Abstract
Populations in seasonal fluctuating environments receive multiple environmental cues and must deal with this heterogenic environment to survive and reproduce. An enlarged literature shows that this situation can be resolved through rapid adaptation in Drosophila melanogaster populations. Long-term monitoring of a population in its natural habitat and quantitative measurement of its responses to seasonal environmental changes are important for understanding the adaptive response of D. melanogaster to temporal variable selection. Here, we use inbred lines of a D. melanogaster population collected at monthly intervals between May to October over a temporal scale spanning three consecutive years to understand the variation in wing size and wing shape over these timepoints. The wing size and shape of this population changed significantly between months and a seasonal cycle of this traits is repeated for three years. Our results suggest that the effects of environmental variables that generated variation in body size between populations such as latitudinal clines, are a selective pressure in a different manner in terms of seasonal variation. Temperature related variable have a significant nonlinear relation to this fluctuating pattern in size and shape, whereas precipitation and humidity have a sex-specific effect which is more significant in males.
Similar content being viewed by others
Introduction
Environmental variables such as temperature, photoperiod and food availability vary over spatial and temporal scales, driving adaptive divergence among populations, and providing information concerning adaptive evolution. Because of this fact, the ecological and evolutionary impact of latitude1,2,3,4, altitude4,5,6,7,8,9 and seasons (10,11 and references therein12,13,14), on organisms’ life history have long been a topic of interest. Natural populations distributed along latitudinal or altitudinal gradients have been widely used for studies of spatial selection, where the most predictable abiotic factor is the temperature between several climatic and ecological factors. In a similar manner, seasonality leads to predictable changes in a variety of environmental parameters over time, and phenotypic and genetic changes in natural populations among seasons demonstrate evolutionary responses to seasonal fluctuations15,16,17,18. Thus, the seasonal variation in life histories of organisms is a type of temporal variation19.
The annual pattern in photoperiod and temperature varies with latitude and seasonal changes in these environmental parameters can form seasonally consistent patterns, e.g., winter versus summer seasons in temperate regions or wet versus dry seasons in tropical regions. Thus, seasonally fluctuating environments can increase the selective pressures as a function of time, in comparison to stable non-seasonal environments. These variations in the measure of seasonality can also be one of the mechanisms where spatial clines are formed and appear as a result of the seasonal phase cline models20. For example, the individuals of a high-latitude Drosophila melanogaster population collected in autumn are genetically and phenotypically similar to low-latitude populations21,22,23. The organism’s evolutionary response to seasonality, which is triggered by a complex and fluctuating environmental pressure, ensures that existing genetic polymorphisms are preserved. In multivoltine species, seasonal selective pressures can change across generations; in this case, natural selection can cause cyclical changes in allele frequencies12,13,14,24,25,26,27,28. The first findings of genetic differences related to seasonal variation reported in Drosophila pseudoobscura29 and Drosophila funebris30. Many further studies have demonstrated cyclic seasonal changes in the chromosomal inversion frequency in species of Drosophila, e.g., D. robusta31, D. persimilis10, D. melanogaster32, D. subobscura33, D. mediopunctata34, suggesting the presence of seasonal adaptation.
Accordingly, seasonality is an important selective force of adaptation in a broad range of invertebrate species, however, it is unclear how populations adapt rapidly to seasonal fluctuations. Recent studies of a seasonal genomic oscillations in Drosophila populations in North America provides an important insight into rapid adaptation to seasons12. Bergland et al.12 identified alleles called ‘fall like’ and ‘spring like’, which have the ability to fluctuate rapidly between seasons, which confirms that the populations rapidly adapt to changing environments. Studies investigating phenotypic variation, such as fecundity, developmental time, stress tolerance22, and immune response25 also show seasonal patterns in common-garden experiments. In the same manner, temperate populations from North America and Europe demonstrate parallel seasonal allele frequency change across the two continents13. Together, these results support that seasonal adaptation could be a general phenomenon of temperate Drosophila populations12,13,14.
Many organisms exhibit clinal patterns of body size35,36,37,38,39,40,41 and well-known of these patterns is Bergmann’s rule35. According to Bergmann’s rule, close species, or individuals of the same species in endotherms are larger in cold climates or higher latitudes than those in warmer climates or lower latitudes. Since its introduction, the rule’s applicability to invertebrates has always been questionable and gave rise to the thoughts of lack of a general pattern in ectotherms for body size clines, yet the result of more than one mechanism for these taxa.
Since body size is correlated with various environmental factors and therefore a strong adaptive morphological character, it has long been accepted as the most significant feature of organisms. Body size is also highly correlated with many life-history traits42 and determines the abundance of species43. For example, in Drosophila species, body size is correlated with developmental time44,45, reproductive success46,47,48 and life span49,50,51. In the light of adaptation, the question that the most researchers are interested in is how and why body size varies through time (i.e., temporal variation) and geography (i.e., spatial variation). In terms of geographic variation, body size increases with latitude in some invertebrate species52, but most other invertebrates do not follow this pattern53. Consequently, the temporal and spatial variation in body size is especially contentious54 because the patterns that we have observed do not have a consensus between ectothermic and endothermic organisms (e.g., between insects and vertebrates).
