 to 400 μg/mL, and n-Butanol from 100 to 400 μg/mL (Table 1).
For the methylvestitol, the MIC values ranged from 25 to 400 μg/mL, medicarpin from 50 to 400 μg/mL, vestitol from 12.5 to 200 μg/mL, neovestitol from 12.5 to 100 μg/mL, oblongifolin B from 3.12 to 50 μg/mL, and guttiferone E from 1.56 to 200 μg/mL (Table 2).
Antibiofilm activity of the BRP crude hydroalcoholic extract and isolated compounds
The BRP crude hydroalcoholic extract reduced the monospecies biofilm formation of the standard strains (ATCC) and their clinical isolates (Fig. 2). Additionally, the number of viable cells in the monospecies biofilm expressed as Log10 CFU/mL decreased (Fig. 2). The lowest MICB50 value obtained for the BRP crude hydroalcoholic extract against the monospecies biofilms was 3.12 μg/mL against A. naeslundii (ATCC 19039) and F. nucleatum (clinical isolate) (Fig. 2e and f). Against the other evaluated monospecies biofilms, the BRP crude hydroalcoholic extract presented MICB50 of 6.25 μg/mL, except for P. intermedia (clinical isolate), against which MICB50 was 12.5 μg/mL. However, even at concentrations above MICB50, we detected viable biofilm cells (Fig. 2a–f).
Antibiofilm activity of Brazilian Red Propolis crude hydroalcoholic extract samples and number of viable cells in monospecies biofilms formed by ATCC strains and clinical isolates included in the study. (a) P. gingivalis (ATCC 49417). (b) P. gingivalis (clinical isolate). (c) P. intermedia (ATCC 15033). (d) P. intermedia (clinical isolate). (e) A. naeslundii (ATCC 19039). (f) F. nucleatum (clinical isolate).
As for the tested isolated compounds, they also reduced monospecies biofilm formation. In the presence of oblongifolin B (Fig. 3), the lowest MICB50 was 0.78 μg/mL against A. naeslundii (ATCC 19039) (Fig. 3c). Against the other evaluated monospecies biofilms, the MICB50 values ranged from 1.56 to 6.25 μg/mL. Oblongifolin B at 6.25 μg/mL eliminated P. gingivalis (clinical isolate) viable cells and, at 12.5 μg/mL, it eliminated P. intermedia (ATCC 15033) and F. nucleatum (clinical isolate) viable cells (Fig. 3a,b and d).
Guttiferone E presented low MICB50 (0.78 μg/mL) against A. naeslundii (ATCC 19,039) (Fig. 4d). Against the other evaluated monospecies biofilms, MICB50 ranged from 1.56 to 25 μg/mL (Fig. 4a,b,c and e). Guttiferone E eliminated all the biofilm cells from a concentration of 3.12 μg/mL against P. gingivalis (clinical isolate), 6.25 μg/mL against P. intermedia (ATCC 15033), 25 μg/mL against F. nucleatum (clinical isolate), and 1.56 μg/mL against A. naeslundii (ATCC 19039). As for P. gingivalis (ATCC 49417), we verified the presence of viable biofilm cells even at concentrations above MICB50 (Fig. 4a).
Antibiofilm activity of guttiferone E and number of viable cells in monospecies biofilms formed by ATCC strains and clinical isolates included in the study. (a) P. gingivalis (ATCC 49417). (b) P. gingivalis (clinical isolate). (c) P. intermedia (ATCC 15033). (d) A. naeslundii (ATCC 19039). (e) F. nucleatum (clinical isolate).
We also assessed the activity of the BRP crude hydroalcoholic extract and isolated compounds against multispecies biofilm formed by standard strains (group 1) and clinical isolates (group 2) (Fig. 5). The BRP crude hydroalcoholic extract had MICB50 of 6.25 μg/mL against the group 1 multispecies biofilm However, even at higher concentrations, viable cells were still found in the biofilm. Similar results were found against the group 2 multispecies biofilm: MICB50 was 6.25 μg/mL, and there also were viable biofilm cells above the MICB50 concentration (Fig. 5A).
Antibiofilm activity of samples of Brazilian Red Propolis crude hydroalcoholic extract, oblongifolin B and guttiferone E and number of viable cells in multispecies biofilms formed by bacteria from groups 1 (standard strains) and 2 (clinical isolates). (A) Crude extract. (B) Oblongifolin B. (C) Guttiferone E.
Concerning oblongifolin B, it had the lowest MICB50 against the group 1 multispecies biofilm (1.56 μg/mL); however, at concentrations above MICB50, cells remained viable in the biofilm. Against the group 2 multispecies biofilm, oblongifolin B presented MICB50 of 50 μg/mL and eliminated all the biofilm cells from the biofilm at this same concentration (Fig. 5B). On the other hand, guttiferone E showed MICB50 of 3.12 μg/mL against the group 1 multispecies biofilm, and 6.25 μg/mL guttiferone E eliminated all the cells from the biofilm. Against the group 2 multispecies biofilm, guttiferone E had higher MICB50 (100 μg/mL), but 50 μg/mL guttiferone E also eliminated all the viable cells from the biofilm (Figs. 5C).
Regarding the control (metronidazole), the MICB50 of monospecies biofilms ranged from 2.95 to 5.9 μg/mL. As for the mixed biofilms, the MICB50 was 2.95 μg/mL for both the biofilm formed by group 1 and the biofilm formed by group 2 (see supplementary material Figures S2 and S3).
Effects of the BRP crude hydroalcoholic extract and isolated compounds on CHIKV replication
To further evaluate the effects of BRP extract and its isolated compounds, BHK 21 cells were treated with each extract at 50, 10 and 2 μg/mL and cell viability was measured 16 h later. The results demonstrated that cells tolerated n-Butanol at 50 μg/mL (98.4%), ethyl acetate at 10 μg/mL (95.9%), while the crude extract, dichloromethane, and hexane at 2 μg/mL (99.3, 99.8, and 100%, respectively), (Table 3). Through the employment of BHK-21 cells infected with CHIKV-nanoluc, the anti-CHIKV activities of each sample were evaluated, at the maximum non-cytotoxic concentrations selected through the viability assay. The results demonstrated that n- Butanol significantly inhibited 69% of CHIKV replication (Fig. 6). The other samples presented no effect on CHIKV infection (Fig. 6).
