Abstract
The subfamily Phyllostominae (Chiroptera, Phyllostomidae) comprises 10 genera of Microchiroptera bats from the Neotropics. The taxonomy of this group is controversial due to incongruities in the phylogenetic relationships evident from different datasets. The genus Lophostoma currently includes eight species whose phylogenetic relationships have not been resolved. Integrative analyzes including morphological, molecular and chromosomal data are powerful tools to investigate the phylogenetics of organisms, particularly if obtained by chromosomal painting. In the present work we performed comparative genomic mapping of three species of Lophostoma (L. brasiliense 2n = 30, L. carrikeri 2n = 26 and L. schulzi 2n = 26), by chromosome painting using whole chromosome probes from Phyllostomus hastatus and Carollia brevicauda; this included mapping interstitial telomeric sites. The karyotype of L. schulzi (LSC) is a new cytotype. The species L. brasiliense and L. carrikeri showed interstitial telomeric sequences that probably resulted from expansions of repetitive sequences near pericentromeric regions. The addition of chromosomal painting data from other species of Phyllostominae allowed phylogeny construction by maximum parsimony, and the determination that the genera of this subfamily are monophyletic, and that the genus Lophostoma is paraphyletic. Additionally, a review of the taxonomic status of LSC is suggested to determine if this species should be reclassified as part of the genus Tonatia.
Similar content being viewed by others
Introduction
The subfamily Phyllostominae (Chiroptera, Phyllostomidae) is composed of 10 genera with 25 species1 that are organized into three tribes: Phyllostomini (Phyllostomus, Tonatia, Mimon, Gardnerycteris, Phylloderma, Lophostoma), Macrophyllini (Trachops, Macrophyllum) and Vampyrini (Chrotopterus, Vampyrum)2. The phylogenetic relationships between the genera of this subfamily and even their positioning in relation to other subfamilies of Phyllostomidae are controversial, as different data sets show different phylogenetic patterns, which result in different taxonomic classifications [for review:2,3,4,5,6,7].
The genus Lophostoma d’Orbigny, 1836 includes eight species: L. silvicola d’Orbigny, 1836; L. brasiliense Peters, 1867; L. carrikeri Allen 1910; L. evotis Davis & Carter 1978; L. occidentalis Davis & Carter 1978; L. schulzi Genoways & Williams 1980; L. yasuni Fonseca & Pinto, 2004 and L. kalkoae Velazco & Gardner 20128, which range from southern Mexico to central Paraguay9,10.
Morphological, molecular and chromosomal data, analyzed under an integrative taxonomy approach, constitute powerful tools for understanding the phylogenetic relationships between groups of organisms. Comparative analysis by chromosomal painting between species, associated with chromosome banding, can elucidate the types of intraspecific and interspecific rearrangements involved in the process of chromosomal differentiation that occurred throughout the evolution of taxa11,12,13. Chromosomal painting data have been successfully used in the reconstruction of karyotypic evolution among several groups of bats, including Phyllostomidae, which resulted in the correct identification of chromosomal homologies, the reconstruction of the phylogeny of the family and interpretation of the ancestral karyotype14,15,16,17,18,19,20,21,22.
Species of the genus Lophostoma show variable rates of karyotypic evolution where some species have highly conserved karyotypes such as L. silvicola (2n = 34, NF = 60; Gardner; Honeycutt et al.; Ribas et al.)20,23,24 and L occidentalis (2n = 34, NF = 62)16,25 On the other hand, the genus also has species with highly rearranged karyotypes: L. brasiliense (2n = 30, NF = 56)23,26,27,28,29, L. carrikeri (2n = 26, NF = 46)23,30 and L. schulzi (2n = 28, NF = 36)24,30,31. Analyzing the species L. schulzi, Baker et al.29 found a karyotype so derived that none of the chromosomal arms proposed as primitive for the family were identified, suggesting that non-Robertsonian rearrangement processes would be involved in the differentiation of these karyotypes. In addition, most of the karyotypes were presented only in Giemsa conventional staining, preventing comparisons that could provide information about the types of chromosomal rearrangements that distinguish them.
In the present work, the karyotypes of three species of the genus Lophostoma (L. schulzi, L. brasiliense and L. carrikeri) were analyzed by chromosome painting using whole chromosome probes from Phyllostomus hastatus and Carollia brevicauda17. Chromosomal painting data for L. occidentalis16, L. silvicola20, Phyllostomus hastatus17, Gardnerycteris crenulatum16, Tonatia bakeri16, Tonatia maresi20, and Macrotus californicus15, were added to the phylogenetic analysis, in order to observe the relationships between Lophostoma species and their position within the Phyllostomini tribe.
Results
Karyotypic description and FISH in Lophostoma schulzi (LSC)
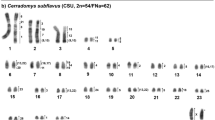
Lophostoma schulzi shows 2n = 26 NF = 34, comprising metacentric (pairs 6 and 9), submetacentric (pair 1), subtelocentric (7 and 8) and 7 acrocentric pairs (2, 3, 4, 5, 10, 11 and 12). The X chromosome presents acrocentric morphology (Fig. 1A). Constitutive heterochromatin (CH) occurs in the pericentromeric region of all chromosomes, and more extensive heterochromatic blocks were observed in pairs 1, 3, 6, 8, 10 and 11 (Fig. 1B). In situ hybridization with telomeric probe occurred only at the telomeres of all chromosomes (Fig. 1C). In situ hybridization with 18S ribosomal DNA probes confirmed the location of the NOR in pair 6 (Fig. 1D). Chromosomal painting using Phyllostomus hastatus (PHA) and Carollia brevicauda (CBR) probes delimited 24 and 39 homologous segments in the genome of Lophostoma schulzi respectively. The number of signals per chromosome pair ranged from one to five.
