Abstract
Adequate intake of micronutrients is necessary to reduce widespread health issues linked to low intake of iron (Fe), zinc (Zn), boron (B), copper (Cu), and manganese (Mn). Because more than two billion people suffer from micronutrient deficiency globally, to address this problem, highly-nutritious ancestral Peruvian crops like tarwi can be an important component of food security. Thus, our work explores the tarwi micronutrient variability to select biofortified genotypes without affecting seed size and weight. Tarwi is a biofortified food because of its seeds' Fe, Zn, and B content. Furthermore, Boron showed a positive correlation between seed size and weight. At the same time, copper showed a negative correlation. Finally, six accessions (P14, P16, P21, T05, T08, and T25) that are biofortified for Fe, Zn, and B with excellent seed size and weight and with adequate levels of Cu and Mn; adding value to Peruvian biodiversity at a low cost is a starting point for a breeding program to prevent micronutrient disorders.
Similar content being viewed by others
Introduction
Tarwi (Lupinus mutabilis Sweet) is a legume cultivated in South American Andes, distributed from 2500 to 4500 m above sea level1. Its domestication occurred between 1800 and 3450 BP, and a cultivated area of 10,000 ha was estimated in Peru ca. 450 BP2. During the colonial and republican times, its consumption decreased and was used only by indigenous communities, maintaining genetic variability until today3.
In recent years, tarwi has positioned itself as a superfood because of its high protein and fatty acid content, comparable to soybean4, which could make it an excellent replacement for animal protein5,6. Unlike other legumes, tarwi is starchless, which is beneficial in reducing the risk of obesity, diabetes, and cardiovascular disease7. Medicinally, tarwi contains quinolizine alkaloids, which are toxic secondary metabolites8. However, quinolizine alkaloids have antileishmanial and antitrypanosomal properties9, whereas sparteine showed in vitro antimicrobial activity against Mycobacterium phlei10 and Mycobacterium tuberculosis11. On the other hand, mature tarwi seeds contain conglutin gamma peptides that were able to increase glucose uptake in patients with type 2 diabetes12,13. Due to these characteristics, the Peruvian government, through Promperu, pointed out that Peruvian Andean grains such as Tarwi have a tremendous potential demand among the APEC economies. In addition, tarwi is a priority in Bolivia, Ecuador, and Chile, while Argentina and Colombia have a medium priority14.
On the other hand, Antúnez de Mayolo15 reported that the diet during the Inca empire was balanced. However, changes in eating habits caused during the colonial and republican times and the lack of foresight of the Peruvian government in food security and nutrition have directly affected the nutritional level of children under five years. According to FAO16, in 1996, the highest prevalence of chronic malnutrition in children under five years was found in rural areas (40.4%), in the sierra (37.8%), in the jungle (33.0%), and in the departments of Huancavelica (50.3%), Pasco (47.2%), Apurímac (46.9%), Ayacucho (43.2%) and Cusco (40.9%). In 2000, Peru reached levels of chronic malnutrition of 33% in children under five years17. Currently, in Peru, 40.1% of children from 6 to 35 months suffer from anemia; almost 700 thousand children under three years of age are anemics out of 1.6 million nationwide18. In this sense, anemia in Peru continues to be a public health problem, mainly affecting children and pregnant women. WHO19 estimates that 42% of children under five and 40% of pregnant women are anemics.
Additionally, the most common micronutrient deficiencies among women and children are associated with deficiencies in calcium, iodine, iron, selenium, and zinc20. Micronutrient deficiency malnutrition is a global problem affecting more than two billion people. Developing countries are the most affected because of the low quality of their diet, characterized by high consumption of carbohydrates but low consumption of vegetables, fruits, animal products, and fish products, which are rich sources of minerals21. Traditional strategies to deliver these nutrients have relied primarily on mineral supplementation, dietary diversification, and food fortification22. An alternative solution to mineral malnutrition is biofortification, which has been defined as increasing the concentration and/or bioavailability of essential elements in the edible portions of crop plants through agronomic intervention or genetic selection23,24,25. It can be used mainly in developing countries to give extra value to its biodiversity at a low cost.
In this sense, highly-nutritious ancestral Peruvian crops like tarwi could play a significant role in food systems to address food security and reach hunger Zero. Nevertheless, the information on Peru's micronutrient composition of tarwi Genbank is scarce. Thus, the corresponding hypothesis evaluated the positive correlation between micronutrient content and tarwi seed traits (seed length, width, and weight of 100 seeds). The objectives of this study were to evaluate 45 tarwi accessions (1) to determine the iron (Fe), zinc (Zn), Boron (B), copper (Cu), and manganese (Mn) seed content; (2) to measure the phenotypic variations in seed length, seed width, and weight of 100 seeds and (3) to assess the correlation between micronutrient content with seed length, seed width, and weight of 100 seeds.
