Abstract
Up to a third of North Americans report using cannabis in the prior month, most commonly through inhalation. Animal models that reflect human consumption are critical to study the impact of cannabis on brain and behaviour. Most animal studies to date utilize injection of delta-9-tetrahydrocannabinol (THC; primary psychoactive component of cannabis). THC injections produce markedly different physiological and behavioural effects than inhalation, likely due to distinctive pharmacokinetics. The current study directly examined if administration route (injection versus inhalation) alters metabolism and central accumulation of THC and metabolites over time. Adult male and female Sprague–Dawley rats received either an intraperitoneal injection or a 15-min session of inhaled exposure to THC. Blood and brains were collected at 15, 30, 60, 90 and 240-min post-exposure for analysis of THC and metabolites. Despite achieving comparable peak blood THC concentrations in both groups, our results indicate higher initial brain THC concentration following inhalation, whereas injection resulted in dramatically higher 11-OH-THC concentration, a potent THC metabolite, in blood and brain that increased over time. Our results provide evidence of different pharmacokinetic profiles following inhalation versus injection. Accordingly, administration route should be considered during data interpretation, and translational animal work should strongly consider using inhalation models.
Similar content being viewed by others
Introduction
With recreational use of cannabis recently becoming legal in Canada and some states across the U.S., as well as medicinal use being legal in many other countries, there is a growing need to better understand the effects of cannabis on brain and behaviour. Up to a third of North Americans over 16 years of age report using cannabis in the prior month1, most commonly through pulmonary (i.e. inhalation) administration2. Animal models provide an extremely valuable approach to studying the effects of cannabis, enabling control over composition, dose, and timing of exposure. Nevertheless, the majority of animal studies to date examine effects of cannabis through parenteral (i.e. intraperitoneal [IP]) injections of delta-9-tetrahydrocannabinol (THC; the primary psychoactive component of cannabis) or cannabinoid receptor 1 (CB1R) agonists, which does not reflect a common route of human cannabis consumption nor reproduce the same physiological or behavioural effects of inhalation.
Inhalation of THC produces a more rapid onset and offset of hypothermia, increases feeding behaviour, decreases locomotion, and fails to induce cross-sensitization to morphine in rats when compared directly to injected THC3,4. Further, while THC injections result in conditioned avoidance, inhalation produces a place preference3 and has reinforcing properties (as demonstrated by robust self-administration) in rats5. The effects of THC inhalation on temperature, appetite, and locomotion in rodents are also typically shown in humans after exposure to cannabis smoke6,7,8. The physiological and behavioural differences between inhalation and injections are likely due to the distinctive pharmacokinetic differences of each route of administration.
Following IP injection, compounds are absorbed primarily through the portal circulation and pass through the liver to undergo metabolism before reaching other organs, such as the brain9,10. Alternatively, inhalation provides rapid delivery of compounds into the blood stream, bypassing initial metabolism by the liver, and resulting in more immediate uptake by highly perfused tissues, including the brain10,11,12. The amount and duration of compound absorption also differs, with injections delivering a single bolus versus inhalation delivering an ongoing infusion. As such, the pharmacokinetic profile of THC, specifically plasma THC concentrations, between injections and inhalation differ in timing, magnitude, and duration.
The most common form of cannabis administration in human is inhalation (smoking or ‘vaping’) with smoking historically being predominant but vaporization becoming increasingly popular, especially in youth13,14,15. Cannabis vape products are highly variable ranging from dried cannabis (most commonly THC concentrations of 10–25%) to concentrated THC distillate (most commonly THC concentrations of 20–25% but can reach upwards of 70–90%)14,16. Controlled inhalation (smoking or vaping) of cannabis cigarettes in humans produces peak plasma THC concentrations 10–15-min after initial administration15,17,18,19,20,21,22 with relatively rapid clearance of THC from plasma. In fact, plasma THC concentrations are only 15–20% of peak at 30-min following cannabis use, 8–10% at 60-min, and 2–3% at 180 min20,23. However, because of individual differences in the number, duration, and spacing of puffs, as well as inhalation volume and hold time, the exact concentration of peak plasma THC in human studies is extremely variable. Peak plasma concentrations of THC range from 60 to 200 ng/mL following inhalation of cannabis flower17,18,19,20,21,23,24, making animal models that can control dose and timing extremely valuable.
Rodent studies utilizing THC injections allow for control over both dose and timing; however, peak plasma THC concentrations are found at a slightly later timepoint following injection than inhalation4,25,26,27. Further, clearance of THC from plasma following IP injections is much slower, with concentrations still roughly 65% of peak at 60-min and 50% at 120 min28. Rodent studies employing IP injections utilize a wide range of dosages (3–20 mg/kg) producing an extensive span of peak plasma THC concentrations from 40 to 200 ng/mL4,25,26,28, suggesting that they are comparable to the range seen in humans following cannabis use. Interestingly, brain THC concentrations following IP administration increase over time, peaking at 60–120 min following initial administration28.