Lophostoma schulzi (LSC): (A) Karyotype with G-banding. The horizontal lines on the right of each pair of chromosomes delimit the hybridization with whole chromosome probes from Phyllostomus hastatus and the horizontal lines on the left, the probes from Carollia brevicauda. H identifies the amplified heterochromatic regions. (B) C-banding pattern showing constitutive heterochromatin regions. (C) FISH with telomeric probes. (D) FISH with 18S ribosomal DNA probe.
Karyotypic description and FISH in Lophostoma brasiliense (LBR)
Lophostoma brasiliense shows 2n = 30 NF = 56, and the autosomal complement consists of 9 pairs of metacentric chromosomes (pairs 4, 5, 6, 7, 8, 11, 12, 13 and 14), three submetacentric pairs (pairs 1, 2, 3) and two subtelocentric pairs (pairs 9 and 10). The X chromosome is subtelocentric of medium size (Fig. 2A). The C-banding technique demonstrated that constitutive heterochromatin is located in the pericentromeric region of all autosomal and sex chromosomes (Fig. 2B). In situ hybridization with telomeric probe showed distal markings, common to the telomeres of all chromosomes, and the detection of an interstitial telomeric sequence (ITS) in pair 1 (Fig. 2C). FISH with 18S rDNA probes showed that the NOR is located in the distal region of the short arm of pair 2 (Fig. 2D). Chromosomal painting using PHA and CBR probes delimited 17 and 24 homologous segments in the genome of Lophostoma brasiliense, respectively. The number of signals per chromosome pair ranged from one to four.
Lophostoma brasiliense (LBR): (A) Karyotype with G-banding. The horizontal lines to the right of each pair of chromosomes delimit the hybridization with whole chromosome probes from Phyllostomus hastatus and the horizontal lines on the left, the probes from Carollia brevicauda. H identifies the amplified heterochromatic regions. (B) C-banding pattern showing constitutive heterochromatin regions. (C) FISH with telomeric probes. Arrows: interstitial telomeric sequences. (D) FISH with 18S ribosomal DNA probe.
Karyotypic description and FISH in Lophostoma carrikeri (LCA)
Lophostoma carrikeri had a diploid number 2n = 26 NF = 48, comprising 10 metacentric pairs of chromosomes (pairs 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12), and two large subtelocentric pairs (pairs 1 and 12). The X chromosome showed acrocentric morphology (Fig. 3A). Constitutive heterochromatin occurs in the pericentromeric region in all autosomes and in the X (Fig. 3B). In situ hybridization with telomeric probe occurred at telomeres and in the pericentromeric region in seven pairs of metacentric chromosomes exhibiting strong signals co-localized to heterochromatic blocks identified by the C-banding pattern (Fig. 3C). In situ hybridization with 18S ribosomal DNA probes identified the location of the NOR on the short arm of pair 2 (Fig. 3D). Chromosome painting using PHA and CBR probes delimited 19 and 26 homologous segments in the genome of Lophostoma carrikeri, respectively. The number of signals per chromosome pair ranged from one to four. No hybridization signal was found in the short arm of the subtelocentric pair 2, which was shown to be heterochromatic by C-banding.
Lophostoma carrikeri (LCA): (A) Karyotype with G-banding. The horizontal lines to the right of each pair of chromosomes delimit the hybridization with whole chromosome probes from Phyllostomus hastatus and the horizontal lines on the left, the probes from Carollia brevicauda. H identifies the amplified heterochromatic regions. (B) C-banding pattern showing constitutive heterochromatin regions. (C) FISH with telomeric probes. (D) FISH with 18S ribosomal DNA probe.
Some examples of the results of hybridizations made in the metaphases of the three species studied are shown in Fig. 4.
Representative images of fluorescence in situ hybridizations with whole chromosome probes from Phyllostomus hastatus (PHA) and Carollia brevicauda (CBR) in metaphases of species of Lophostoma. The probes used in LSC (A–C), LCA (G–I) and LBR (D–F) are indicated in white in the lower corner of each image.
Phylogenetic analysis
The chromosome paintings by Sotero-Caio et al.15,16 were made using whole chromosome probes from Macrotus californicus. Once we mapped the Glossophaga soricina genome with the PHA and CBR whole chromosome probes21 and Sotero-Caio et al.16 mapped the same species with human and MCA probes, it was possible to compare the distribution of the probes and determine their homeology (Fig. 5). For the purposes of our phylogenetic analysis, we converted the information from MCA to PHA (Supplementary Table 1) based on Fig. 5, in order to build a unified data matrix. A single tree was recovered by our maximum parsimony analysis (Fig. 6). The Phyllostomini genera formed a monophyletic group. The genus Lophostoma, however, is paraphyletic. One of the branches brings together the species of Tonatia and L. schulzi. The other branch aggregates the other species studied here, where one of the branches brings together L. brasiliense and L. carrikeri; the other branch is divided in two, being L. occidentalis and L. silvicola in one of the branches and Phyllostomus hastatus and Gardnerycteris crenulatum in the other branch. The consistency index (CI) was 0.88, the retention index (RI) = 0.8594 and the homoplasy index (HI) = 0.12. Bootstrap values ranged from 61 to 100.