However, the majority of scientific evidence indicates that temperature is the primary cause for the observed clinal pattern. For example; studies on the thermal adaptation under the laboratory conditions show that body size evolution is concordant with latitudinal sorting in Drosophila55,56. Many other ecological and environmental parameters, such as rainfall, humidity and UV exposure, vary along latitudes. Recent studies have attempted to identify the ecological and environmental factors that may have resulted in latitudinal clines for body size40,57,58. Furthermore, observed latitudinal body size clines in Drosophila are suggested to be the direct result of adaptation59,60,61. Common-garden experiments show that clines in body size are continuous, providing a shred of clear evidence that it has a genetic basis62.
Adaptation to new environments usually occurs through the adaptation of polygenic characters, as selection alters the optimal values of life history traits in new environment. One of the best studied polygenic morphological characters in Drosophila is body size63,64 and previous studies showed positive correlation between size related traits such as thorax length, body weight and wing size in (e.g.,45,65,66), although wing size is the most commonly used proxy of body size67. Moreover, as body size is correlated with many life history traits such as lifespan49,50,51, mating behavior68 and flight ability69, any of the morphological traits related to body size can be used as a “model trait” to track changes in traits correlated with body size.
In this study, we investigate putative seasonal selection pressures on wing size and shape using inbred lines derived from a temperate D. melanogaster population collected at monthly intervals between May to October over a temporal scale spanning three consecutive years from 2014 to 2016. Our findings show a significant effect of seasonality on both morphological traits. The pattern of seasonal variation in wing morphology is consistent across years, implying rapid seasonal adaptation to changing climatic factors across seasons.
Material and methods
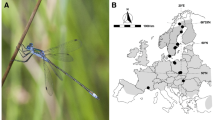
The wild population of Drosophila melanogaster was collected from an orchard in Yeşilöz, Turkey (40.30° N, 32.34° E) monthly from June to October in 2014 and May to October in 2015 and 2016. Every collection occurred approximately with a 30-day interval with traps hung always in the same location for 1–2 days. Isofemale lines were established upon collection and were maintained at standard conditions; 25 °C and 60–65% humidity on a 12:12 h light/dark cycle on a standard cornmeal-agar-sugar-yeast medium.
After two generations, inbred lines were established from isofemale lines through 20 generations of full-sib mating which is considered in a theoretically inbreeding level of f ≅ 170. A minimum of 10 inbred lines per collection month and year were used for the wing measurements, summing up to a total of 166 inbred lines in this study. One exception to this is June 2014 sampling time-point, which has been phenotype only for 6 lines due to low survival upon inbreeding.
Wing measurements
To obtain flies for the wing size and shape measurements, we put 25 adult flies of each sex in 3 replicate bottles containing 50 mL of standard medium. To prevent overcrowding the parents were discarded after 3 days from the bottles. Upon eclosion, at least 25 flies of each sex moved into egg-collection chambers with %2 agar medium, and a pile of yeast paste at 25 °C. After 6–8 h, 150 eggs are collected from the surface with fine forceps and transferred evenly to 3 replicate vials containing 7 mL standard medium. The collected eggs are allowed to develop under standard conditions, as described above. Three days after eclosion adult flies from every replicate vial were collected and stored in ethanol. These standard conditions are used for minimizing the environmental effects on body size.
A minimum of 15 individuals per sex have been dissected from 166 inbred lines, summing up to a total of 5377 wings being measured. The left wing was removed with fine forceps and mounted on a microscope slide in Entellan® (Merck Millipore). All wings were photographed by using a camera attached stereo microscope (Leica S9i) and then digitized as jpeg format.
Analysis
For the estimation of wing shape, 11 landmarks were digitized of the left wing (Fig. 1) of each fly using tpsUtil and tpsDig271,72. The tps files were imported to the MorphoJ software version 1.07a73. To remove the position and orientation variations from the coordinates the raw landmark coordinates were aligned and superimposed using a Procrustes Fit function and wing shape variation was analyzed. The effects of sex and sampling months on shape were tested with Procrustes ANOVA. MANOVA was performed on shape coordinates (Procrustes coordinates) to test whether the population diverged in shape by months. To maximize the differences between groups by comparing mean shapes a Canonical variate analysis (CVA)73 was used to analyze discrimination between groups where month used as a classifier. The pairwise differences in shape were analyzed by using a permutation test (10.000 rounds) with Procrustes distances, which is the sum of the distance between the landmarks. Additionally, a discriminant function analysis (DFA) was used to find the shape differences between sampling months. To test for allometry (relationship of body size to shape) a multivariate regression of shape (the Procrustes coordinates as dependent variable) on size (centroid size as independent variable) was performed, pooled within sub-groups of lines, with a permutation test with 10,000 rounds for testing statistical significance. All analyses were performed using MorphoJ version 1.07a73.
Wing centroid sizes (computed from raw data of landmarks) were used as a measure of wing size. Male and female flies were analyzed separately due to the significant sexual dimorphic differences in body size. The centroid size (CS) data were first analyzed by a Shapiro–Wilk normality test. As the data did not follow a normal distribution, analyses were continued by nonparametric tests. To determine the difference between the three replicate of wing measurements, a Kruskal–Wallis test was performed. Since there was no significant difference between replicas (p > 0.05), the data was pooled. Differences among months of CS were computed with the Kruskal–Wallis test followed by Dunn test with Bonferroni correction for multiple comparisons and pairwise tests using the “ggstatsplot” package74. All analyses were performed in R software75 version 4.1.2.