For the isolated substances (medicarpin, neovestitol, vestitol, oblongifolin B, methylvestitol and, guttiferone E), BHK-21 cells were treated with concentrations of each compound ranging from 32 to 0.5 μg/mL. As an outcome, the treatment with compounds in concentrations over 3 µg/mL presented cell viability rates higher than 80% (Table 4), and the highest non-cytotoxic concentration of each compound was selected for the antiviral assay. Since medicarpin, neovestitol and vestitol at 14 µg/mL presented cytotoxicity (Table 4), and at 3 µg/mL showed no antiviral activity (Supplementary Figure S4), the alternative concentration of 11 µg/mL was selected to the further assays. Therefore, the antiviral activity of medicarpin, neovestitol and vestitol was tested at 11 µg/mL, guttiferone E and oblongifilin B at 6 µg/mL, and methylvestitol at 14 µg/mL. The compounds medicarpin, neovestitol and vestitol inhibited CHIKV replication in vitro in 86%, 94%, and 97% respectively (Fig. 7).
Toxicity assessment in Caenorhabditis elegans
To assess the toxicity of the BRP crude hydroacoholic extract and isolated compounds in an in vivo system, the technique for determining the lowest concentration capable of killing 50% (LC50) of the larvae in relation to the incubation time was employed. Figure 8 shows the toxicity evaluation of the BRP crude hydroalcoholic extract, oblongifolin B, and guttiferone E as a function of time and concentration. The LC50 of the BRP crude hydroalcoholic extract and oblongifolin B was 1500 μg/mL, determined on the second day of incubation (Fig. 8A and B). On the other hand, guttiferone E had LC50 of 750 μg/mL, determined on the last day of incubation (Fig. 8C).
Discussion
For years, propolis has been used to treat infections in folk medicine, and its antimicrobial potential has been demonstrated by the scientific community15. This biological potential can be related to its differentiated chemical composition.
Sesquiterpenes, pterocarpans, and isoflavans characterize Brazilian red propolis. Red propolis chemical composition is much different from other propolis types, such as brown propolis, which is characterized by hydrocarbons, aldehydes, and monoterpenes; and green propolis, which is characterized by polycyclic aromatic hydrocarbons, sesquiterpenes, and naphthalene derivatives31.
Vestitol, neovestitol, and medicarpin have been reported as major compounds in red propolis From Canavieiras, Bahia State, Brazil. On the other hand, formononetin, calycosin, biochanin A, and isoliquiritigenin were detected at lower concentrations17. Guttiferone E and oblongifolin B were described as chemical markers of red propolis14, but they appear to be at lower concentrations in the studied sample compared to the isoflavans. The triterpenes β-amyrin and glutinol have also been described in BRP from this location14.
According to Rios and Recio32 and Gibbons33, MIC values below 100 µg/mL for crude hydroalcoholic extract or below 10 µg/mL for isolated compounds are considered promising when evaluating the antibacterial activity of plant extracts, essential oils, and compounds isolated from natural sources. On the basis of these criteria and considering the MIC values presented here for all the evaluated BRP samples, the BRP crude hydroalcoholic extract and the isolated compounds guttiferone E and oblongifolin B displayed the best inhibition activity against most of the evaluated bacteria.
The red propolis dichloromethane fraction was not tested since the selection was based on the effect of the individual constituents of each fraction. The main compounds of hexane fraction, oblongifolin B and guttiferone E, displayed good activity at the individual testing, compared with the dichloromethane fraction individual compounds.
These samples showed antibacterial activity mainly against P. gingivalis (ATCC 49417), considered the most clinically important species in the development of periodontal disease34 and F. nucleatum (clinical isolate) bacteria, also considered a relevant pathogen since it worsens gingival inflammation and tooth loss35. These results demonstrated the relevance of these natural products in periodontal disease control and treatment. In this paper, the BRP crude hydroalcoholic extract, fractions (n-hexane, dichloromethane, ethyl acetate, and n-butanol), and isolated compounds (methylvestitol, medicarpin, vestitol, neovestitol, oblongifolin B, and guttiferone E) were analyzed for their antibacterial activity against clinical isolates and the ATCC strains. The ATCC strains are more stable from a genetic viewpoint and would thus represent the bacterium species, thereby enabling comparison with other investigations. The in vitro assay furnishes a reliable indication of how the microorganism responds to the target agent, and extrapolation of the results for that species or even genus should be accepted. Clinical isolates (also known as wild strains) are bacteria that can have their metabolism altered by environmental conditions and their genetics modified by circulation in the population, which would justify the relevance of evaluating these two types of strains.
The MIC values (1.56–400 µg/mL) for the other evaluated bacteria were significantly lower as compared to literature data. Bueno-Silva et al.29 evaluated the antibacterial activity of the crude extract and isolated compounds neovestitol and vestitol obtained from BRP from the same botanical origin against A. naeslundii (ATCC 12104), and they reported MIC values of 25, 25, and 50 µg/mL, respectively. Here, neovestitol and vestitol were not promising against several of the evaluated periodontal bacteria. Another point to emphasize is that the MIC value for the crude extract reported by Bueno-Silva et al.29 against A. naeslundii (ATCC 12104) resembled the value we obtained against A. naseslundii (ATCC 19039), suggesting a species susceptibility relation for the crude extract.