Animal models utilizing vapor delivery of THC or whole cannabis extract have recently been validated3,4,25 and are able to control for dose and timing of exposure while also employing the most common route of cannabis consumption in humans. Several rodent studies have found plasma THC concentrations of 100–200 ng/mL following 30-min of exposure to 100–200 mg/mL of THC vapor4,25,26,29. Similar to human inhalation, plasma THC concentrations peak at around 15-min30 with relatively rapid plasma clearance as suggested by concentrations of ~ 30% of peak at 60-min and 8–10% at 120 min4,25,26. Finally, opposite to injection, brain THC concentrations following inhalation peak at 15-min following initial administration and decrease over time30.
In preclinical studies, dosing of THC is typically determined by whether it produces blood THC concentrations in the desired range seen in humans following cannabis consumption. However, whether route of administration influences how much THC, or its metabolites, accumulates in the brain and activate central CB1R is not understood. As such, it is not clear if injections of THC that produce similar blood THC levels as those seen following inhalation are indeed comparable in how much impact they have on activation of the central cannabinoid system. Consideration of the metabolism of THC is also incredibly important in this context and is often not measured in most analyses even though the metabolites of THC are highly bioactive, undoubtedly influenced by route of administration, and known to be significantly impacted by sex25,27,29,31. In the liver, THC is hydroxylated by cytochrome P450 enzymes into 11-hydroxy-THC (11-OH-THC), which is subsequently oxidized by the same group of enzymes to create 11-Nor-9-carboxy-THC (THC-COOH) and is excreted in urine11. The concentrations of THC metabolites are very important factors to consider when examining the impacts of THC administration, as 11-OH-THC is also psychoactive, is at least equipotent if not more potent than THC, and diffuses more readily into the brain than THC32,33,34. Thus, differences in the generation and central accumulation of 11-OH-THC are not trivial and can have a robust impact on the outcome of studies given its ability to be as, if not more, efficacious than THC in activating CB1R. THC-COOH is detectable for weeks, lacks any known psychoactivity, yet may possess anti-inflammatory and analgesic effects32,35.
In our attempts to develop more translationally relevant models of THC and cannabis administration, we examined if there were pharmacokinetic differences in the metabolism and accumulation of THC between inhalation and injection administration that could help ascertain if these approaches are interchangeable or if there are differences that need to be considered when interpreting animal research data through a translational lens. To this extent, we utilized inhalation and injection paradigms, in both male and female rats, that produced comparable peak plasma THC concentrations to see if these different routes of administration resulted in differential pharmacokinetics or central accumulation of THC and metabolites. To quantify concentrations of THC, 11-OH-THC and THC-COOH, we also developed our own analytical approach using mass spectrometry-liquid chromatography, which allowed us to quantify these molecules in both plasma and brain tissue.
Methods
Animals and housing
Adult male (n = 62) and female (n = 66) Sprague–Dawley rats were obtained from Charles Rivers Laboratories (St. Constant, QC, Canada). Rats were pair-housed in clear polycarbonate cages with in-cage shelters and aspen-chip bedding, as well as ad libitum access to water and standard laboratory chow, and were acclimated to a standard colony room (12 h light–dark cycle; constant temperature of 21 ± 1 °C). Following ~ 1 week of acclimation rats were split into two administration groups (injection [parenteral] and inhaled [pulmonary]) and each group was further sub-divided according to five timepoints (15, 30, 60, 90, and 240-min) (n = 6 per timepoint per administration group unless otherwise specified). All animal experiments were carried out in compliance with ARRIVE guidelines, and were performed in accordance with the Canadian Council on Animal Care (CCAC) guidelines and were approved (protocol #: AC19-0024) by the University of Calgary Animal Care Committee.
Injections of THC
Dosing for both injection and inhalation studies was based on pilot work establishing doses that produced roughly comparable blood THC levels in the range of 60–100 ng/mL. Age matched (90–110 days of age) male (438 ± 32 g, n = 26) and female (275 ± 12 g, n = 30) rats received a single injection of THC intraperitoneally (dose of 2.5 mg/kg in a volume of 2 mL/kg). THC (100 mg/mL in 100% EtOH from Toronto Research Chemicals) was stored at − 20 °C until dissolved into a 1:1:8 solution of dimethylsulfoxide (DMSO), Tween 80, and 0.9% saline respectively. DMSO is commonly used in dilution of injectable drugs and can cause toxicity in concentrations > 10% or at lower concentration with ocular or oral exposure36; importantly, our study diluted to only 1% DMSO. All injections occurred between 0900 and 1200 h; following injections rats were euthanized via decapitation at five different timepoints (15, 30, 60, 90 and 240-min, referred to as INJ-15, INJ-30, INJ-60, INJ-90 and INJ-240 hereafter; n = 6 per group for females and n = 5–6 per group for males); trunk blood was collected, and brains were extracted for hippocampus dissection. The hippocampus was chosen as the brain structure for analysis as it is an important site for many of the cognitive and emotional effects of cannabinoids, has a high density of cannabinoid receptors and is a brain structure whose isolation and dissection is consistent and straightforward. Blood samples were collected in EDTA tubes and stored on ice until centrifuged at 10,000 rpm for 10 min at 4 °C. Plasma was collected and stored at − 80 °C until analysis. Dissected hippocampi were immediately frozen on dry ice and then stored at − 80 °C until analysis.