To test the relationship between climatic variables and centroid size we used climatic variables recorded monthly by the Turkish State Meteorological Service in Güdül, 6 km far from the sampling point (Table 1). Monthly mean temperature (Tmean), monthly mean maximum temperature (Tmax), monthly mean minimum temperature (Tmin), monthly mean humidity (Hmean), monthly mean maximum humidity (Hmax), monthly mean minimum humidity (Hmin) and total monthly precipitation (Ptotal) variables were used. The analyses were split in two parts: (i) using the climatic data for 15 days before the collection dates, (ii) using the climatic data for the collection days. Our preliminary results supported a strong relationship with climatic variables prior the collection dates and analyses were performed with these variables (given in Table 1).
We performed a nonparametric regression analysis using Generalized Additive Models (GAMs)76 to estimate temporal trends in wing CS and its relationships to climatic variables. GAMs provide a capture of linear and non-linear relationships between variables77. Thus, the model allows better prediction of trends in the means of the variables and associated uncertainties. As the climatic categories were collinear, we have adjusted several models with a single independent variable. The dependent variable was CS and independent variables were monthly mean temperature (Tmean), monthly mean maximum temperature (Tmax), monthly mean minimum temperature (Tmin), monthly mean humidity (Hmean), monthly mean maximum humidity (Hmax), monthly mean minimum humidity (Hmin) and total monthly precipitation (Ptotal). Models were run using “mgcv” package (version 3.6.2)78 in R75 with residual marginal likelihood (REML) smoothness selection78,79. The corresponding R function is gam(CS ~ s(temperature related variable), method = 'REML', data) and gam(CS ~ s(humidity and precipitation related variable), method = 'REML', data). For CS analyses, final model selection was determined by calculating the Akaike Information Criterion (AIC) for all models where the type of smooth terms specify thin plate regression splines (default smooth for s terms) or cubic regression splines (bs = “cs”).
Results
Wing size variation
We studied the effects of the monthly changing environments on wing size and shape in a temperate population from Yeşilöz, Turkey collected over successive years and months. Inbred lines from each collection month were used for size measurements. While size is a sexual dimorphic trait and CS differ significantly between sexes (χ2 = 3985.8, p < 0.0001) (Fig. S1), male and females wing measurements were analyzed separately.
The mean and standard errors and coefficients of variation for wing measurements for each year and month were given in Table 2 and we found evidence of seasonally fluctuating wing size which is repeated over three years. Mean wing centroid size was smaller in May and rises from June to July, which is followed by smaller sizes in August to September then an increase in October (Table 2, Fig. 2). This fluctuating pattern is repeating every year, except for October 2016, where the wing sizes are decreasing and that was not consistent with the pattern seen in previous years (Fig. 2). Inbred lines originating from June and July are one average bigger than other months in both sexes (Table 2). The coefficients of variation were particularly higher in some months (Table 2). This might be due to a greater genetic heterogeneity between inbred lines in October 2014, June 2015, August 2015 and May 2016.
Kruskal–Wallis test exhibited a significance between months in females (χ2 = 138.3, df = 5, p-value < 0.0001, Fig. 2) as also in males (χ2 = 144.5, df = 5, p-value < 0.0001, Fig. 2). Pairwise comparisons performed with Dunn test showed a significant difference between months except May–August, May–September, May–October, June-July and August-October in females. Whereas pairwise comparisons between months in males shows that only June and July differ significantly (p < 0.0001) from other months, all other comparisons are not significant (Fig. 2).
Wing shape variation
We digitized 11 landmarks to evaluate differences in wing shape between months. Procrustes ANOVAs detected significant differences in shape among sex (F = 466, 17, df = 18, p < 0.0001) and months (F = 20.99, df = 90, p < 0.0001). The MANOVA on Procrustes coordinates was found to be highly significant, suggesting that wing shape varies significantly among months in females (F = 11.59, df = 90, p < 0.0001) and males (F = 12.08, df = 90, p < 0.0001).
The canonical variate analysis provided a clear discrimination of the months defined by the two first canonical axes which respectively accounted for 38.97% and 26.20% of the among-month variance in females (total 65.17%) and 35.40% and 25.36% of the among-month variance in males (total 60.76%). The results of the canonical variate analysis with Mahalanobis distance and Procrustes distances showed morphological variability across the months (Table S1). Permutation tests (10,000 rounds) for Procrustes distances and Mahalanobis distances among months were significant in all comparisons (Table S1) where May and October lines exhibited difference less than others. The highest Procrustes distance in shapes were found among May and July, where the lowest distance was found between May and October in both sexes. Furthermore, there was a significant difference between sexes (Mahalanobis distance among groups = 2.1648, p < 0.0001) within the population (Figs. S2, S3). The multivariate regression coefficient showed that the effect of size (centroid size) on shape (Procrustes coordinates) was highly significant (P < 0.0001). In addition, we observed a moderate amount of allometry that accounted for 22.06% of the total shape variance. In other words, line specific shape variation explained by size differences.