Santos et al.36 evaluated the antibacterial activity of the aqueous hydroalcoholic extract and fractions (hexane, dichloromethane, and ethyl acetate) obtained from a different type of propolis, from the region of “Cachoeira da Prata”, Minas Gerais—Brazil, which is also collected from Apis mellifera bees. The tested bacteria were periodontal F. nucleatum (ATCC 10953), P. gingivalis (ATCC 33277), and P. intermedia (ATCC 25611). The extract gave MIC values of 1024, 256, and 256 µg/mL against F. nucleatum (ATCC 10953), P. gingivalis (ATCC 33277), and P. intermedia (ATCC 25611), respectively. As for the fractions, the MIC values ranged from 512 to > 1024 µg/mL. The MIC results against these bacteria were higher than those presented here for the BRP crude hydroalcoholic extract and fractions against the same bacterial species but from different strains: we found MIC values of 100, 3.12, and 6.25 µg/mL for the crude extract against F. nucleatum (ATCC 10953), P. gingivalis (ATCC 49417), and P. intermedia (ATCC 15033), respectively. As for the fractions, we found MIC values ranging from 12 to 400 µg/mL against the same bacteria. Therefore, compared to the results described by these authors, the BRP crude hydroalcoholic extract and fractions employed here were more effective against periodontal bacteria.
Additionally, these authors compared the MIC results they obtained with the crude extract and fractions by ANOVA analysis. They did not find any differences in the antibacterial activity of the fractions or extract against the evaluated bacteria. This corroborates our results: the BRP crude hydroalcoholic extract was more promising than the fractions and gave better values against all the evaluated bacteria, while the fractions presented antibacterial activity against only two bacteria (P. gingivalis ATCC 49417 and clinical isolate).
Shabbir et al.37 evaluated the activity of the crude propolis extract collected in Skardu (Pakistan) originating from Robinia pseudoacacia, Elegnus agustifolia (Russian olive), and Acacia modesta, collected from Apis mellifera bees. The natural products afforded MIC values ranging from 64 to 512 µg/mL against P. gingivalis and P. intermedia clinical isolates. Our results suggested that BRP is more effective than the propolis used by Shabbir et al.37 since the MIC values we obtained for the BRP crude hydroalcoholic extract against P. gingivalis and P. intermedia clinical isolates were lower (12.5 and 6.25 µg/mL, respectively). Therefore, BRP proved to have more promising activity than propolis from other countries given that it is composed of unique compounds that do not normally occur in other types of propolis15.
Inhibiting biofilm formation by these bacteria can contribute to reducing periodontitis. Indeed, Al-Ahmad et al.38 described that A. naeslundii and F. nucleatum play the roles of initial colonizing bacterium and late colonizer, respectively. The latter bacterium was shown to be present at rates greater than 50% after 62 h of biofilm formation, contributing to increased inflammation and tooth loss38.
Other studies have evaluated the BRP monospecies antibiofilm activity against other types of bacteria. de Souza Silva et al.39 evaluated the antibiofilm activity of the BRP crude hydroalcoholic extract (BRP collected in the same region as the BRP used in our study) coated on polymeric nanoparticles against Staphylococcus aureus (ATCC 25923), Staphylococcus aureus (ATCC 33591), Staphylococcus aureus (ATCC 43300), and Pseudomonas aeruginosa (ATCC 27853). The free BRP crude hydroalcoholic extract and the extract coated on nanoparticles inhibited the biofilm formed by the Gram-positive strains more effectively as compared to the biofilm formed by the Gram-negative strains, with biofilm inhibitory concentration values ranging from 15.6 to 125 μg/mL against the S. aureus strains and from 100 to 1560 μg/mL against the P. aeruginosa strain. These results corroborated our findings given that we verified the lowest MICB50 values against the Gram-positive bacterium A. naeslundii (ATCC 19039) 3.12 μg/mL for the crude extract and 0.78 μg/mL for the isolated compounds oblongifolin B and guttiferone E.
Miranda et al.13 evaluated the antibiofilm activity of the crude hydroalcoholic extract of BRP from the same botanical origin as the BRP used here. These authors divided the evaluated bacteria into complexes (Actinomyces, purple, yellow, green, orange, red, and others). At extract concentrations of 800 and 1,600 μg/mL, the authors showed a 40 and 45% decrease in the metabolic activity of the multispecies biofilms formed by these complexes, respectively. de Figueiredo et al.30 also evaluated the antibiofilm activity of the BRP crude extract at 1600, 800, and 400 μg/mL and obtained 56, 56, and 57% reduction in the biofilm metabolic activity, respectively. Our study did not assess eradication, but it evaluated the ability of the BRP samples to inhibit biofilm formation. According to Wei et al.40 inhibiting biofilm formation is much more important than erradicating it because biofilm formation inhibition prevents bacterial growth and, hence, bacterial maturation. The results presented here demonstrated that the BRP samples inhibited multispecies biofilm formation by periodontal bacteria by at least 50%. Oblongifolin B gave the lowest MICB50 value (1.56 μg/mL) against the multispecies biofilm formed by the ATCC strains. As for the multispecies biofilm formed by the clinical isolates, the lowest MICB50 value was 6.25 μg/mL. These results suggested that the isolated compounds oblongifolin B and guttiferone E inhibited all the viable cells of most monospecies and multispecies biofilms formed by the bacteria included in this study. This pointed out that the BRP samples can inhibit biofilm formation and reach the cells within this bacterial community, eliminating them and leaving only the glycoprotein conjugate without live cells13.
It is worth mentioning that the MICB50 values found in this paper are relatively low, especially for clinical isolates that are generally more resistant and demand higher concentrations. However, at the lowest concentration capable of inhibiting biofilm formation by at least 50%, the so-called MICB50, we demonstrated an inhibition of at least 50% of the biofilm. In other words, this did not correspond to total inhibition of the biofilm, which would probably require a higher concentration.