Results and discussions
Micronutrient profile of Lupinus mutabilis
It is critical to have high variability among micronutrients to identify biofortified genotypes for Fe, Zn26, B27, Cu, and Mn28. Thus, Fig. 1A shows greater Fe, Zn, B, Cu, and Mn variability in L. mutabilis. Moreover, there was a higher Fe, B, Cu, and Mn content variability in the early-flowering accessions, while Zn variability was higher in late-flowering genotypes (Fig. 1A and Table S2). Additionally, there were statistically significant differences in the accessions' micronutrient concentrations of Fe, Zn, B, Cu, and Mn (Tables 1 and 2). For early-flowering accessions, the Fe concentration was between 46.67 and 88 mg kg−1, whereas the range for late-flowering accessions was 44.67–70.33 mg kg−1. Without employing a plant breeding strategy, the seed iron concentration in L. mutabilis exceeds 40 mg kg−1 of Fe29; it can be considered a biofortified food. The highest Fe concentration observed in genotypes P03 (Table 1) and T05 (Table 2) was higher than that reported by Sanca30, Rodriguez31, and Villacres et al.32. In contrast, the P03 accession was only higher than Villacres et al.33. However, the iron concentrations reported by Ortega-David et al.34 were superior to this study.
The Zn concentration in early-flowering accessions was between 35.67 and 52.67 mg kg−1 and for late-flowering accessions between 35.33 and 48.67 mg kg−1, being considered as Zn-biofortified29,35 as well. P07 and T15 genotypes showed the highest Zn concentrations (Tables 1 and 2). The Zn content reported in L. mutabilis by Villacres et al.33, Rodriguez31, and Ortega-David et al.34 were lower than those of P07 and T15. However, the higher Zn concentrations over P07 and T15 were reported by Sanca30 and Villacres et al.32.
The high levels of Fe and Zn observed in tarwi (Fig. 1A) could be explained by Zhao et al.36 work. He suggests a possible link between grain protein and the levels of the two trace elements under the control of a NAC transcription factor (NAM-B1) that accelerates senescence and increases the remobilization of nutrients (N, Fe, and Zn) from leaves to developing grains37. Though Zhao's findings need to be validated in tarwi, they are relevant to this study because the tarwi seed protein content is higher than 40%6.
Due to massive reports on B-deficiency in cropping systems38, biofortification is a sustainable option to increase the yield and quality of crops39. Boron levels in food are critical because it is involved in the formation and hardness of bone structure40. Under this consideration, Boron concentration in the late and early-flowering accessions was between 20.33 and 29.33 mg kg−1 and 16.67 and 34 mg kg−1, respectively (Tables 1 and 2), where T15 and P12 are unique genotypes to generate B biofortified cultivars. Although the T15 genotype showed high levels of B and Zn, its iron level was 55.33 mg kg−1. Since the interaction between B and Zn affects Fe transport and Fe content in organs41.
Despite the significant variability in Cu content, genetic selection should focus on a balanced intake of this micronutrient (≤ 10 mg kg−1) because consuming foods rich in Cu may increase the incidence of neurodegenerative pathologies like Alzheimer's42. According to Schilsky43, the adequate concentration of Cu within a balanced diet should not be higher than 11 mg kg−1. Thus, Cu concentration in the late and early-flowering groups of tarwi is between 7 and 11 mg kg−1 and 5 and 10.67 mg kg−1, respectively (Table 1 and 2). According to the Schilsky43 criterion, T08, T12, T14, P03, and P07 genotypes have an adequate Cu concentration for a balanced diet, unlike tarwi genotypes studied by Villacres et al.33, Rodriguez31 and Ortega-David et al.34.
Like Cu, a high intake of manganese can cause neurodegenerative problems44. According to Falah et al.45, the Mn content in a balanced diet should contain 18 mg kg−1 for women and 22 mg kg−1 for men. Tarwi Mn content was between 20.67 and 47.67 mg kg−1 (Tables 1 and 2), where the late-flowering genotypes T03 and T04 presented the lowest Mn concentration (Table 2). However, the highest Mn concentrations were observed in P09 (47.67 mg kg−1), P22 (42.33 mg kg−1), T10 (39.67 mg kg−1), T12 (39 mg kg−1), T13 (41.67 mg kg−1) and P07 (40.67 mg kg−1) accessions, see Table 1 and 2. Similarly, Ortega-David et al.34 reported genotypes with higher concentrations of Mn in L. mutabilis.