Phylogenetic tree obtained using chromosomal characters from maximum parsimony in the PAUP program for representatives of the genus Lophostoma. The blue numbers below the branches represent bootstrap values. The numbers in black refer to the apomorphies described in Supplementary Table 1. The numbers in red refer to the apomorphies that present homoplasy and are repeated in the branches in which they occur. MCA Macrotus californicus; PHA Phyllostomus hastatus, GCR Gardnerycteris crenulatum, LOC Lophostoma occidentalis, LSI Lophostoma silvicola, LBR Lophostoma brasiliense, LCA Lophostoma carrikeri, TMA Tonatia maresi, TBA Tonatia bakeri, LSC Lophostoma schulzi.
Discussion
Chromosomal differences in Lophostoma
The data on the karyotype of the specimens of Lophostoma brasiliense (2n = 30 NF = 56) and Lophostoma carrikeri (2n = 26 NF = 46) analyzed here are in agreement with the studies previously carried out23, in specimens collected in Peru and for specimens collected in Suriname24,29,32. We described a new cytotype for Lophostoma schulzi 2n = 26 (6 sm/4 st + 16 a) for individuals collected in Juruti, State of Pará, Brazil (present study) that differs from the L. schulzi cytotype 2n = 28 (4 sm/6 st + 18 a), for individuals collected in Suriname24,30. Comparative analysis between L. schulzi karyotypes from Suriname and Brazil showed that the difference is due to a centric fusion/fission rearrangement. Chromosomal variations for populations of this species had not yet been reported, although they have already been recorded for other phyllostomid19,29,33,34,35,36.
Most Phyllostomidae species have a bi-armed X chromosome, and this condition is considered basal for the family28,37,38. The acrocentric form of X has been reported only for some genera, such as Micronycteris and Mesophylla26,27,28, a form admitted as a homoplasic character in these species because they are not closely related. In Lophostoma, the acrocentric X chromosome is found only in L. carrikeri and L. schulzi [Refs.16,20,24,25, present work], similarly suggesting a homoplasic character, since these species are phylogenetically far apart (Fig. 6).
Distribution of heterochromatin and telomeric sites in Lophostoma
A pericentromeric pattern of constitutive heterochromatin distribution was observed in L. brasiliense (Fig. 2B), which is commonly found in phyllostomid bats38,39,40,41. Unusual distribution was observed in the karyotype of L. schulzi (Fig. 1B) and L. carrikeri (Fig. 3B), which have extensions of heterochromatin beyond the pericentromeric region.
The chromosomal location of telomeric sequences by FISH has been determined in many groups of animals42,43,44,45. In addition to distal markings, interstitial telomeric sites (ITS) were also reported46,47,48. Telomeric hybridization was observed here at the ends of all chromosomes of species of the genus Lophostoma. Additionally, ITS were observed in L. brasiliense (Fig. 2C) and L. carrikeri (Fig. 3C), where we identified strong signals co-located with heterochromatic blocks marked by the C-positive band pattern, which suggests that these sequences are present as satellite DNA components in the centromeric region and not as a result of fusion processes. However, the presence/absence of large C-positive band blocks is not directly related to the presence/absence of telomeric sequences. We found extensive blocks with positive C-banding in the karyotype of L. schulzi, but no ITS was observed in the individuals analyzed in this study, exemplifying the heterogeneity of the repetitive DNA that makes up the heterochromatic regions in the mammalian genome46.
18S ribosomal DNA location
When analyzing the location of the 18S ribosomal DNA in Phyllostomidae species mapped using chromosome painting and 18S rDNA probes16,20,21,22, it is very common to find its mapping in different places, an observation already found in literature16. Regarding the genus Lophostoma, five species were studied using these probes. In four, LCA, LBR, LSC (current study) and L. silvicola (LSI)20 the 18S ribosomal DNA mapped into a block of heterochromatin. The only exception is L. occidentalis (LOC)16, where the 18S rDNA maps to chromosomes corresponding to pairs 5 and 19 of Macrotus californicus (PH1 and PHA13, respectively, see Fig. 5). This seems to suggest that the 18S rDNA was located in a heterochromatic block in the Lophostoma ancestral karyotype. However, a DNA sequence study is necessary to verify whether all heterochromatic blocks harboring 18S DNA in Lophostoma are homologous.
Comparative chromosomal mapping in species of the genus Lophostoma
Through chromosome painting, using PHA and CBR whole chromosome probes, it was possible to establish the homeologies between the karyotypes of the species analyzed here. Additionally, we added the data previously obtained with these same probes in L. silvicola20 and the analysis with MCA probes in L. occidentalis16, where we converted the MCA data to PHA, as illustrated in Fig. 5. Lophostoma karyotypes were highly variable, from highly conserved forms (LOC) to karyotypes with many rearrangements (LSC).
Considering the PHA karyotype as a reference, as it is close to the ancestral karyotype proposed for Phyllostomidae18,22, we show that the LOC and LSI karyotypes are very similar to the PHA karyotype, differing only by the fission of PHA13. In addition to presenting autapomorphies, the LBR and LCA karyotypes share the syntenic arrangement PHA13/1/6 (pair 1 in both species). These species and also LOC and LSI share with PHA the pairs PHA1, 2, 3, 4, 7, 12 and a metacentric X. Finally, the LSC karyotype is the most differentiated in relation to other species, as most chromosomes are acrocentric and show a greater number of rearrangements in relation to the ancestral and PHA karyotypes. These data confirm the observation by Baker et al.29, that LSC has a derived karyotype in which none of the chromosomal arms proposed as primitive for the family were identified by G-banding.