Propolis bioactive components such as flavonoids, esters, alcohols, essential oils, and other organic compounds have already been demonstrated to display antiviral activity against viruses such as herpes viruses (HSV-1 and HSV-2), sindbis virus, parainfluenza virus, cytomegalovirus, HIV, and Varicella zoster (HSV-1 and HSV-2), sindbis virus, parainfluenza virus, cytomegalovirus, HIV, and Varicella zoster41,42. In addition to the BRP antibacterial and antibiofilm activities demonstrated in this study, we evaluated the anti-CHIKV activity of the BRP crude hydroalcoholic extract, fractions, and pure substances. We assessed the BHK-21 cell viability in the presence of the BRP samples by the MTT assay. In drug discovery, samples are considered non-toxic when the cell viability rate is above 50%, moderately cytotoxic when the cell viability rate varies between 25 and 50%, and highly cytotoxic when the cell viability rate is less than 25%43. In this study, all samples evaluated showed cell viability equal to or higher than 80% at a concentration of 50 μg/mL for the crude extract and fractions and 3 μg/mL for isolated compounds (Tables 3 and 4). All the BRP samples evaluated in this study provided BHK-21 cell viability equal to or higher than 80% (Tables 3 and 4), which was higher than the cell viability found by Rufatto et al. 20, (between 14.5 and 46%).
Regarding the infection assays, the isolated compounds neovestitol and vestitol furnished the most promising results, with virus infection inhibition rates of 94 and 97%, respectively (Fig. 7). Even though several natural compounds with antiviral activity, including anti-CHIKV activity, have been described, neovestitol and vestitol have not had their antiviral potential screened. Our results showed higher inhibition rates than those reported in other studies with natural molecules, such as the study of Pohjala et al.44, who obtained an infection inhibition limit of at most 75% when they screened 356 compounds, being 123 of them natural compounds. To the best of our knowledge, there are no reports on the anti-CHIKV activity of BRP or isolated compounds. This shows the importance of capitalizing the BRP potential as candidate for antiviral treatment. Our study has pioneered evaluation of the BRP anti-CHIKV activity and has achieved expressive inhibition rates, paving the way for the development of antivirals against CHIKV as well as other viruses.
For BRP to be safely applicable, its toxicity must be evaluated in different experimental models. The murine model is the most used in vivo model to assess the toxicity of treatments, but it has disadvantages such as high cost, difficult maintenance, and delay in obtaining results, among others45. Therefore, here we evaluated toxicity by using another in vivo model, the nematode C. elegans, a complete animal with digestive, reproductive, endocrine, and neuromuscular systems. Apart from being small, having a short life cycle, and being easy to maintain, C. elegans possesses 60–80% genetic homology with humans46. In this context, we evaluated the most promising BRP samples for their toxicity against C. elegans. The lowest concentration capable of killing at least 50% of the larvae (LC50) was 1500 μg/mL for the BRP crude hydroalcoholic extract and oblongifolin B and 750 μg /mL for gutiferon E. These values were significantly higher than all the MIC and MICB50 concentrations reported in this study.
Moreover, below this concentration, even after the larvae had been exposed to BRP samples for two days, LC50 was not reached, demonstrating the non-toxic profile of these natural products. Interistingly, the LC50 values of the BRP samples against C. elegans obtained in this study were higher than the LC50 values of other types of Brazilian propolis evaluated against C. elegans. For example, Campos and collaborators (2015)47 reported that propolis samples possessed LC50 of 461.8 μg/mL. Here, the BRP concentrations determined as toxic were high, above the highest MIC value (400 μg /mL). Therefore, propolis is not toxic at the concentrations used in this study and can be safely employed at concentrations below 1500 and 750 μg /mL. The results obtained here are extremely relevant, because through different methodologies the antibacterial activity of Brazilian red propolis was demonstrated against a panel of periodontopathogenic bacteria. Another point to highlight is the anti-CHIKV activity of the BRP isolated compounds. Chikungunya infection has a high incidence and severity, therefore, the search for new treatment options is highly desirable. Our results constitute an initial step for further studies of BRP as an alternative approach for treating various infectious diseases.
Conclusion
The Brazilian red propolis used in this study has antibacterial activity against a panel of periodontopathogenic bacteria. Furthermore, it’s crude extract and isolated compounds oblongifolin B and guttiferone E at concentrations similar to or slightly above the MIC concentrations inhibits monospecies and multispecies biofilms by over 50%. Medicarpin, neovestitol, and vestitol strongly inhibit CHIKV infection in vitro. Besides, toxicity tests on C. elegans demonstrated that the crude extract, oblongifolin B, and guttiferone E are non-toxic, proving to be safe and promising so that in the future, these samples of propolis can be used as medicine.
Methods
Crude hydroalcoholic extract, fractions, and isolated compounds
BRP was collected in Canavieiras Bahia State, Brazil, in March 2019 at the Canavieiras Beekeepers Association (COAPER). BRP was frozen and extracted with 70% hydroalcoholic ethanol solution, as described by Santiago et al.48. The BRP crude hydroalcoholic extract was partitioned with organic solvents (hexane, dichloromethane, ethyl acetate, and n-butanol). Authentic standards from BRP (7-O-methylvestitol, medicarpin, vestitol, neovestitol, oblongifolin B, and guttiferone E) previously isolated by our research group were used to characterize the samples17.
Chromatographic analysis of BRP extract and its fractions were performed on a Waters 2695 HPLC instrument, coupled to a 2998 photodiode array detector (PDA), with Empower 3 software as a controller. Chromatographic profiles were carried out on a Supelco Ascentis Express C-18 (150 × 4.6 mm, 2.7 µm) column. Mobile phase with water (A) (0.1% formic acid) and acetonitrile (B) was used as follows: 10 → 100% of B until 80 min; 100% of B in 89 min; 100 → 10% in 90 min, maintaining the condition until 95 min. The injections were performed on a flow rate of 1 mL/min, a 40 °C, and an injection volume of 10 µL. Chromatograms were recorded at 275 nm.
For the antibacterial, antiviral, and toxicicity assays were used the crude hydroalcoholic extract of BRP, fractions in dichloromethane, hexane, ethyl acetate, n-butanol, as well as the isolated compounds guttiferone E, oblongifolin B, methylvestitol, medicarpin, vestitol, and neovestitol.