In contrast, Rodriguez31 reported accessions with lower amounts of Mn for this species. On the other hand, Villacres et al.33 affirm that the Mn content can be reduced by up to 50% during the debittering process of seeds, turning them into safe foods45. In this sense, the maximum Mn concentration in seeds without debittering should not exceed 36 mg.kg−1 to be included in a balanced diet. Furthermore, more than 90% of absorbed manganese is mainly excreted via the bile into the feces46.
Seed morphological traits
Since crop domestication, the yield has been one of the most important agronomic traits in plant breeding47. Yield components include seed traits such as seed size (SL and SW) and 100-SW48; these characteristics showed considerable variability in early-flowering accessions than in late-flowering accessions (Fig. 1B,C) and were statistically significant differences for each group (Table 1 and 2). In tarwi, there is a high correlation between 100-SW and yield49. The 100-SW ranged from 20.80 to 30.64 g for the early-flowering genotypes (Table 1) and 18.31 to 27.61 g for late-flowering genotypes (Table 2), with the accessions P14, P16, P21, and T12 exhibiting the largest 100-SW. Furthermore, tarwi 100-SW was higher than Lupinus angustifolius L., Lupinus cosentinii G., Lupinus digitatus F., Lupinus hispanicus B&R., Lupinus luteus L. and Lupinus micranthus G.50. However, our findings on 100-SW were similar to Aguilar-Angulo51 but higher than De La Cruz52, Huisa53, Cayo54, Buircell and Cowling55, Plata56, Mendoza57, Aguilar58, Aquino59, Atchison et al.2 and Barda et al.60.
Seed size is an important agronomic trait because of its importance for consumers and industry61. Thus the genotypes with large seed sizes (AS and LS) were P14, P16, T02, and T22 (Tables 1 and 2). This pattern was also observed in eight genotypes by Cayo54. Additionally, P14 and P16 genotypes displayed excellent seed morphological characteristics (AS, LS, and 100-WS) with a high content of micronutrients (Fig. 1A), demonstrating the crop's excellent potential for food and feed industries.
Selection of tarwi accessions
The first three components in the principal component analysis (PCA) account for 72.2% of the total variability (Table 3). The weight of 100 seeds (100-SW), seed length (SL), seed width (SW), and Boron content (B) are related to PC1 (41.2%). The Mn and Zn contents are closely related to PC2 (17.4%), and the iron content (Fe) is closely related to PC3 (13.6%). In PC1, the morphological characteristics showed a high correlation with B (Table 3, Fig. 2, and Table S3), which can be attributed to B's role in seed development and size62, which can boost crop yields63. The high B concentration in L. mutabilis may be due to the high efficiency of B transport to the seeds. Furthermore, B can modulate the absorption and translocation of nutrients to the seed64, allowing a higher absorption of Fe, Zn, and Cu65 but less in Mn66.
However, the relationship between Cu and seed size was inverse and significant; because, in high concentrations, copper reduces the seed size, impacting grain yield and quality67. However, the only two micronutrients with a significant and positive correlation were Mn and Zn (Table S3), and Biplot-PCA corroborates it (Fig. 2). This may show a synergistic effect between these two micronutrients68, suggesting a cotransport of Mn and Zn.
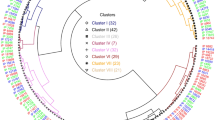
Since 100-WS is highly associated with yield in L. mutabilis49 and seed size can affect it, the Mahalanobis cluster analysis was used to select the best genotypes. Thus, the late-flowering accessions were divided into 12 accessions with 100-SW > 23 g and 13 accessions with 100-SW < 23 g (Fig. 3A). Simultaneously, 20 early-flowering accessions were divided into two subgroups, 2 genotypes with 100-SW < 21 g and 18 genotypes with 100-SW > 21 g (Fig. 3C). To narrow down the selection, results based on the micronutrient criteria (Fe > 40 mg kg−1, Zn > 28 mg kg−1, B > 13 mg kg−1, Cu < 9 mg kg−1 and Mn < 30 mg kg−1) and a mean coefficient of variation of micronutrients (MCVmicronutrient ≤ 10%) were used and allowed to select three late-flowering accessions (T05, T08 and T25) and three early-flowering accessions (P14, P16 and P21) that were biofortified for Fe, Zn and B with adequate nutritional requirements in Cu and Mn and excellent seed size and weight (Fig. 3C,D and Table S4), unlike than Villacres et al.33, Rodríguez31 and Ortega-David et al.34. Furthermore, a MCVmicronutrient value of ≤ 10% among the selected accessions could be due to a high specificity of the proteins associated with the absorption, translocation, remobilization and/or storage of these micronutrients. Therefore, a better understanding of these processes is required to explain the micronutrient homeostasis of tarwi. Moreover, our data demonstrated that the selection of micronutrient-rich tarwi genotypes was achieved without affecting the morphological traits of the seeds (Fig. 3 and Table S4). Finally, its unique micronutrient levels make it a suitable grain to be included in a healthy diet.