Tonatia bakeri × Tonatia maresi
Ribas et al.20 mapped the Tonatia saurophila karyotype collected in the Brazilian Amazon with PHA and CBR whole chromosome probes. Sotero-Caio et al.16 mapped the same species, collected in Central America, with MCA whole chromosome probes. Recently the specimens of T. saurophila were reclassified49, considering only specimens from Jamaica as belonging to this species, while specimens located in Central America and northeast of South America were considered to belong to the species Tonatia bakeri (TBA), while specimens from the Amazon would belong to the species Tonatia maresi (TMA), with the Andes being the barrier that separates these new species. The presence of karyotypic differences could be an additional diagnostic element to define these species. Thus, we converted the probe mapping data from MCA to PHA in TBA by Sotero-Caio et al.16 using Fig. 5 and compared with the results we described earlier for TMA20. Sotero-Caio et al.16 had already noticed the difference in morphology of the TBA4 pair (equivalent to TMA6) between the karyotypes, suggesting a pericentric inversion. After comparing the mapping results of the two species, we found three chromosomal rearrangements between these karyotypes: (1) the difference between TBA4 and TMA6 is actually due to an insertion of a segment of the PHA10 chromosome (= MCA3) into the short arm of TBA4; (2) Insertion of a segment of the PHA7 chromosome (= MCA4) into the long arm of TBA3 (= TMA5); (3) Inversion in the long arm of TBA2 (= TMA4), where the syntenic group corresponding to PHA1 (= MCA5, MCA10) are joined in TMA and separated (MCA5 and MCA10) in TBA. Therefore, these differences reinforce the specific status of the Central American (TBA) and South American (TMA) taxa and were included in the data matrix (Supplementary Table 1) for the construction of the phylogeny (Fig. 6).
Phylogenetic relationships
The genus Lophostoma is paraphyletic, as it includes PHA and GCR in the same branch as LOC and LSI, while the other Lophostoma representatives are located in more basal branches (Fig. 6). It is possible that this result is a consequence of the chromosomal conservatism of PHA, GCR, LOC and LSI, since the karyotypes of these two species of Lophostoma differ from PHA and GCR only by the fission of the PHA13 chromosome. Therefore, there would not be a phylogenetic signal significant enough to separate these genera, as already observed20. Regarding the internal branches, the association of PHA with GCR occurs because their karyotypes does not show any difference with the techniques used here. This close relationship had already been observed in other phylogenetic studies using molecular data50,51,52. Likewise, the sister species relationship between LOC and LSI is evidenced by the shared fission of PHA13. These two species are close, as LOC was considered a subspecies of LSI until it received its specific recognition53, again explaining the absence of rearrangements between their karyotypes. The branch joining LBR and LCA has a strong phylogenetic signal, composed of the PHA13/1/6 syntenic association exclusive to these species, although the karyotypes are not identical as in the case of LOC x LSI, due to LBR and LCA autapomorphies. The association of LBR with LCA has also been observed in molecular phylogeny studies52,53. Finally, the association between Tonatia maresi, Tonatia bakeri and L. schulzi results from the shared synapomorphies between these taxa, including PHA16q/3, PHA2p/12p, PHA4p/13p, and PHA9 fission. Lophostoma species were once considered part of the genus Tonatia, being later separated into the current genus54. This separation results from molecular studies that place the two taxa in different branches50,51,54. However, the presence of four synapomorphic associations uniting LSC, TBA and TMA reinforce the phylogenetic proximity of these species. Consequently, a review of the taxonomic status of LSC would be important considering the possibility of this species being reclassified as part of the genus Tonatia.
The results of the present analysis are broadly in line with other studies on the evolution of Lophostoma, with the exception of its paraphyly and the proximity of LSC to the genus Tonatia. Studies including the other tribes of Phyllostominae can be expected to shed light on the phylogenetic relationships found here.
Materials and methods
Ethics declarations
All experimental protocols were approved by the Ethics Committee from Para Federal University (Comitê de Ética Animal da Universidade Federal do Pará) under Permit 68/2015. All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). The Cytogenetics Laboratory from Centro de Estudos Avançados da Biodiversidade (UFPA) has permit number 19/2003 from the Ministry of Environment for sample transport and permit 52/2003 for using the samples for research. Sample collections were authorized by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) and Secretaria de Estado de Meio Ambiente do Pará (SEMA-PA) under permit 020/2005 (Registration: 207419).
Analyzed specimens
Cytogenetic analyzes were performed on samples collected in the Brazilian Amazon region (Table 1). Bats were captured from natural populations with the aid of mist nets. Chromosomal preparations and tissue biopsies were sent to the Cytogenetics Laboratory of the Federal University of Pará, in Belém. The specimens were fixed in 10% formaldehyde, preserved in 70% ethanol and deposited in the mammal collection of the Museu Paraense Emilio Goeldi.
Chromosomal preparations and banding
Chromosomal preparations were obtained by the direct bone marrow extraction method55. G-banding patterns were obtained using trypsin solution56, with subsequent incubation in saline solution (0.5 × SSC) at 60 °C and staining with Wright’s solution57. C-banding was performed according to literature58. Karyotypes were arranged in decreasing order of chromosome size.
Fluorescence in situ hybridization (FISH)
FISH using telomeric probe labeled with digoxigenin (All Human Telomere—Oncor), and detected with FITC-Cy3 labelled antibody, was performed according to the protocol provided by the manufacturer. The 18S rDNA probes were amplified by PCR using the probes NXS1 and NS8, as described in literature59. The probes were labeled with dUTP-biotin labelled antibody by nick translation and later detected with avidin-Cy3 or FITC labelled antibodies.