Climatic effects for size and shape of wing
To find the environmental factor that led to this repetitive pattern across years we applied a Generalized Additive Model (GAM) analysis to assess the relationship between wing centroid size and the climatic variables.
To estimate the relationship between the dependent and independent variables, the GAM analysis was used. The findings of these relationships were estimated as linear and nonlinear effects using smoothing splines (Fig. S4), results presented in Table 3. Results in Table 3 showed that some models resulted with the effective degrees of freedom (edf) values near to 1, the measures suggest a linear pattern. Whereas edf values were larger than 1 suggested a nonlinear pattern in the relationship between the dependent and independent variable. Furthermore, the model output has indicated that all spline terms of climate variables were significant variables of wing size (Table 3).
Temperature related variables have a nonlinear relationship with wing size. Temperature related variables fit well with a cubic regression spline (model selection made by AIC criterion). On the other hand, humidity has a linear effect (edf = 1) on male and females in terms of both mean and minimum values. However, precipitation has a nonlinear effect in females, but a linear effect in males (Table 3).
Discussion
Seasonal fluctuations are main sources for rapidly changing environments in temperate regions and recent studies provided evidence for rapid change in SNP frequencies among seasons12,13,14. Previous studies also revealed that seasonal variation in adaptive phenotypic traits like thermal and starvation tolerance22, and innate immunity25 in D. melanogaster populations and all together suggesting that many loci respond to seasonal selection. This evidence raises new questions about seasonal adaptation and adaptive phenotypic traits. This is main reason to carry out this study to investigate the presence of seasonal variation in wing size and shape. We have investigated the seasonal variation in wing size and shape in a temperate Drosophila population. Our results show that wing size and shape changed significantly between months and the observed seasonal cycle is repeated for three consecutive years. We show that temporal selection acts along seasons in the Yeşilöz population of D. melanogaster and wing size and shape exhibit a rapid response among months. Our analysis reveals evidence for temporal varying selection on wing size and shape; and this suggesting differences within population between month from this geographic region.
Body size is characterized by a complex genetic nature, that a large number of genes are involved, and the alleles have small effects of this trait. It is hard to find the adaptive polymorphism for such traits to towards more simple traits with alleles of large effects. Furthermore, the methodology used in this study provides measurements of inbred lines derived monthly from the population, reflecting a random genotypic sample in the population. Our results of the panel of inbred lines support that the source of seasonal variation in wing size and shape is an outcome of rapid seasonal adaptation, as the frequency of some alleles of different loci vary between months and contribute to the variation of size. It is likely that many ecological and environmental variables that changed between months are interacted to generate variation in body size and strong selection occurs between months for this morphological trait.
The wing size and shape variations depend on the amount of genetic variation in the population. The fluctuating response to selection shows that the genetic variation is maintained in the population but allele frequency for loci responsible for size and shape are changed rapidly through seasons. This might partially explain the difference within months and between inbred lines. For example, some individuals of an inbred line might have outliers within a month, however a general pattern persists. Previous studies showed that SNP frequencies in D. melanogaster populations vary seasonally12,13,14 and transposable elements (TEs) were significantly associated with seasons80. Thus, it is possible that selection favors size and / or size related traits depended on changing environmental variables as our wing size and shape results support.
Temperature has also direct environmental effects on body size in Drosophila and other ectotherms and it is known as the major driver for body size clines35,59,60,62,81,82,83,84,85. Our results indicated that wing size and shape significantly affected by monthly temperature, humidity and precipitation. Accelerated precipitation and humidity have a significant linear relationship with increased wing size especially on males (Table 3). In contrast to this, temperature has a significant non-linear relationship with wing size (Table 3). Our results show that seasonally varying precipitation and humidity have a higher selective pressure on body size than temperature. Increased humidity and precipitation promote larger body size significantly (Table 3, Fig. S4). Our results are not consistent with some other studies about the relationship between size and precipitation and humidity. For example, the wing size of Bactrocera tryoni, an Australian endemic horticultural pest species, is also affected by precipitation but shows a negative relationship with precipitation seasonality86. Stilwell et al.40 proposed that the latitudinal cline in body size of the seed—feeding beetle Stator limbatus generated by humidity and seasonality then temperature, and the results support humidity for a better candidate to explain the body size clines where body size increased with decreased humidity. In a similar manner Gibb et al.87 showed that the body size declines as precipitation increases in ant species. Experimental evolution at low and high humidity showed in larger wing areas at low humidity conditions in D. melanogaster88. For D. simulans and D. mercatorum, Przybylska et al.89 found that the flies were larger in the dry season. Generally, the relationship between humidity and body size is explained by desiccation tolerance. Larger body size decreased the surface-to-volume ratio, and this is correlated with increased water content90. Selection for increased desiccation tolerance showed increased wet weight and lipid content in D. melanogaster91. Such proving’s support the relationship between size and desiccation tolerance moreover humidity. However, our results are inconsistent with this pattern. The study with field collected Anopheles albimanus populations have exhibited a positive correlation between wing size and relative humidity92 which is consistent with our findings. The inconsistency among the studies with humidity and body size point out the need for further work to understand the relationship between humidity and also precipitation and body size. It is known that humidity has a large impact of some traits in D. melanogaster93,94,95 and that rainfall is associated with adaptation in D. melanogaster natural populations96. Moreover, our results reveal that humidity and precipitation have a greater impact on males than females where temperature selection acts the same direction in both sexes. This sex-specific effect for precipitation and humidity could be explained by the selection in nature on body size in the context of fitness advantage. Body size is a sexual dimorphic trait but the genetic basis of body size is shared in both sexes. But it is known that these genes have diverge effects by sex on body size in D. melanogaster97,98. Like body size, lifespan and ageing share also a same genetic basis in both sexes but sex-specific effects of lifespan were recorded for D. melanogaster99 and D. simulans100. Archer et al.100 demonstrated that the sexual and natural selection have sex-specific effects in D. simulans where males evolve grater baseline mortality than females under high temperature conditions100. Natural selection may be a determinant sex differences for wing size in Yeşilöz population where precipitation and humidity favors larger size in males may due the differences in fitness outcomes.