Cluster analysis and selection of late-flowering and early-flowering tarwi genotypes. (A and C) cluster analysis and selected material according to their micronutrient content (Fe > 40 mg kg−1, Zn > 28 mg kg−1, B > 13 mg kg−1, Cu < 9 mg kg−1 and Mn < 30 mg kg−1) and mean-coefficient of variation for micronutrient (MCVmicronutrient) ≤ 10% (B and D) description of the best genotypes.
Materials and methods
Plant material
In 2014, the regeneration of the tarwi Genbank was carried out in the Santa Ana experimental station at INIA-Huancayo. The INIA data on days to flowering was taken when the plot was at 50% of flowering. Thus, 20 early-flowering (86–122 days) and 25 late-flowering (140–166 days) accessions of Lupinus mutabilis S. used in this study were given in 2015 to Universidad Nacional Agraria La Molina under a material transfer agreement (ATMG 001–2015). The germplasm used was collected in Cuzco, Cajamarca, Ancash, Junin and Huanuco. Finally, Lupin Descriptors69 was used to characterize tarwi seed (Table S1). All the measurements for this study were done between 2019 and 2020 at Universidad Nacional Agraria La Molina.
Sample preparation
All samples were weighted with a Henkel balance (± 0.01) and dried until constant weight in an oven at 70 °C. Dry samples were ground with a Thomas Model 4 Wiley® Mill (Thomas Scientific) until a homogeneous mass was obtained. All samples were kept in sealed containers to avoid contact with atmospheric humidity.1 g of ground sample was weighed with an OHAUS PA313 balance (± 0.001 g) and used for sample digestion.
Analysis of the micronutrients profile (Fe, Zn, B, Cu, and Mn) and seed size and weight
In L. mutabilis seeds, Fe, Zn, Cu, and Mn concentrations were measured by the wet digestion method70. The samples were digested in a nitroperchloric mixture and measured by atomic absorption spectrometry (Perkin Elmer Analyst 200). The curcumin-acetic acid method72 determined boron (B) concentration. The micronutrient concentration was expressed in mg kg−1.
However, seed length (SL), seed width (SW), and the weight of 100 seeds (100-SW) were determined according to Pereira et al.71. Seed size was expressed in millimeters (mm), and the weight of 100 seeds in grams (g). All experiments were done in triplicate. The Pearson correlation analysis was performed in Excel to determine the relationship between variables. Finally, boxplots, bar-graphs, mean, standard deviation, and coefficient of variation (CV %) were calculated using the statistical InfoStat analysis system (www.infostat.com.ar) software. Duncan’s multiple range test was used to separate the differences in the mean scores at a significance level of P < 0.05.
Selection of tarwi accessions
The data in early and late-flowering genotypes of L. mutabilis (Table S2) were standardized to estimate the genetic distance matrix using the Euclidean distance72. The analysis was carried out using InfoStat software (www.infostat.com.ar), and PCA and Biplot of PCA consistency were verified by cophenetic correlation coefficient73.
To identify the best traits, the cluster analysis was done first, where Mahalanobis distance was used as the distance matrix74 with InfoStat software (www.infostat.com.ar); and to narrow the selection results based on healthy eating, the micronutrient criteria: Fe > 40 mg kg−1 29, Zn > 28 mg kg−1 29, B > 13 mg kg−1 75, Cu < 9 mg kg−1 76, and Mn < 30 mg kg−1 77 and mean-coefficient of variation for micronutrient (MCVmicronutrient) of less or equal than 10%78 were used.
Conclusions
Without employing a plant breeding strategy, Fe, Zn, and B content in tarwi seeds exceed the 40 mg kg−1, 28 mg kg−1, and 13 mg kg−1, respectively, and it can be considered biofortified food. Moreover, Boron stands out as an important micronutrient because of its positive correlation with seed size and seed weight because B is involved in the development and size of the seeds. However, Cu and seed size showed a negative correlation, helping the selection of genotypes with good seed morphological traits and adequate Cu concentration (≤ 10 mg kg−1). These results do provide partial validation of the hypothesis.