Bacterial strains, Chikungunya virus and animal model employed in the study
The periodontopathogenic bacterial strains employed in the antibacterial and antibiofilm activity assays were obtained from the American Type Culture Collection (ATCC); their respective clinical isolates were obtained from human periodontal infections. The strains included Porphyromonas gingivalis (ATCC 49417 and clinical isolate), Fusobacterium nucleatum (ATCC 10953 and clinical isolate), Prevotella intermedia (ATCC 15033 and clinical isolate), and Actinomyces naeslundii (ATCC 19039 and clinical isolate). These bacteria are part of the collection of the Antimicrobial Assays Laboratory (LEA, abbreviation in Portuguese) of the Federal University of Uberlândia (UFU) and were cryopreserved at − 20 °C. For the in vivo toxicity assays, the mutant strain Caenorhabditis elegans AU37, obtained from the Genetics Center (CGC, University of Minnesota), was used.
For the antiviral assays, a CHIKV expressing the Nanoluciferase reporter (CHIKV-nanoluc) based on the CHIKV LR2006PYY1 strain (East/Central/South African genotype) was rescued49. The protocols were carried out as described previously50.
Determination of the minimum inhibitory concentration51
The antibacterial activity of the BRP crude hydroalcoholic extract, fractions, and isolated compounds were evaluated by the broth microdilution method, in triplicate. The assays were conducted in 96-well microplates; the methodology recommended by the Clinical and Laboratory Standards Institute52, with modifications, was followed. The inoculum was standardized to the McFarland 0.5 scale and diluted to a bacterial concentration of 1.5 × 106 CFU/mL in the wells. To prepare the samples, the BRP crude hydroalcoholic extract, fractions, or isolated compounds were solubilized in 5% dimethyl sulfoxide (DMSO) and diluted in Brucella broth supplemented with hemin (5.0 mg/mL) and menadione (1.0 mg/mL); a twofold serial dilution with concentrations ranging from 0.195 to 400 µg/mL was used. Control of 5% DMSO was performed, and the solvent did not interfere with bacterial growth at this concentration. It was also performed the following controls: inoculum (all the bacteria used in the test + the culture medium), to observe the viability of the bacteria; broth, to guarantee that the culture medium is sterile; and BRP sample, to guarantee that this solution is sterile. The microplates were incubated in an anaerobic chamber (Don WhitleyScientific, Bradford, U.K.) under anaerobic conditions (80% N2, 10% CO2, and 10% H2) at 37 °C for 72 h. Rezasurin was used to reveal bacterial growth—the blue color indicated absence of bacterial growth, and the pink color indicated presence of bacteria53. As a control technique, metronidazole from 0.0115 to 5.9 μg/mL was used against the control bacteria Bacteroides fragilis (ATCC 25285) and Bacteroides thetaiotaomicron (ATCC 29741)52.
Evaluation of antibiofilm activity monospecies and multispecies by Minimum Inhibitory Concentration of Biofilm (MICB50)
To assess the antibiofilm activity, the BRP samples that presented the most promising MIC results against four or more bacteria were submitted to the Minimum Inhibitory Concentration of Biofilm (MICB50) assay. MICB50 is defined as the lowest concentration of the microbial agent that can inhibit biofilm formation by at least 50%40 and is calculated using the following equation:
Here, MICB50 was determined as described in the CLSI guidelines (2007)52, with modifications. First, the capacity of the analyzed strains to grow in the sessile mode was verified. All the strains at 1.5 × 106 CFU/mL formed monospecies and multispecies biofilms after incubation at 37 °C for 72 h (data not shown).
For the monospecies biofilms, 100 μL of each bacterium inoculum at 1.5 × 106 CFU/mL was added to the well with the propolis samples to be evaluated at concentrations from 0.195 to 400 µg/mL (crude hydroalcoholic extract, oblongifolin B and guttiferone E). The microplates were incubated in an anaerobic chamber at 37 °C for 72 h. For the multispecies biofilms, the main periodontopathogenic bacteria found in the oral biofilm were selected and divided into two groups: group 1 consisted only of the standard bacteria (P. gingivalis ATCC 49417, P. intermedia ATCC 15033, and A. naeslundii ATCC 19039), while group 2 was composed only by the P. gingivalis, P. intermedia, and F. nucleatum clinical isolates. The antibiofilm activity of the most promising BRP samples was evaluated against the multispecies biofilm formed by group 1 bacteria and against the multispecies biofilm composed by group 2 bacteria. For this purpose, 33.33 μL of each evaluated bacterium, totaling 100 μL of bacterial inoculum, at 1.5 × 106 CFU/mL was added to the wells with the propolis samples to be evaluated at concentrations from 0.195 to 400 µg/mL (crude hydroalcoholic extract, oblongifolin B and guttiferone E). The microplates were incubated under the same conditions as the monospecies biofilm microplates. The standard antibiotic metronidazole was used as a control at concentrations from 0.0115 to 5.9 μg/mL with MIC50 (see supplementary material, Figures S2 and S3). Control of 5% DMSO was performed, and the solvent did not interfere with bacterial growth at this concentration. It was also performed the following controls: inoculum (all the bacteria used in the test + the culture medium), to observe the viability of the bacteria; broth, to guarantee that the culture medium is sterile; and BRP sample, to guarantee that this solution is sterile. After incubation, the supernatant culture was withdrawn, and the planktonic cells were removed by washing the wells with ultrapure distilled water. Monospecies and multispecies biofilms were fixed with methanol and stained with 2% crystal violet54. The reading was performed in a microplate reader (GloMax®) at 595 nm. Reading was performed in a microplate reader (GloMax®) at 595 nm. The experiments were carried out in triplicate and independent events.