Populations in seasonal fluctuating environments receive multiple environmental cues and must deal with this heterogeneous environment to survive and reproduce. A growing body of literature shows that this situation is resolved through rapid adaptation in D. melanogaster populations. This study shows that body size, an important fitness component, is one of the changing phenotypic traits across seasons. Our results suggest that the effects of environmental variables that generated variation in body size among populations such as latitudinal clines, act as a selective pressure in a different manner in seasonal variation within population. Long-term monitoring of a population in its natural habitat and quantitative measurement of its responses to seasonal environmental changes are important for understanding the adaptive response of population to temporal selection. Future studies should focus on the sex-specific effects of environmental variables to better understand the impact of the environment on the evolution of differences in sexual traits.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Roff, D. Optimizing development time in a seasonal environment: the ‘ups and downs’ of clinal variation. Oecologia 45(2), 202–208 (1980).
Mittelbach, G. G. et al. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 10(4), 315–331 (2007).
Kapun, M., Fabian, D. K., Goudet, J. & Flatt, T. Genomic evidence for adaptive inversion clines in Drosophila melanogaster. Mol. Biol. Evol. 33(5), 1317–1336 (2016).
Rajpurohit, S., Zhao, X. & Schmidt, P. S. A resource on latitudinal and altitudinal clines of ecologically relevant phenotypes of the Indian Drosophila. Sci. Data 4(1), 1–6 (2017).
Hawkins, B. & DeVries, P. J. Altitudinal gradients in the body sizes of Costa Rican butterflies. Acta Oecol. 17, 185–194 (1996).
Sørensen, J. G., Norry, F. M., Scannapieco, A. C. & Loeschcke, V. Altitudinal variation for stress resistance traits and thermal adaptation in adult Drosophila buzzatii from the New World. J. Evol. Biol. 18(4), 829–837 (2005).
Pitchers, W., Pool, J. E. & Dworkin, I. Altitudinal clinal variation in wing size and shape in African Drosophila melanogaster: one cline or many?. Evolution 67(2), 438–452 (2013).
Klepsatel, P., Gáliková, M., Huber, C. D. & Flatt, T. Similarities and differences in altitudinal versus latitudinal variation for morphological traits in Drosophila melanogaster. Evolution 68(5), 1385–1398 (2014).
Ayhan, N., Güler, P. & Onder, B. S. Altitudinal variation in lifespan of Drosophila melanogaster populations from the Firtina Valley, northeastern Turkey. J. Therm. Biol. 61, 91–97 (2016).
Dobzhansky, T. & Ayala, F. J. Temporal frequency changes of enzyme and chromosomal polymorphisms in natural populations of Drosophila. Proc. Natl. Acad. Sci. USA 70(3), 680–683 (1973).
Williams, C. M. et al. Understanding evolutionary impacts of seasonality: An introduction to the symposium. Integr. Comp. Biol. 57(5), 921–933 (2017).
Bergland, A. O., Behrman, E. L., O’Brien, K. R., Schmidt, P. S. & Petrov, D. A. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet. 10(11), e1004775 (2014).
Machado, H. E. et al. Broad geographic sampling reveals the shared basis and environmental correlates of seasonal adaptation in Drosophila. Elife 10, e67577 (2021).
Rodrigues, M. F., Vibranovski, M. D. & Cogni, R. Clinal and seasonal changes are correlated in Drosophila melanogaster natural populations. Evolution 75(8), 2042–2054 (2021).
Hairston, N. G. & Dillon, T. A. Fluctuating selection and response in a population of freshwater copepods. Evolution 44(7), 1796–1805 (1990).
Grant, P. R. & Grant, B. R. Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296(5568), 707–711 (2002).
Brown, C. R., Brown, M. B. & Roche, E. A. Fluctuating viability selection on morphology of cliff swallows is driven by climate. J. Evol. Biol. 26(5), 1129–1142 (2013).
Bergland, A. O., Tobler, R., González, J., Schmidt, P. & Petrov, D. Secondary contact and local adaptation contribute to genome-wide patterns of clinal variation in Drosophila melanogaster. Mol. Ecol. 25(5), 1157–1174 (2016).
Haldane, J. B. S. & Jayakar, S. D. Polymorphism due to selection of varying direction. J. Genet. 58(2), 237–242 (1963).