100-WS, along with micronutrient criteria for healthy eating and MCVmicronutrient ≤ 10% allowed to select six accessions (P14, P16, P21, T05, T08, and T25) that were biofortified for Fe, Zn and B with excellent seed size and weight and adequate levels of Cu and Mn. These genotypes will serve as the basis for a breeding program to boost micronutrient content, which will help avoid micronutrient problems and reduce healthcare expenditures by promoting healthy eating. Lastly, fostering well-being and ensuring healthy eating/living is crucial for sustainable development. Biofortification has a crucial role in reducing health costs by promoting healthy eating. However, it has limitations because the technology does not address the high-cost analysis to evaluate large seed banks in megadiverse countries. Thus, other technologies such as Micro-XRF and benchtop TXRF spectrometers could be an alternative to reduce analysis costs and build a high-throughput mineral-nutrient phenotyping platform.
Data availability
All relevant data are within the paper and its supporting Information files.
References
Taco-Taype, N. & Zúñiga-Dávila, D. Efecto de la inoculación de plantas de Tarwi con cepas de Bradyrhizobium spp. aisladas de un lupino silvestre, en condiciones de invernadero. Revista peruana de biología. 27, 35–42 (2022).
Atchison, G. W. et al. Lost crops of the Incas: Origins of domestication of the Andean pulse crop tarwi Lupinus mutabilis. Am. J. Bot. 103, 1592–1606 (2016).
Peru Origins. Tarwi (Lupinus Mutabilis). https://peruorigins.com/tarwi/ (2022).
Guilengue, N., Alves, S., Talhinhas, P. & Neves-Martins, J. Genetic and genomic diversity in a tarwi (Lupinus mutabilis Sweet) germplasm collection and adaptability to Mediterranean climate conditions. Agronomy 10, 21 (2020).
Repo-Carrasco-Valencia, R., Basilio-Atencio, J., Luna-Mercado, G. I., Pilco-Quesada, S. & VidaurreRuiz, J. Andean ancient grains: Nutritional value and novel uses. Biol. Life Sci. Forum. https://doi.org/10.3390/blsf2021008015 (2022).
Gulisano, A., Alves, S., Martins, J. N. & Trindade, L. M. Genetics and breeding of Lupinus mutabilis: An emerging protein crop. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.01385 (2019).
Chen, Y., She, Y., Zhang, R., Wang, J. & Zhang, X. Use of starch-based fat replacers in foods as a strategy to reduce dietary intake of fat and risk of metabolic diseases. Food Sci. Nutr. 8, 16–22 (2020).
Frick, K. M., Kamphuis, L. G., Siddique, K. H. M., Singh, K. B. & Foley, R. C. Quinolizidine alkaloid biosynthesis in lupins and prospects for grain quality improvement. Front. Plant Sci. https://doi.org/10.3389/fpls.2017.00087 (2017).
Chirinos-Arias, M. C. Andean Lupin (Lupinus mutabilis Sweet) a plant with nutraceutical and medicinal potential. Revista Bio. Ciencias. 3, 163–172 (2015).
Wink, M. Chemical defense of leguminosae. Are quinolizidine alkaloids part of the antimicrobial defense system of lupins?. Zeitschrift für Naturforschung C. 39, 548–552 (1984).
Hidalgo, M. et al. Evaluation of in vitro suceptibility to spartein in four strain of Mycobacterium tuberculosis. Rev. Peru Med Exp Salud Publica. 39, 77–82 (2022).
Muñoz, E. B., Luna-Vital, D. A., Fornasini, M., Baldeón, M. E. & Gonzalez de Mejia, E. Gamma-conglutin peptides from Andean lupin legume (Lupinus mutabilis Sweet) enhanced glucose uptake and reduced gluconeogenesis in vitro. J. Funct. 45, 339–347 (2018).
Bryant, L., Rangan, A. & Grafenauer, S. Lupins and health outcomes: A systematic literature review. Nutrients 14, 327 (2022).
Jacobsen, S. & Mujica, A. Geographical distribution of the Andean lupin (Lupinus mutabilis Sweet). Plant Genet. Resour. Newslett. 155, 1–8 (2008).
Antunez de Mayolo, S. Nutricion en el antiguo Peru. Banco Central de la Republica. Lima, Peru. 127 (1981).
FAO. Perfiles nutricionales por paises: Peru. (ed. FAO) 36 p. (2000).
UNICEF. Estado Mundial de la Infancia 2019 incluye a Perú entre las experiencias exitosas de lucha contra la desnutrición crónica infantile. https://www.unicef.org/peru/nota-de-prensa/estado-mundial-infancia-nutricion-alimentos-derechos-peru-experiencias-exitosas-desnutricion-cronica-infantil-reporte (2022).