Whole chromosome probes from Carollia brevicauda and Phyllostomus hastatus17 were amplified and labeled by DOP-PCR60,61 and hybridized following previously described17,61. Briefly, slides were incubated with pepsin enzyme solution, washed in 2× SSC solution and serially dehydrated with ethanol (70%, 90% and 100%). The slides were then aged in an oven at 65 °C for two hours, denatured in formamide 70%/2× SSC for 50 s and mounted with hybridization solution (14 µl of solution containing: 50% formamide, 2 × SSC, 10% dextran sulfate and 1–3 µl of PCR product) for three days. After hybridization and stringency washing, biotin-labeled probes were detected with avidin-Cy3 or avidin-FITC (1 µg/ml; Amersham). The slides were mounted with Vectashield antifading solution (Vector Lab) and DAPI (4ʹ,6-diamidino-2-phenylindole) and the images were captured with the aid of the Zeiss Axiocam CCD camera controlled by the Axiovision 3.0 software, coupled to a Zeiss Axioplan 2 microscope. Chromosomes were identified according to their morphology and banding patterns interpreted from DAPI (4ʹ,6-diamidino-2-phenylindole) staining images edited in grayscale format.
Phylogenetic analysis
We used syntenic segments and shared chromosomal associations to establish a matrix of characters (Supplementary Table 1), which were coded based on presence or absence, to be used in the maximum parsimony analysis in PAUP Version 4a, build 16962. All characters were weighted with the same weight, based on the equal probability of occurrence of chromosomal rearrangements. We searched the most parsimonious phylogenetic tree, which was obtained using the exhaustive search. The robustness of each node was evaluated by bootstrap estimation of 1000 repetitions. Macrotus californicus15 was used as outgroup and as ingroup the three species studied here and L. occidentalis16, L. silvicola20, Phyllostomus hastatus17, Gardnerycteris crenulatum16, Tonatia maresi20, and Tonatia bakeri16.
Data availability
All relevant data are within the paper and in the Supplementary Table 1. Data can be requested from the corresponding author.
Abbreviations
- 2n:
-
Diploid number
- a:
-
Acrocentric chromosome
- BAC:
-
Bacterial artificial chromosome
- CBR:
-
Carollia brevicauda
- CH:
-
Constitutive heterochromatin
- Cy3:
-
Cyanine 3-methine
- DAPI:
-
4',6-Diamidino-2-phenylindole
- DOP-PCR:
-
Degenerate oligonucleotide-primed polymerase chain reaction
- dUTP:
-
2ʹ-Deoxyuridine 5ʹ-triphosphate
- FISH:
-
Fluorescence in situ hybridization
- FITC:
-
Fluorescein isothiocyanate
- FN:
-
Fundamental number
- GCR:
-
Gardnerycteris crenulatum
- GSO:
-
Glossophaga soricina
- ITS:
-
Interstitial telomeric sequences
- LBR:
-
Lophostoma brasiliense
- LCA:
-
Lophostoma carrikeri
- LOC:
-
Lophostoma occidentalis
- LSC:
-
Lophostoma schulzi
- LSI:
-
Lophostoma silvicola
- MCA:
-
Macrotus californicus
- sm:
-
Submetacentric chromosome
- st:
-
Subtelocentric chromosome
- NOR:
-
Nucleolar Organization Region
- PAUP:
-
Phylogenetic analysis using parsimony
- PHA:
-
Phyllostomus hastatus
- rDNA:
-
Ribosomal DNA
- SSC:
-
Saline sodium citrate
- TBA:
-
Tonatia bakeri
- TMA:
-
Tonatia maresi
References
Simmons, N. B. & Cirranelo, A. L. Bats of the World: A Taxonomic and Geographic Database. https://batnames.org (2022). Accessed 20 May 2022.
Baker, R. J., Solari, S., Cirranello, A. L. & Simmons, N. B. Higher level classification of phyllostomid bats with a summary of DNA synapomorphies. Acta Chiropterol. 18, 1–38. https://doi.org/10.3161/15081109ACC2016.18.1.001 (2016).
Wetterer, A. L., Rockman, M. V. & Simmons, N. B. 2000 Phylogeny of phyllostomid bats (Mammalia: Chiroptera): Data from diverse morphological systems, sex chromosomes and restriction sites. Bull. Am. Mus. Nat. Hist. 248, 1–200. https://doi.org/10.1206/0003-0090(2000)248%3c0001:POPBM%3e2.0.CO;2 (2000).
Baker, R. J., Porter, C. A., Patton, J. C. & Van Den Bussche, R. A. Systematics of bats of the family Phyllostomidae based on RAG2 DNA sequences. Occas. Pap. Mus. Texas Tech Univ. 202, 116 (2000).
Baker, R. J., Porter, C. A., Patton, J. C. & Van Den Bussche, R. A. Diversification among New World leaf-nosed bats: An evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence. Occas. Pap. Mus. Texas Tech Univ. 230, 1–32 (2003).
Datzmann, T., von Helversen, O. & Mayer, F. Evolution of nectarivory in phyllostomid bats (Phyllostomidae Gray, 1825, Chiroptera: Mammalia). BMC Evol. Biol. 10, 165. https://doi.org/10.1186/1471-2148-10-165 (2010).
Rojas, D., Vale, A., Ferrero, V. & Navarro, L. When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Mol. Ecol. 20, 2217–2228. https://doi.org/10.1111/j.1365-294X.2011.05082.x (2011).