Rhomberg, L. R. & Singh, R. S. Evidence for a link between local and seasonal cycles in gene frequencies and latitudinal gene clines in a cyclic parthenogen. Genetica 78(1), 73–79 (1986).
Cogni, R. et al. The intensity of selection acting on the couch potato gene—spatial–temporal variation in a diapause cline. Evolution 68, 538–548 (2014).
Behrman, E. L., Watson, S. S., O'brien, K. R., Heschel, M. S., & Schmidt, P. S. Seasonal variation in life history traits in two Drosophila species. J. Evol. Biol. 28(9), 1691–1704 (2015).
Cogni, R. et al. Variation in Drosophila melanogaster central metabolic genes appears driven by natural selection both within and between populations. Proc. Biol. Sci. 282, 20142688 (2015).
Wittmann, M. J., Bergland, A. O., Feldman, M. W., Schmidt, P. S. & Petrov, D. A. Seasonally fluctuating selection can maintain polymorphism at many loci via segregation lift. Proc. Natl. Acad. Sci. USA 114(46), E9932–E9941 (2017).
Behrman, E. L., et al. Rapid seasonal evolution in innate immunity of wild Drosophila melanogaster. P. Roy. Soc. B Biol. Sci. 285(1870), 20172599 (2018).
Rudman, S. M. et al. Microbiome composition shapes rapid genomic adaptation of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 116(40), 20025–20032 (2019).
Dowle, E. J. et al. Genome-wide variation and transcriptional changes in diverse developmental processes underlie the rapid evolution of seasonal adaptation. Proc. Natl. Acad. Sci. USA 117(38), 23960–23969 (2020).
Garcia‐Elfring, et al. Using seasonal genomic changes to understand historical adaptation to new environments: Parallel selection on stickleback in highly‐variable estuaries. Mol. Ecol. 30(9), 2054–2064 (2021).
Dobzhansky, T. Genetics of natural populations IX. Temporal changes in the composition of populations of Drosophila pseudoobscura. Genetics 28(2), 162 (1943).
Dubinin, N. P. & Tiniakov, G. G. Seasonal cycles and the concentration of inversions in populations of Drosophila funebris. Am. Nat. 79(785), 570–572 (1945).
Stalker, H. D. & Carson, H. L. Seasonal variation in the morphology of Drosophila robusta Sturtevant. Evolution 3(4), 330–343 (1949).
Stalker, H. D. Chromosome studies in wild populations of Drosophila melanogaster. II. Relationship of inversion frequencies to latitude, season, wing-loading and flight activity. Genetics 95(1), 211–223 (1980).
Rodriguez-Trelles, F., Alvarez, G. & Zapata, C. Time-series analysis of seasonal changes of the O inversion polymorphism of Drosophila subobscura. Genetics 142(1), 179–187 (1996).
Ananina, G. et al. Chromosomal inversion polymorphism in Drosophila mediopunctata: seasonal, altitudinal, and latitudinal variation. Genet. Mol. Biol. 27, 61–69 (2004).
Bergmann, K. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe. Gottinger Studien 3, 595–708 (1847).
Graves, G. R. Bergmann’s rule near the equator: latitudinal clines in body size of an Andean passerine bird. Proc. Natl. Acad. Sci. USA 88(6), 2322–2325 (1991).
Partridge, L. & Coyne, J. A. Bergmann’s rule in ectotherms: Is it adaptive?. Evolution 51(2), 632–635 (1997).
Ashton, K. G. Patterns of within-species body size variation of birds: strong evidence for Bergmann’s rule. Global Ecol. Biogeogr. 11(6), 505–523 (2002).
Hallas, R., Schiffer, M. & Hoffmann, A. A. Clinal variation in Drosophila serrata for stress resistance and body size. Genet. Res. 79(2), 141–148 (2002).
Stillwell, R. C., Morse, G. E. & Fox, C. W. Geographic variation in body size and sexual size dimorphism of a seed-feeding beetle. Am. Nat. 170(3), 358–369 (2007).
Clauss, M., Dittmann, M. T., Müller, D. W., Meloro, C. & Codron, D. Bergmann′ s rule in mammals: A cross-species interspecific pattern. Oikos 122(10), 1465–1472 (2013).
Stearns, S. C. The evolution of life histories (Oxford Univ. Press, 1992).
Blackburn, T. M. & Gaston, K. J. Linking patterns in macroecology. J. Anim. Ecol. 70(2), 338–352 (2001).
Robertson, F. W. The ecological genetics of growth in Drosophila 6. The genetic correlation between the duration of the larval period and body size in relation to larval diet. Genet. Res. 4(1), 74–92 (1963).
Partridge, L., Langelan, R., Fowler, K., Zwaan, B. & French, V. Correlated responses to selection on body size in Drosophila melanogaster. Genet. Res. 74(1), 43–54 (1999).
Partridge, L. & Farquhar, M. Lifetime mating success of male fruitflies (Drosophila melanogaster) is related to their size. Anim. Behav. 31(3), 871–877 (1983).
Lefranc, A. & Bundgaard, J. The influence of male and female body size on copulation duration and fecundity in Drosophila melanogaster. Hereditas 132(3), 243–247 (2000).