MINSA (Ministry of health - Peru). Situacion actual de la anemia. https://anemia.ins.gob.pe/situacion-actual-de-la-anemia-c1 (2022).
WHO. Anemia. https://www.who.int/es/health-topics/anaemia#tab=tab_1 (2022).
Galani, Y. J. H., Orfila, C. & Gong, Y. Y. A review of micronutrient deficiencies and analysis of maize contribution to nutrient requirements of women and children in Eastern and Southern Africa. Crit. Rev. Food Sci. Nutr. 62, 1568–1591 (2022).
White, P. J. & Martin, R. B. Biofortifying crops with essential mineral elements. Trends Plant Sci. 10, 586–593 (2005).
White, P. J. & Martin, R. B. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182, 49–84 (2009).
Waters, B. M. & Sankaran, R. P. Moving micronutrients from the soil to the seeds: genes and physiological processes from a biofortification perspective. Plant Sciences. 180, 562–574 (2011).
Brooker, R. W. et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 206, 107–117 (2015).
Ducsay, L. et al. Possibility of selenium biofortification of winter wheat grain. Plant Soil Environ. 62, 379–383 (2016).
Kumar, S. & Pandey, G. Biofortification of pulses and legumes to enhance nutrition. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e03682 (2020).
Diehn, T. A. et al. Boron demanding tissues of Brassica napus express specific sets of functional Nodulin26-like Intrinsic Proteins and BOR 1 transporters. Plant J. 100, 68–82 (2019).
Jayalakshmi, V. A., Reddy, T. & Nagamadhuri, K. V. Genetic diversity and variability for protein and micro nutrients in advance breeding lines and chickpea varieties grown in Andhra Pradesh.". Legume Res. Int. J. 42, 768–772 (2019).
Bouis, H. & Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob Food Sec. 12, 49–58 (2017).
Sanca, D. Composición nutricional de diez genotipos de lupino (L. mutabilis y L. albus) desamargados por proceso acuoso. Thesis. Universidad Nacional Agraria La Molina. (2015).
Rodríguez, A. Evaluación “in vitro” de la actividad antibacteriana de los alcaloides del agua de desamargado del chocho (Lupinus mutrabilis Sweet). Thesis. Escuela Superior Politécnica de Chimborazo, Ecuador (2009).
Villacres, E. et al. Germination, an effective process to in-crease the nutritional value and reduce non-nutritive factors of lupine grain (Lupinus mutabilis Sweet). Int. J. Food Sci. Nutr. Eng. 5, 163–168 (2015).
Villacres, E., Rubio, A., Egas, L., Segovia, G. Usos alternativos del chocho: Chocho (Lupinus mutabilis Sweet) alimento andino redescubierto. IOP publishing: repositorio. https://repositorio.iniap.gob.ec/handle/41000/298 (2006).
Ortega-David, E. A., Rodríguez, A. D. & Burbano, A. Z. Caracterización de semillas de lupino (Lupinus mutabilis) sembrado en los Andes de Colombia. Acta Agronómica. 59, 111–118 (2010).
White, P. J. & Broadley, M. R. Physiological limits to zinc biofortification of edible crops. Front Plant Sci. 80, 1–11 (2011).
Zhao, F., Su, Y. H., Dunham, S. J. & Rakszegiet, M. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 49, 290–295 (2009).
Uauy, C., Distelfeld, A., Fahima, T., Blechl, A. & Dubcovsky, J. A NAC Gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 24, 1298–1301 (2006).
Shorrocks, V. M. The occurrence and correction of boron deficiency. Plant Soil 193, 121–148 (1997).
D’Imperio, M. et al. Boron biofortification of Portulaca oleracea L. through soilless cultivation for a new tailored crop. Agronomy. 10, 999–1013 (2020).
Boyacioglu, O., Orenay-Boyacioglu, S., Yildirim, H. & Korkmaz, M. Boron intake, osteocalcin polymorphism and serum level in postmenopausal osteoporosis. J. Trace Elem. Med. Biol. 48, 52–56 (2018).
Oliveira Araújo, E., Ferreira Dos Santos, E. & Camacho Oliveira, M. A. Boron-zinc interaction in the absorption of micronutrients by cotton. Agronomía Colombiana. 36, 51–57 (2018).
Squitti, R., Siotto, M. & Polimanti, R. Low-copper diet as a preventive strategy for Alzheimer’s disease. Neurobiol. Aging 2, 40–50 (2014).