Camacho, M. A., Chavéz, D. & Buneo, S. F. A taxonomic revision of the Yasuni Round-eared bat, Lophostoma yasuni (Chiroptera: Phyllostomidae). Zootaxa 4114, 246–260. https://doi.org/10.11646/zootaxa.4114.3.2 (2016).
Simmons, N. B. Order Chiroptera. In Mammal Species of the World: Taxonomic and Geographic Reference 3rd edn (eds Wilson, D. E. & Reeder, D. M.) 312–529 (Johns Hopkins University Press, 2005).
Williams, S. L. & Genoways, H. H. Subfamily Phyllostominae Gray, 1825. In Mammals of South America. Marsupials, Xenarthrans, Shrews, and Bats (ed. Gardner, A. L.) 255–300 (The University of Chicago Press, 2008).
Wienberg, J. & Stanyon, R. Comparative painting of mammalian chromosomes. Curr. Opin. Genet. Dev. 7, 784–791. https://doi.org/10.1016/S0959-437X(97)80041-X (1997).
Ferguson-Smith, M. A., Yang, F. & O’Brien, P. C. M. Comparative mapping using chromosome sorting and painting. ILAR J. 39, 68–76. https://doi.org/10.1093/ilar.39.2-3.68 (1998).
Ferguson-Smith, M. A. & Trifonov, V. Mammalian karyotype evolution. Nat. Rev. Genet. 8, 950–962 (2007).
Sotero-Caio, C. G. et al. Chromosomal homologies among vampire bats revealed by chromosome painting (Phyllostomidae, Chiroptera). Cytogenet. Genome Res. 132, 156–164. https://doi.org/10.1159/252F000321574 (2011).
Sotero-Caio, C. G. et al. Chromosomal evolution among leaf-nosed nectarivorous bats-evidence from cross-species chromosome painting (Phyllostomidae, Chiroptera). BMC Evol. Biol. 13, 276. https://doi.org/10.1186/1471-2148-13-276 (2013).
Sotero-Caio, C. G. et al. Integration of molecular cytogenetics, dated molecular phylogeny, and model-based predictions to understand the extreme chromosome reorganization in the neotropical genus Tonatia (Chiroptera: Phyllostomidae). BMC Evol. Biol. 15, 220. https://doi.org/10.1186/s12862-015-0494-y (2015).
Pieczarka, J. C. et al. Reciprocal chromosome painting between two South American bats: Carollia brevicauda and Phyllostomus hastatus (Phyllostomidae, Chiroptera). Chromosome Res. 13, 339–347. https://doi.org/10.1007/s10577-005-2886-0 (2005).
Pieczarka, J. C. et al. A phylogenetic analysis using multidirectional chromosome painting of three species (Uroderma magnirostrum, U. bilobatum and Artibeus obscurus) of subfamily Stenodermatinae (Chiroptera-Phyllostomidae). Chromosome Res. 21, 383–392. https://doi.org/10.1007/s10577-013-9365-9 (2013).
Ribas, T. F. A. et al. Two new cytotypes reinforce that Micronycteris hirsuta Peters, 1869 does not represent a monotypic taxon. BMC Genet. 14, 119. https://doi.org/10.1186/1471-2156-14-119 (2013).
Ribas, T. F. A. et al. Phylogenetic reconstruction by cross-species chromosome painting and G-banding in four species of Phyllostomini tribe (Chiroptera, Phyllostomidae) in the Brazilian Amazon: an independent evidence for monophyly. PLoS ONE 10(3), e0122845. https://doi.org/10.1371/journal.pone.0122845( (2015).
Gomes, A. J. B. et al. Chromosomal evolution and phylogeny in the Nullicauda group (Chiroptera, Phyllostomidae): Evidence from multidirectional chromosome painting. BMC Evol. Biol. 18, 62. https://doi.org/10.1186/s12862-018-1176-3 (2018).
Benathar, T. C. M. et al. Karyotype, evolution and phylogenetic reconstruction in micronycterinae bats with implications for the ancestral karyotype of Phyllostomidae. BMC Evol. Biol. 19, 98. https://doi.org/10.1186/s12862-019-1421-4 (2019).
Baker, R. J. et al. (eds) Feeding Habits. Biology of Bats of the New World Family Phyllostomidae 293–350 (The Museum Texas Tech University, 1997).
Honeycutt, R. L., Baker, R. J. & Genoways, H. H. Results of the alcoa foundation-suriname expeditions III. Chromosomal data for bats (Mammallia: Chiroptera) from suriname. Ann. Carnegie Mus. 49, 237–250 (1980).
Baker, R. J., Fonseca, R. M., Parish, D. A., Phillips, C. J. & Hoffmann, F. G. New bat of the genus Lophostoma (Phyllostomidae: Phyllostominae) from Northwestern Ecuador. Occas. Pap. Mus. of Texas Tech Univ. 232, 1–16. https://doi.org/10.5962/bhl.title.156948 (2004).
Baker, R. J. & Hsu, T. C. Further studies on the sex-chromosome systems of the American leaf-nosed bats (Chiroptera, Phyllostomidae). Cytogenetics 9, 131–138 (1970).
Baker, R. J. Comparative cytogenetics of the New World leaf-nosed bats (Phyllostomidae). Period. Biol. 75, 37–45 (1973).
Baker, R. J. Karyology. In Biology of the Bats of the New World Family Phyllostomatidae Vol. 16 (eds Baker, R. J. et al.) 1107–1155 (The Museum of Texas Tech University, 1979).