Long, T. A., Pischedda, A., Stewart, A. D. & Rice, W. R. A cost of sexual attractiveness to high-fitness females. PLoS Biol. 7(12), e1000254 (2009).
Partridge, L. & Fowler, K. Direct and correlated responses to selection on age at reproduction in Drosophila melanogaster. Evolution 46(1), 76–91 (1992).
Rodriguez, C., Fanara, J. J. & Hasson, E. Inversion polymorphism, longevity, and body size in a natural population of Drosophila buzzatii. Evolution 53(2), 612–620 (1999).
Norry, F. M. & Loeschcke, V. Temperature-induced shifts in associations of longevity with body size in Drosophila melanogaster. Evolution 56(2), 299–306 (2002).
Karan, D., Dubey, S., Moreteau, B., Parkash, R. & David, J. R. Geographical clines for quantitative traits in natural populations of a tropical Drosophilid: Zaprionus indianus. Genetica 108(1), 91–100 (2000).
Shelomi, M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 180(4), 511–519 (2012).
Blackburn, T. M., Gaston, K. J. & Loder, N. Geographic gradients in body size: a clarification of Bergmann’s rule. Divers. Distrib. 5(4), 165–174 (1999).
Cavicchi, S., Guerra, D., Natali, V., Pezzoli, C., & Giorgi, G. Temperature‐related divergence in experimental populations of Drosophila melanogaster. II. Correlation between fitness and body dimensions. J. Evol. Biol. 2(4), 235–251 (1989).
Partridge, L., Barrie, B., Fowler, K. & French, V. Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48(4), 1269–1276 (1994).
Jones, J. et al. Multiple selection pressures generate adherence to Bergmann’s rule in a Neotropical migratory songbird. J. Biogeogr. 32(10), 1827–1833 (2005).
Stillwell, R. C., Moya-Laraño, J. & Fox, C. W. Selection does not favor larger body size at lower temperature in a seed-feeding beetle. Evolution 62(10), 2534–2544 (2008).
Imasheva, A. G., Bubli, O. A. & Lazebny, O. E. Variation in wing length in Eurasian natural populations of Drosophila melanogaster. Heredity 72(5), 508–514 (1994).
van’t Land, J., P. van Putten, H. Villarroel, A. Kamping & W. van Delden Latitudinal variation in wing length and allele frequencies for Adh and α-Gpdh in populations of Drosophila melanogaster from Ecuador and Chile. Dros. Info. Serv. 76, 156 (1995).
Loeschcke, V., Bundgaard, J., & Barker, J. S. F. Variation in body size and life history traits in Drosophila aldrichi and D. buzzatii from a latitudinal cline in eastern Australia. Heredity 85(5), 423–433 (2000).
Gilchrist, A. S. & Partridge, L. A comparison of the genetic basis of wing size divergence in three parallel body size clines of Drosophila melanogaster. Genetics 153(4), 1775–1787 (1999).
Turner, T. L., Stewart, A. D., Fields, A. T., Rice, W. R. & Tarone, A. M. Population-based resequencing of experimentally evolved populations reveals the genetic basis of body size variation in Drosophila melanogaster. PLoS Genet. 7(3), e1001336 (2011).
Pitchers, W. et al. A multivariate genome-wide association study of wing shape in Drosophila melanogaster. Genetics 211(4), 1429–1447 (2019).
Reeve, E. C. R. Genetical aspects of size allometry. P. Roy. Soc. B-Biol. Sci. 137(889), 515–518 (1950).
Cowley, D. E. & Atchley, W. R. Development and quantitative genetics of correlation structure among body parts of Drosophila melanogaster. Am. Nat. 135(2), 242–268 (1990).
Reeve, E. C. R. & Robertson, F. W. Studies in quantitative inheritance. J. Genet. 51(2), 276–316 (1953).
Menezes, B. F., Vigoder, F. M., Peixoto, A. A., Varaldi, J. & Bitner-Mathé, B. C. The influence of male wing shape on mating success in Drosophila melanogaster. Anim. Behav. 85(6), 1217–1223 (2013).
Ray, R. P., Nakata, T., Henningsson, P. & Bomphrey, R. J. Enhanced flight performance by genetic manipulation of wing shape in Drosophila. Nat. Commun. 7(1), 1–8 (2016).
Falconer D.S., & Mackay T.F.C. Introduction to Quantitative Genetics. (4th ed Benjamin Cummings, Longmans Green: Harlow, UK. 1996).
Rohlf, F. J. Comparative methods for the analysis of continuous variables: geometric interpretations. Evolution 55(11), 2143–2160 (2001).
Rohlf, F. J. The tps series of software. Hystrix 26(1), (2015).
Klingenberg, C. P. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11(2), 353–357 (2011).
Patil, I. Visualizations with statistical details: The “ggstatsplot” approach. J. Open Source Softw. 6(61), 3167 (2021).
R Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria 2021).
Wood, S. N., Pya, N. & Säfken, B. Smoothing parameter and model selection for general smooth models. J. Am. Stat. Assoc. 111(516), 1548–1563 (2016).
Pedersen, E. J., Miller, D. L., Simpson, G. L. & Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 7, e6876 (2019).
Wood, S. N. Generalized additive models: an introduction with R 2nd edn. (Chapman and Hall/CRC, 2017).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73(1), 3–36 (2011).