Schilsky, M.L. Management of Wilson Disease (A Pocket Guide), 1st ed.; Publisher: Humana Press, Farmington, CT, USA. 154–196 (2018).
Martins, A. C. et al. Manganese in the diet: Bioaccessibility, adequate intake, and neurotoxicological effects. J. Agric. Food Chem. 46, 12893–12903 (2020).
Falah, S. A. & Saja, N. M. Essential trace elements and their vital roles in human body. Indian J. Adv. Chem. Sci. 3, 127–136 (2017).
National institutes of health. Manganese. Fact Sheet for Health Professionals. IOP Publishing ods.od.nih.gov. https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional/. (2021).
Savadi, S. Molecular regulation of seed development and strategies for engineering seed size in crop plants. Plant Growth Regul. 84, 401–422 (2018).
Ge, L. et al. (2016) Increasing seed size and quality by manipulating BIG SEEDS1 in legume species. Proc Natl Acad Sci. 113, 12414–12419 (2016).
Zou, L. Effects of gradual and sudden heat stress on seed quality of Andean lupin, Lupinus mutabilis. Thesis. University of Helsinki. https://helda.helsinki.fi/handle/10138/16501 (2009).
Buircell, B.J., Cowling, A.W. Genetic Resources in Lupins (eds. Gladstones, J.S., Atkins, C.A., Hamblin, J.) (United Kingdom: CAB International, 1998).
Aguilar-Angulo, L. A. Evaluación del rendimiento de grano y capacidad simbiótica de once accesiones de tarwi (Lupinus mutabilis Sweet), bajo condiciones de Otuzco-La Libertad (Universidad Nacional Agraria La Molina, 2015).
De La Cruz, N. Caracterización fenotípica y de rendimiento preliminar de ecotipos de tarwi (Lupinus mutabilis sweet), bajo condiciones del Callejón de Huaylas – Ancash (Universidad Nacional Agraria la Molina, 2018).
Huisa, J. Evaluación del comportamiento agronómico de catorce accesiones del ensayo nacional de tarwi (Lupinus mutabilis sweet.) en el CIP Camacani Puno – Perú”. Thesis. Universidad Nacional Agraria la Moina (2018).
Cayo, B. Evaluación del comportamiento agronómico de ocho genotipos selectos de tarwi (Lupinus mutabilis sweet) bajo condiciones del CIP. CAMACANI - UNA – PUNO. Thesis. Universidad Nacional del Altiplano (2020).
Buircell, B.J., Cowling, A.W. Lupin. Lupinus spp. Promoting the conservation and use of underutilized and ne-glected crops (eds. Gladstones, J.S., Atkins, C.A., Hamblin, J.) (United Kingdom: CAB International, 1998).
Plata, J. Comportamiento Agronómico de dos Variedades de tarwi (Lupinus mutabilis Sweet), bajo tres densidades de siembra en la comunidad Marka Hilata Carabuco (Universidad San Andres, 2016).
Mendoza, C. Rendimiento de ecotipos regionales y variedades de tarwi (Lupínus mutabilis Sweet.) en el valle del Mantaro, Jauja, Junín. Thesis. Universidad Nacional Agraria la Moina (2020).
Aguilar, S. Sistemas de producción de Lupinus mutabilis Sweet ‘chocho’ en terrazas y laderas con fertilización fosfatada en Cajamarca. Dissertation. La Molina National Agrarian University (2011).
Aquino, S. Sustentabilidad del cultivo de tarwi (Lupinus mutabilis sweet) en la zona altoandina del Valle del Mantaro (Universidad Nacional Agraria la Molina, 2018).
Barda, M. S., Chatzigeorgiou, T., Papadopoulos, G. K. & Bebeli, P. J. Agro-morphological evaluation of Lupinus mutabilis in two locations in greece and association with insect pollinators. Agriculture https://doi.org/10.3390/agriculture11030236 (2021).
Herniter, I. A., Jia, Z. & Kusi, F. Market preferences for cowpea (Vigna unguiculata [L.] Walp) dry grain in Ghana. African J Ag Res. 14, 928–934 (2019).
Dordas, C. Foliar boron application affects lint and seed yield and improves seed quality of cotton grown on calcareous soils. Nutr. Cycl. Agroecosyst. 76, 19–28 (2006).
Kristek, S. et al. Effect of various rates of boron on yield and quality of high-grade sugar beet varieties. Listy Cukrovarnické a Řepařské. 4, 146–150 (2018).
Thomas, C. L. et al. Root morphology and seed and leaf ionomic traits in a Brassica napus L. diversity panel show wide phenotypic variation and are characteristic of crop habit. BMC Plant Biol. 16, 214–232 (2016).