Baker, R. J., Haiduk, M. W., Robbins, L. W., Cadena, A. & Koop, B. F. Chromosomal studies of south American bats their systematic implication. In Mammalian Biology in South America (eds Mares, M. A. & Genoways, H. H.) 303–327 (Pymatuning Laboratory of Ecology Pittsburg, 1982).
Baker, R. J., Genoways, H. H. & Seyfarth, P. A. Results of the alcoa foundation-suriname expeditions. VI. Additional chromosomal data for bats (Mammalia: Chiroptera) from suriname. Ann. Carnegie Mus. 50, 333 (1981).
Genoways, H. H. & Williams, S. L. Results of the alcoa foundation-suriname expeditions. I. A new species of bat of the genus Tonatia (Mammalia: Phyllostomatidae). Ann. Carnegie Mus. 49, 203–211 (1980).
Baker, R. J. & Bickham, J. W. Karyotypic evolution in bats: Evidence of extensive and conservative chromosomal evolution in closely related taxa. Syst. Zool. 29, 239–253. https://doi.org/10.1093/sysbio/29.3.239 (1980).
Baker, R. J. & Lopez, G. Chromosomal variation in bats of the genus Uroderma (Phyllostomidae). J. Mammal. 51, 786–789. https://doi.org/10.2307/1378302 (1970).
Baker, R. J. & Bass, R. A. Evolutionary relationship of the Brachyphyllinae to Glossophaginae genera Glossophaga and Monophyllus. J. Mammal. 60, 364–372. https://doi.org/10.2307/1379808 (1979).
Silva, A. M., Marques-Aguiar, S., Barros, R. M. S., Nagamachi, C. Y. & Pieczarka, J. C. Comparative cytogenetic analysis in the species Uroderma magnirostrum and U. bilobatum (cytotype 2n = 42) (Phyllostomidae, Sternodermatinae) in the Brazilian Amazon. Genet. Mol. Biol. 28, 248–253. https://doi.org/10.1590/S1415-47572005000200012 (2005).
Gomes, A. J. B., Rodrigues, L. R. R., Rissino, J. D., Nagamachi, C. Y. & Pieczarka, J. C. Biogeographical karyotypic variation of Rhinophylla fischerae (Chiroptera, Phyllostomidae) suggests the occurrence of cryptic species. Compar. Cytogenet. 4, 79–85. https://doi.org/10.3897/compcytogen.v4i1.24 (2010).
Patton, J. C. & Baker, R. J. Chromosomal homology and evolution of phyllostomatoids bats. Syst. Zool. 27, 449–462. https://doi.org/10.1093/sysbio/27.4.449 (1978).
Rodrigues, L. R. R. et al. Chromosome comparison between two species of Phyllostomus (Chiroptera-Phyllostomidae) from Eastern Amazonia, with some phylogenetic insights. Genet. Mol. Biol. 3, 593–599 (2000).
Varella-Garcia, M., Morielle-Versute, E. & Taddei, V. A. A survey of cytogenetic data on Brazilian bats. Rev. Bras. de Genet. 12, 761–793 (1989).
Santos, N. & Sousa, M. J. Use of fluorochromes chromomycin A3 and DAPI to study constitutive heterochromatin and NORs in four species of bats (Phyllostomidae). Caryologia 51, 265–278. https://doi.org/10.1080/00087114.1998.10797418 (1998).
Barros, H. M. D., Sotero-Caio, C. G., Santos, N. & Souza, M. J. Comparative cytogenetic analysis between Lonchorhina aurita and Trachops cirrhosus (Chiroptera, Phyllostomidae). Genet. Mol. Biol. 32(4), 748–752. https://doi.org/10.1590/S1415-47572009005000095 (2009).
Lee, C., Sasi, R. & Lin, C. C. Interstitial localization of telomeric DNA sequences in the Indian muntjac chromosomes: Further evidence for tandem chromosome fusions in the karyotypic evolution of the Asian muntjacs. Cytogenet. Cell Genet. 63, 156–159. https://doi.org/10.1159/000133525 (1993).
Fagundes, V., Scalzi-Martin, J. M., Hozier, K. & Yonenaga-Yassuda, Y. ZOO-FISH of microdissection DNA library and G-banding patterns reveal the homeology between the Brazilian rodents Akodon cursor and A. montensis. Cytogenet Cell Genet. 78, 224–228. https://doi.org/10.1159/000134662 (1997).
Pellegrino, K. C. M., Rodrigues, M. T. & Yonenaga-Yassuda, Y. Chromosomal evolution in the Brazilian lizards of genus Leposoma (Squamata, Gymnophthalmidae) from Amazon and Atlantic rain forests: Banding patterns and FISH of telomeric sequences. Hereditas 131, 15–21. https://doi.org/10.1111/j.1601-5223.1999.00015.x (1999).
Faria, K. C., Marchesin, S. R. C., Moreira, P. R. L., Beguelini, M. R. & Morielle-Versute, E. New insights into telomeric DNA sequence (TTAGGG)n location in bat chromosomes. Genet. Mol. Res. 8(3), 1079–1084. https://doi.org/10.4238/vol8-3gmr637 (2009).
Meyne, J. et al. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequences in vertebrate chromosomes. Chromosoma 99, 3–10. https://doi.org/10.1007/BF01737283 (1990).
Ventura, K., Silva, M. J. J., Fagundes, V., Christoff, A. U. & Yonenaga-Yassuda, Y. Non-telomeric sites as evidence of chromosomal rearrangement and repetitive (TTAGGG)n arrays in heterochromatic and euchromatic regions in four species of Akodon (Rodentia, Muridae). Cytogenet. Genome Res. 115, 169–175. https://doi.org/10.1159/000095238 (2006).