Kapun, M. et al. Genomic analysis of European Drosophila melanogaster populations reveals longitudinal structure, continent-wide selection, and previously unknown DNA viruses. Mol. Biol. Evol. 37(9), 2661–2678 (2020).
David, J., Bocquet, C. & De Scheemaeker-Louis, M. Genetic latitudinal adaptation of Drosophila melanogaster: new discriminative biometrical traits between European and equatorial African populations. Genet. Res. 30(3), 247–255 (1977).
Coyne, J. A. & Beecham, E. Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics 117(4), 727–737 (1987).
Capy, P., Pla, E., & David, J. R. Phenotypic and genetic variability of morphometrical traits in natural populations of Drosophila melanogaster and D. simulans. I. Geographic variations. Genet. Sel. Evol. 25(6), 517–536 (1993).
James, A. C., Azevedo, R. B. & Partridge, L. Cellular basis and developmental timing in a size cline of Drosophila melanogaster. Genetics 140(2), 659–666 (1995).
Flatt, T. Life-history evolution and the genetics of fitness components in Drosophila melanogaster. Genetics 214(1), 3–48 (2020).
Zhou, Y., Rodriguez, J., Fisher, N. & Catullo, R. A. Ecological drivers and sex-based variation in body size and shape in the Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). Insects 11(6), 390 (2020).
Gibb, H. et al. Habitat disturbance selects against both small and large species across varying climates. Ecography 41(7), 1184–1193 (2018).
Kennington, W. J., Killeen, J. R., Goldstein, D. B. & Partridge, L. Rapid laboratory evolution of adult wing area in Drosophila melanogaster in response to humidity. Evolution 57(4), 932–936 (2003).
Przybylska, M. S., Roque, F. & Tidon, R. Drosophilid species (Diptera) in the Brazilian Savanna are larger in the dry season. Ann. Entomol. Soc. Am. 107(5), 994–999 (2014).
Chown, S. L. & Gaston, K. J. Exploring links between physiology and ecology at macro-scales: The role of respiratory metabolism in insects. Biol. Rev. 74(1), 87–120 (1999).
Telonis-Scott, M., Guthridge, K. M. & Hoffmann, A. A. A new set of laboratory-selected Drosophila melanogaster lines for the analysis of desiccation resistance: response to selection, physiology and correlated responses. J. Exp. Biol. 209(10), 1837–1847 (2006).
Gómez, G. F., Márquez, E. J., Gutiérrez, L. A., Conn, J. E. & Correa, M. M. Geometric morphometric analysis of Colombian Anopheles albimanus (Diptera: Culicidae) reveals significant effect of environmental factors on wing traits and presence of a metapopulation. Acta Trop. 135, 75–85 (2014).
Service & P. M., Hutchinson, E. W., MacKinley, M. D., & Rose, M. R,. Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol. Zool. 58(4), 380–389 (1985).
Al-Saffar, Z. Y., Grainger, J. N. R. & Aldrich, J. Temperature and humidity affecting development, survival and weight loss of the pupal stage of Drosophila melanogaster, and the influence of alternating temperature on the larvae. J. Therm. Biol. 21(5–6), 389–396 (1996).
Aggarwal, D. D. et al. Rapid effects of humidity acclimation on stress resistance in Drosophila melanogaster. Comp. Biochem. Phys. A 166(1), 81–90 (2013).
Bogaerts-Márquez, M., Guirao-Rico, S., Gautier, M. & González, J. Temperature, rainfall and wind variables underlie environmental adaptation in natural populations of Drosophila melanogaster. Mol. Ecol. 30(4), 938–954 (2021).
Carreira, V. P., Mensch, J. & Fanara, J. J. Body size in Drosophila: genetic architecture, allometries and sexual dimorphism. Heredity 102(3), 246–256 (2009).
Carreira, V. P., Soto, I. M., Mensch, J. & Fanara, J. J. Genetic basis of wing morphogenesis in Drosophila: Sexual dimorphism and non-allometric effects of shape variation. BMC Dev. Biol. 11(1), 1–16 (2011).
Parker, G. A. et al. Genetic basis of increased lifespan and postponed senescence in Drosophila melanogaster. G3 - Genes Genom. Genet. 10(3), 1087–1098 (2020).
Archer, C. R. et al. Sex-specific effects of natural and sexual selection on the evolution of life span and ageing in Drosophila simulans. Funct. Ecol. 29(4), 562–569 (2015).
Acknowledgements
We are grateful to Esra Durmaz, Utku Perktaş, Nuran Çiçek and Alper Orhan for helpful comments. This study was funded by the Scientific and Technological Research Council of Turkey (TUBITAK) Grant No 214Z238.
Author information
Authors and Affiliations
Contributions
B.Ş.Ö. conceived the research, conducted data analysis, wrote the original draft, revised the manuscript and administered the project. C.F.A. and B.Ş.Ö. collected field samples and performed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Önder, B.Ş., Aksoy, C.F. Seasonal variation in wing size and shape of Drosophila melanogaster reveals rapid adaptation to environmental changes. Sci Rep 12, 14622 (2022). https://doi.org/10.1038/s41598-022-18891-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18891-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.