Dursun, A. et al. Effects of boron fertilizer on tomato, pepper and cucumber yields and chemical composition. Commun Soil Sci Plant Anal. 1, 1576–1593 (2010).
Sotiropoulos, T. E., Therios, T. N., Dimassi, K. N., Bosabalidis, A. & Kofidis, G. Nutritional status, growth, CO2 assimilation, and leaf anatomical responses in two kiwifruit species under boron toxicity. J Plant Nutr. 25, 1249–1261 (2002).
Muccifora, S. & Bellani, L. Effects of copper on germination and reserve mobilization in Vicia sativa L. seeds. Environ. Pollut. 179, 68–74 (2013).
Kobraee, S. Effect of foliar fertilization with zinc and manganese sulfate on yield, dry matter accumulation, and zinc and manganese contents in leaf and seed of chickpea (Cicer arietinum). J. Appl. Biol. Biotechnol. 7, 20–28 (2019).
IBPGR (1981) Lupin descriptors. https://www.bioversityinternational.org/fileadmin/bioversity/publications/Web_version/103/ (1981).
Zasoski, R. J. & Burau, R. G. A rapid nitric-perchloric acid digestion method for multi-element tissue analysis. Commun. Soil Sci. Plant Anal. 8, 425–436 (1997).
Pereira, T., Coelho, C. M. M., Bogo, A., Guidolin, A. F. & Miquelluti, D. J. Diversity in common bean landraces from south Brazil. Acta Bot. Croat. 1, 79–92 (2009).
Pujar, M., Govindaraj, M., Gangaprasad, S., Kanatti, A. & Shivade, H. Genetic variation and diversity for grain iron, zinc, protein and agronomic traits in advanced breeding lines of pearl millet [Pennisetum glaucum (L.) R Br] for biofortification breeding. Genet. Resour. Crop Evol. 67, 2009–2022 (2020).
Lira, J. P. E. et al. Safflower genetic diversity based on agronomic characteristics in Mato Grosso state, Brazil, for a crop improvement program. Genet. Mol. Res. 1, 1–12 (2021).
de Sá, S. F. et al. Genetic diversity via REML-BLUP of ex situ conserved macauba [Acrocomia aculeata (Jacq.) Lodd. ex Mart.] ecotypes. Genet. Resour. Crop Evol. 68, 3193–3204 (2021).
Kuru, R., Yilmaz, S., Tasli, P. N., Yarat, A. & Sahin, F. Boron content of some foods consumed in Istanbul, Turkey. Biol. Trace Elem. Res. 187, 1–8 (2019).
Shokunbi, O., Adepoju, O., Mojapelo, P., Ramaite, I. & Akinyele, I. Copper, manganese, iron and zinc contents of Nigerian foods and estimates of adult dietary intakes. J. Food Compos. Anal. 82, 103–245 (2019).
Norwegian scientific committee for food and environment. Assessment of dietary intake of manganese in rela-tion to tolerable upper intake. IOP Publishing wkm. www.vkm.no. (2018).
Gil, V., Guzmán, L. & Quintero, E. Caracterización de la variabilidad morfológica de un “genotipo local” de maíz y dos de sus selecciones. Centro Agrícola. 4, 79–83 (2004).
Acknowledgment
The authors would also like to thank Abelardo Calderon and Jose De Vega for their critical reading.
Plant material declaration
The authors confirm that no collection of plant or seed specimens was practiced in the present study. Moreover, the plant material was provided by INIA (Instituto Nacional de Innovación Agraria) and is under the Material Transfer Agreement ATMG-001-2015. This study also complies with relevant institutional, national, and international guidelines and legislation, and no genotyping data have been analyzed or generated during the study.
Funding
This research was funded by PROCIENCIA grant numbers 177–2015-FONDECYT.
Author information
Authors and Affiliations
Contributions
Conceptualization, G.Z.; data curation, M.V. and G.Z.; formal analysis, M.V., and G.Z.; funding acquisition, J.J, and G.Z.; investigation, M.V., J.J. and G.Z.; methodology, M.V., and G.Z.; project administration, G.Z.; software, M.V.; supervision, G.Z.; validation, M.V., and G.Z.; visualization, M.V., and G.Z.; writing-original draft, M.V., and G.Z.; writing-review & editing, M.V., and G.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vera-Vega, M., Jimenez-Davalos, J. & Zolla, G. The micronutrient content in underutilized crops: the Lupinus mutabilis sweet case. Sci Rep 12, 15162 (2022). https://doi.org/10.1038/s41598-022-19202-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19202-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.