Evaluation of the inhibition of biofilm formation by counting microorganism
This assay was performed for monospecies and multispecies biofilms according to de Souza Silva et al.39, as described below. Two microplates were incubated, one for MICB50 determination, and the other for microorganism count. After the microorganism count microplate was incubated, the supernatant was withdrawn, and the planktonic cells were removed by washing the wells with ultrapure distilled water. Subsequently, supplemented Brucella broth was added to all the microplate wells, and the biofilm was detached from the well after an ultrasound bath. Then, tenfold serial dilutions were performed in each well of a 96-well microplate, and 50 μL of each well, corresponding to each dilution avaliated was placed on two plates of Brucella agar supplemented with horse blood (5%), hemin (5.0 mg/mL), and menadione (1.0 mg/mL). Each of the plates were fractionated into eight parts, as described by Harrison et al.55 and incubated in an anaerobic chamber for 37 °C. After 72 h, the Colony Forming Units (CFU) count was performed in each plate. The results were expressed as Log10 (CFU/mL), and the assays were independently performed in triplicate.
Mammalian cells for antiviral assays
The BHK-21 cells (fibroblasts derived from Syrian golden hamster kidney; ATCC CCL-10) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) supplemented with 100 U/mL penicillin (Hyclone Laboratories), 100 mg/mL streptomycin (Hyclone Laboratories), 1% dilution of stock of non-essential amino acids (Hyclone Laboratories), and 1% fetal bovine serum (FBS, Hyclonen Laboratoires) in a humidified 5% CO2 incubator at 37 °C.
Cell viability through MTT for antiviral assays
BHK-21 cell viability in the presence of the tested BRP samples was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (Sigma–Aldrich) assay. BHK-21 cells were cultured in 48-well microplates and treated with different concentrations of the tested BRP sample at 37 °C for 16 h. Then, media containing the tested BRP sample was removed from the 48-well microplate. Next, 1 mg/mL MTT solution was added to each well, incubated for 30 min, and replaced with 300 μL of DMSO to solubilize the formazan crystals. Absorbance was measured at 490 nm on a Glomax microplate reader (Promega). Cell viability was calculated according to the equation (T/C) × 100%, where T and C represent the optical density of the treated well and control groups, respectively. DMSO was used as untreated control50.
Antiviral activity against CHIKV infection in vitro
For initial screening of the anti-CHIKV activity of the BRP crude hydroalcoholic extract and isolated compounds, HK-21 cells were seeded at a density of 5 × 104 cells per well in 48-well microplates 24 h before the infection. CHIKV-nanoluc at a multiplicity of infection56 of 0.1 and the tested isolated compound or extract were simultaneously added to the cells. The cells were harvested in Renilla luciferase lysis buffer (Promega) 16 h post-infection (h.p.i.), and virus replication was quantified by measuring nanoluciferase activity with the Renilla luciferase Assay System (Promega). The CHIKV replication rates were calculated according to the equation (T/C) × 100%, where T and C represent the optical density of the treated well and control groups, respectively. DMSO 0.1% was used as untreated control.
Toxicity assessment in Caenorhabditis elegans
Toxicity evaluation was performed for the most promising BRP samples in the CIM, using the in vivo model of C. elegans, according to Andrade et al.57 and Singulani et al.58. The C. elegans AU37 mutant strain was cultivated in Nematode Growth Medium (NGM) plates seeded with Escherichia coli OP50 and incubated at 16 °C for 72 h. After incubation, the NGM plates containing larvae and eggs were washed with M9 buffer, and the supernatant was placed in 15-mL conical tubes. A bleaching solution (hypochlorite + NaOH) was further added, to kill the adult larvae. The eggs were placed in NGM plates and incubated again at 15 °C for 24 h. Later, the NGM plates containing the larvae at the L1/L2 stages were washed with M9 buffer, and the supernatant was transferred to NGM plates seeded with E. coli OP50 and incubated at 16 °C for 24 h. After synchronization, 20 µL of the NGM plate contents containing from 10 to 20 L4 stage larvae was added to each well of a 96-well flat-bottomed microplate and incubated at 16 °C for 72 h. The BRP crude hydroalcoholic extract was evaluated from 750 to 6000 μg/mL, and the isolated compounds oblongifolin B and guttiferone E were evaluated from 5.85 to 1500 μg/mL. DMSO was used as solvent (final concentration ≤ 1%).
Larvae were counted every 24 h for three consecutive days under an inverted microscope. Larvae with movement were considered alive and static even after touching they were considered dead. For each sample, the lowest concentration that was able to kill 50% of the larvae, called Lethal Concentration (LC50), was determined according to time.
Statistical analysis
Individual experiments were performed in triplicate, and all the assays were performed a minimum of three times to confirm the reproducibility of the results. Differences between the means of the readings were compared by analysis of variance (one-way or two-way ANOVA) or Student’s t-test conducted with the software Graph Pad Prism 8.0 (Graph Pad Software). The p values ≤ than 0.05 were considered statistically significant.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Ellwanger, J. H. et al. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An. Acad. Bras. Cienc. 92, e20191375. https://doi.org/10.1590/0001-3765202020191375 (2020).
Papapanou, P. N. et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 45(Suppl 20), S162–S170. https://doi.org/10.1111/jcpe.12946 (2018).
Mehrotra, N. & Singh, S. Periodontitis (StatPearls Publishing, 2020).
Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 75, 7–23. https://doi.org/10.1111/prd.12221 (2017).
Cooley, L. & Teng, J. Anaerobic resistance: Should we be worried?. Curr. Opin. Infect. Dis. 32, 523–530. https://doi.org/10.1097/QCO.0000000000000595 (2019).
Silva, L. A. & Dermody, T. S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Invest. 127, 737–749. https://doi.org/10.1172/JCI84417 (2017).
Reilly, J. M. et al. Postmortem chikungunya diagnosis: A case report and literature review. Am. J. Forensic Med. Pathol. 41, 48–51. https://doi.org/10.1097/PAF.0000000000000519 (2020).
Vairo, F. et al. Chikungunya: Epidemiology, pathogenesis, clinical features, management, and prevention. Infect. Dis. Clin. North Am. 33, 1003–1025. https://doi.org/10.1016/j.idc.2019.08.006 (2019).