Calixto, M. S. et al. Patterns of rDNA and telomeric sequences diversification: Contribution to repetitive DNA organization in Phyllostomidae bats. Genetica 142, 49–58. https://doi.org/10.1007/s10709-013-9753-2 (2014).
Basantes, M. et al. Systematics and taxonomy of Tonatia saurophila Koopman & Williams, 1951 (Chiroptera, Phyllostomidae). ZooKeys 915, 59–86. https://doi.org/10.3897/zookeys.915.46995 (2020).
Porter, C. A., Hoofer, S. R., Van Den Bussche, R. A., Lee, T. E. & Baker, R. J. Systematic of round-eared bats (Tonatia and Lophostoma) based on nuclear and mitochondrial DNA sequences. J. Mammal. 84(3), 791–808. https://doi.org/10.1644/BME-010 (2003).
Hooffmann, F. G., Hoofer, S. R. & Baker, R. J. Molecular dating of the diversification of phyllostominae bats based on nuclear and mitochondrial DNA sequences. Mol. Phylogenet. Evol. 49, 653–658. https://doi.org/10.1016/j.ympev.2008.08.002 (2008).
Hurtado, N. & D’Elia, G. Taxonomy of the genus Gardnerycteris (Chiroptera: Phyllostomidae). Acta Chiropterol. 20(1), 99–115. https://doi.org/10.3161/15081109ACC2018.20.1.007 (2018).
Velazco, P. M. & Cadenillas, R. On the identity of Lophostoma silvicolum occidentalis (Davis & Carter, 1978) (Chiroptera: Phyllostomidae). Zootaxa 2962, 1–20. https://doi.org/10.11646/ZOOTAXA.2962.1.1 (2011).
Lee, T. E., Hoofer, S. R. & Van Den Bussche, R. A. Molecular phylogenetics and taxonomic revision of the genus Tonatia (Chiroptera: Phyllostomidae). J. Mammal. 83(1), 49–57. https://doi.org/10.1644/1545-1542(2002)083%3c0049:MPATRO%3e2.0.CO;2 (2002).
Baker, R. J., Hamilton, M. & Parish, D. A. Preparations of Mammalian karyotypes under field conditions. Occas. Pap. Mus. Texas Tech Univ. 228, 1–7. https://doi.org/10.5962/bhl.title.1156987 (2003).
Seabright, M. A rapid banding technique for human chromosomes. Lancet 2, 971–972. https://doi.org/10.1016/s0140-6736(71)90287-x (1971).
Verma, R. S. & Babu, A. Human Chromosomes: Principles and Techniques 2nd edn. (Mcgraw-Hill Inc, 1995).
Sumner, A. T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 75, 304–306. https://doi.org/10.1016/0014-4827(72)90558-7 (1972).
Hatanaka, T. & Galetti, P. M. Jr. Mapping of 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica 122, 239–244. https://doi.org/10.1007/s10709-004-2039-y (2004).
Telenius, H. et al. Cytogenetic analysis by chromosome painting using DOP-PCR amplified flow-sorted chromosomes. Genes Chromosomes Cancer 4, 257–263. https://doi.org/10.1002/gcc.2870040311 (1992).
Yang, F., Carter, N. P., Shi, L. & Ferguson-Smith, M. A. A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma 103, 642–652. https://doi.org/10.1007/BF00357691 (1995).
Swofford, D. L. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4a (Build 169) (Sinauer Associates Sunderland, 2002).
Acknowledgements
The authors are grateful to members of Sapopema, Conservación Internacional do Brasil, Rio and Aotus Consultoria Ambiental for their logistical support for the collection of samples. To Dr. Anderson José Baia Gomes for suggestions and help with the phylogenetic analysis; to MSc. Jorge Rissino, to MSc. Shirley Nascimento and Maria da Conceição Mandú for assistance in laboratory work.
Funding
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support on a project coordinated by CYN (Edital Pró-Amazônia Proc 047/2012); the FAPESPA for financial support (Edital Vale—Proc 2010/110447) and Banco Nacional de Desenvolvimento Econômico e Social—BNDES (2.318.697.0001) on a project coordinated by JCP; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support on Productivity Grants by CYN (305880/2017-9) and JCP (305876/2017-1). This study is part of the Doctoral Thesis in Genetic and Molecular Biology of NKNS who is recipient of a CNPq Doctor Scholarship.
Author information
Authors and Affiliations
Contributions
N.K.N.S.: Conceptualization; Data Curation; Formal analysis; Investigation; Methodology; Visualization; Writing original draft; Writing review and editing. C.Y.N.: Data Curation; Formal analysis; Funding acquisition; Resources; Visualization; Writing review and editing. L.R.R.R.: Formal analysis; Investigation; Writing review and editing. P.C.M.O.: Investigation; Methodology; Visualization; Writing review and editing. F.Y.: Investigation; Methodology; Writing review and editing. M.A.F.-S.: Investigation; Methodology; Resources; Visualization; Writing review and editing. J.C.P.: Data Curation; Formal analysis; Funding acquisition; Project administration; Resources; Supervision; Visualization; Writing review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Silva, N.K.N., Nagamachi, C.Y., Rodrigues, L.R.R. et al. Chromosome painting and phylogenetic analysis suggest that the genus Lophostoma (Chiroptera, Phyllostomidae) is paraphyletic. Sci Rep 12, 19514 (2022). https://doi.org/10.1038/s41598-022-21391-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21391-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.