Department, H. S. Vol. 53 (ed Ministry of Health) (2022).
WHO. (World Health Organization, 2018).
Valli, M. et al. Development of a natural products database from the biodiversity of Brazil. J. Nat. Prod. 76, 439–444. https://doi.org/10.1021/np3006875 (2013).
Batista, L. L. et al. Comparative study of topical green and red propolis in the repair of wounds induced in rats. Rev. Col. Bras. Cir. 39, 515–520. https://doi.org/10.1590/s0100-69912012000600012 (2012).
Miranda, S. L. F. et al. Brazilian red propolis reduces orange-complex periodontopathogens growing in multispecies biofilms. Biofouling 35, 308–319. https://doi.org/10.1080/08927014.2019.1598976 (2019).
Ccana-Ccapatinta, G. V. et al. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The botanical sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules https://doi.org/10.3390/molecules25092060 (2020).
Reis, J. H. O. et al. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasound-assisted extraction. PLoS ONE 14, e0219063. https://doi.org/10.1371/journal.pone.0219063 (2019).
Boeing, T. et al. The gastroprotective effect of red propolis extract from Northeastern Brazil and the role of its isolated compounds. J. Ethnopharmacol. 267, 113623. https://doi.org/10.1016/j.jep.2020.113623 (2021).
Aldana-Mejia, J. A. et al. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 198, 114029. https://doi.org/10.1016/j.jpba.2021.114029 (2021).
Bueno-Silva, B., Marsola, A., Ikegaki, M., Alencar, S. M. & Rosalen, P. L. The effect of seasons on Brazilian red propolis and its botanical source: Chemical composition and antibacterial activity. Nat. Prod. Res. 31, 1318–1324. https://doi.org/10.1080/14786419.2016.1239088 (2017).
Freires, I. A., de Alencar, S. M. & Rosalen, P. L. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 110, 267–279. https://doi.org/10.1016/j.ejmech.2016.01.033 (2016).
Rufatto, L. C. et al. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 214, 74–82. https://doi.org/10.1016/j.micres.2018.05.003 (2018).
Ayres, D. C., Marcucci, M. C. & Giorgio, S. Effects of Brazilian propolis on Leishmania amazonensis. Mem. Inst. Oswaldo Cruz 102, 215–220. https://doi.org/10.1590/s0074-02762007005000020 (2007).
Dantas Silva, R. P. et al. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 12, e0172585. https://doi.org/10.1371/journal.pone.0172585 (2017).
do Nascimento, T. G. et al. Comprehensive multivariate correlations between climatic effect, metabolite-profile, antioxidant capacity and antibacterial activity of Brazilian red propolis metabolites during seasonal study. Sci. Rep. 9, 18293. https://doi.org/10.1038/s41598-019-54591-3 (2019).
Morsy, A. S. et al. Effect of Brazilian red propolis administration on hematological, biochemical variables and parasitic response of Santa Ines ewes during and after flushing period. Trop. Anim. Health Prod. 45, 1609–1618. https://doi.org/10.1007/s11250-013-0406-3 (2013).
Regueira-Neto, M. D. S. et al. Antitrypanosomal, antileishmanial and cytotoxic activities of Brazilian red propolis and plant resin of Dalbergia ecastaphyllum (L) Taub. Food Chem. Toxicol. 119, 215–221. https://doi.org/10.1016/j.fct.2018.04.029 (2018).
Sena-Lopes, A. et al. Chemical composition, immunostimulatory, cytotoxic and antiparasitic activities of the essential oil from Brazilian red propolis. PLoS ONE 13, e0191797. https://doi.org/10.1371/journal.pone.0191797 (2018).
Sinott, F. A. et al. Essential oil from Brazilian Red Propolis exhibits anthelmintic activity against larvae of Toxocara cati. Exp. Parasitol. 200, 37–41. https://doi.org/10.1016/j.exppara.2019.03.014 (2019).
Silva-Beltran, N. P., Balderrama-Carmona, A. P., Umsza-Guez, M. A. & Souza Machado, B. A. Antiviral effects of Brazilian green and red propolis extracts on Enterovirus surrogates. Environ. Sci. Pollut. Res. Int. 27, 28510–28517. https://doi.org/10.1007/s11356-019-07458-z (2020).
Bueno-Silva, B. et al. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 61, 4546–4550. https://doi.org/10.1021/jf305468f (2013).
de Figueiredo, K. A. et al. Brazilian Red propolis is as effective as amoxicillin in controlling red-complex of multispecies subgingival mature biofilm in vitro. Antibiotics (Basel) https://doi.org/10.3390/antibiotics9080432 (2020).
Ribeiro, V. P. et al. Chemical characterization of Brazilian propolis using automated direct thermal desorption-gas chromatography-mass spectrometry. J. Sci. Food Agric. 102, 4345–4354. https://doi.org/10.1002/jsfa.11788 (2022).
Rios, J. L. & Recio, M. C. Medicinal plants and antimicrobial activity. J Ethnopharmacol 100, 80–84. https://doi.org/10.1016/j.jep.2005.04.025 (2005).
Gibbons, S. Phytochemicals for bacterial resistance–strengths, weaknesses and opportunities. Planta Med. 74, 594–602. https://doi.org/10.1055/s-2008-1074518 (2008).
Yoshimasu, Y. et al. Rapid bactericidal action of propolis against porphyromonas gingivalis. J. Dent. Res. 97, 928–936. https://doi.org/10.1177/0022034518758034 (2018).
Han, Y. W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147. https://doi.org/10.1016/j.mib.2014.11.013 (2015).
Santos, F. A. et al. Antibacterial activity of Brazilian propolis and fractions against oral anaerobic bacteria. J. Ethnopharmacol. 80, 1–7. https://doi.org/10.1016/s0378-8741(02)00003-x (2002).
Shabbir, A., Rashid, M. & Tipu, H. N. Propolis, a hope for the future in treating resistant periodontal pathogens. Cureus 8, e682. https://doi.org/10.7759/cureus.682 (2016).
Al-Ahmad, A. et al. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 56, 681–687. https://doi.org/10.1099/jmm.0.47094-0 (2007).
de Souza Silva, T. et al. Green and Red brazilian propolis: Antimicrobial potential and anti-virulence against ATCC and clinically isolated multidrug-resistant bacteria. Chem Biodivers 18, e2100307. https://doi.org/10.1002/cbdv.202100307 (2021).
Wei, G. X., Campagna, A. N. & Bobek, L. A. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J. Antimicrob. Chemother. 57, 1100–1109. https://doi.org/10.1093/jac/dkl120 (2006).
Gonzalez-Burquez, M. J. et al. Comparison between in vitro antiviral effect of Mexican propolis and three commercial flavonoids against canine distemper virus. Evid. Based Complement Alternat. Med. 2018, 7092416. https://doi.org/10.1155/2018/7092416 (2018).
Labska, K., Plodkova, H., Pumannova, M. & Sensch, K. H. Antiviral activity of propolis special extract GH 2002 against Varicella zoster virus in vitro. Pharmazie 73, 733–736. https://doi.org/10.1691/ph.2018.8672 (2018).
de Carvalho, F. M. A. et al. Brazilian red propolis: Extracts production, physicochemical characterization, and cytotoxicity profile for antitumor activity. Biomolecules https://doi.org/10.3390/biom10050726 (2020).
Pohjala, L. et al. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS ONE 6, e28923. https://doi.org/10.1371/journal.pone.0028923 (2011).
Hunt, P. R. & The, C. The C. elegans model in toxicity testing. J Appl Toxicol 37, 50–59. https://doi.org/10.1002/jat.3357 (2017).
Ruszkiewicz, J. A. et al. C. elegans as a model in developmental neurotoxicology. Toxicol. Appl. Pharmacol. 354, 126–135. https://doi.org/10.1016/j.taap.2018.03.016 (2018).
Campos, J. F. et al. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jatai). Evid. Based Complement Alternat. Med. 2015, 296186. https://doi.org/10.1155/2015/296186 (2015).
Santiago, M. B. et al. Brazilian red propolis presents promising anti-H. pylori activity in in vitro and in vivo assays with the ability to modulate the immune response. Molecules https://doi.org/10.3390/molecules27217310 (2022).
Matkovic, R. et al. The host DHX9 DExH-box helicase is recruited to Chikungunya virus replication complexes for optimal genomic RNA translation. J. Virol. https://doi.org/10.1128/JVI.01764-18 (2019).
de Oliveira, D. M. et al. Organometallic complex strongly impairs Chikungunya virus entry to the host cells. Front. Microbiol. 11, 608924. https://doi.org/10.3389/fmicb.2020.608924 (2020).
Shaughnessy, M. K. et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 32, 201–206. https://doi.org/10.1086/658669 (2011).
CLSI. Vol. Approved Standard M11-A7, CLSI (Clinical and Laboratory Standards Institute Wayne, PA, 2007).
Sarker, S. D., Nahar, L. & Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42, 321–324. https://doi.org/10.1016/j.ymeth.2007.01.006 (2007).
Stepanovic, S. et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115, 891–899. https://doi.org/10.1111/j.1600-0463.2007.apm_630.x (2007).
Harrison, J. J., Turner, R. J. & Ceri, H. High-throughput metal susceptibility testing of microbial biofilms. BMC Microbiol. 5, 53. https://doi.org/10.1186/1471-2180-5-53 (2005).
Moise, A. R. & Bobis, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, main plant sources for bioactive properties in green and red Brazilian propolis. Plants (Basel) https://doi.org/10.3390/plants9111619 (2020).
Andrade, G. et al. Brazilian Copaifera species: Antifungal activity against clinically relevant Candida species, cellular target, and in vivo toxicity. J Fungi (Basel) https://doi.org/10.3390/jof6030153 (2020).
Singulani, J. L. et al. Activity of gallic acid and its ester derivatives in Caenorhabditis elegans and zebrafish (Danio rerio) models. Future Med. Chem. 9, 1863–1872. https://doi.org/10.4155/fmc-2017-0096 (2017).
Funding
This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grant # 2017/04138–8), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG # 12138—Scholarship granted; APQ-03385–18 and APQ-01487–22), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES Finance Code 001—Scholarship granted and Prevention and Combat of Outbreaks, Endemics, Epidemics and Pandemics—Finance Code #88881.506794/2020–01) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Grant # 307974/2019–7).
Author information
Authors and Affiliations
Contributions
N.B.S.S. designed the experiments, functions/Writing—original draft; writing—proofreading and editing. J.H.S. designed the experiments and carried out the experiments. M.B.S. designed the experiments and carried out the experiments. J.R.S.A. designed the experiments and carried out the experiments. D.O.S.M. designed the experiments and analysed the results. R.A.S. designed the experiments and carried out the experiments. I.A.S. Analysed the results and Writing—proofreading and editing. J.A.A. designed the experiments and carried out the experiments and Analysed the results and Writing—proofreading and editing. A.C.J. Analysed the results and Writing—proofreading and editing. R.P.S. Writing—proofreading and editing, methodology, supervision. S.R.A. Acquisition of financing, writing—proofreading and editing. R.C.S.V. Writing—proofreading and editing, acquisition of financing. J.K.B. Writing—proofreading and editing, acquisition of financing. R.H.P. Methodology. C.H.G.M. Conceptualization, formal analysis, methodology, supervision, validation; visualization, functions/Writing—original draft; writing—proofreading and editing. All authors gave the final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, N.B.S., de Souza, J.H., Santiago, M.B. et al. Potential in vitro anti-periodontopathogenic, anti-Chikungunya activities and in vivo toxicity of Brazilian red propolis. Sci Rep 12, 21165 (2022). https://doi.org/10.1038/s41598-022-24776-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24776-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.