Passive inhaled delivery of THC
Male (423 ± 19 g, n = 30) and female (274 ± 16 g, n = 30) rats received a single (15-min) session of inhaled exposure to a THC-dominant cannabis extract (100 mg/mL; 95% THC from Aphria Inc., ON, CND) via a validated4,5,25 vapor inhalation system (La Jolla Alcohol Research Inc., CA, USA). THC-dominant cannabis extract was stored at room temperature until diluted to a concentration of 100 mg/mL THC in polyethylene glycol (PEG-400). PEG is commonly added to cannabis and nicotine-based vaping products for human consumption and is generally recognized as safe by the FDA37. PEG-400 can cause toxicity in rats upon exposure > 8 h38, but importantly our study exposes animals for only 15-min. Both DMSO and PEG-400 are common solvents used for diluting water-insoluble substances (i.e. cannabinoids), but whether different vehicles alter the detection of cannabinoids and their metabolites through mass spectrometry remains relatively unknown. As the goal of our study is to compare the pharmacokinetics and central accumulation of THC and metabolites across routes of administration the most common vehicle for each route was utilized (DMSO for injection and PEG-400 for inhalation).
The vapor inhalation system uses machinery similar to electronic cigarettes to deliver distinct “puffs” of cannabis vapor within airtight chambers. Chamber airflow is controlled by a vacuum compressor (i.e. a “pull” system) that draws room ambient air through an intake valve at a constant rate of 1 L per minute. At set intervals (as controlled by MedPC IV software [Med Associates, ST. Albans, VT, USA]) THC-dominant cannabis extract is vaporized (utilizing a SMOK TFV8 X-baby atomizer [Shenzhen IVPS Technology Co., Shenzhen, China] at 40-watts) and combines with the constant ambient air flow for delivery into the chamber. Air (and vapor) are evacuated through the back of the chamber via the vacuum compressor, filtered and ventilated out of the building (Fig. 1 for illustrated depiction of the vapor delivery system). In this study, THC vapor was delivered through a 10-s “puff” every 2 min for 15-min. “Puffing” profiles vary greatly in human cannabis consumption with self-titration to reach desired “high”23, therefore studies examining the effects of inhaled cannabis often control exposure through both percentage of THC and an allotted inhalation time of ~ 10-min15,20. Our delivery schedule of 15-min was chosen to similarly reflect this previous research, as well as pilot testing showed similar peak levels to cannabis inhalation in humans17,18,19,20,21,23. In accordance with the injected animals, all inhalation sessions occurred between 0900 and 1200 h, and following the conclusion of the vapor session rats were euthanized via decapitation at five different timepoints (15 [immediate], 30, 60, 90 and 240-min, referred to as INH-15, INH-30, INH-60, INH-90 and INH-240 hereafter; n = 6 per group for females and males). Trunk blood and brains were collected and stored as previously described.
Vapor delivery system. (A) Illustration of passive vapor delivery system: Schematic of vapor apparatus components with direction of air-flow (adapted from Freels et al.5). Briefly, the vapor inhalation system uses machinery similar to electronic cigarettes to deliver distinct “puffs” of vapor within airtight chambers. A vacuum compressor pulls ambient room air through an intake valve at a constant rate of 1 L per minute. At set intervals THC-dominant cannabis extract is vaporized combining with the constant ambient air flow for delivery into the chamber. Air (and vapor) are evacuated through the back of the chamber via the vacuum compressor, filtered and ventilated out of the building. (B) Image of passive vapor exposure: Picture of a male SD rat within the vapor apparatus.
Body temperature
Body temperature was taken via a rectal thermometer immediately prior to euthanasia for all rats at the 30, 60, 90, and 240-min timepoints. As peak THC and metabolite levels were imperative to measure immediately following inhalation, blood and brain collection were prioritized at the 15-min timepoint. Further, hypothermic onset following THC exposure has been shown to occur at 30-min following inhalation3. Body temperature following THC administration was compared to control animals. Male (n = 6) and female (n = 6) control animals were exposed to either vehicle vapor (PEG-400 alone) for 15-min or to vehicle injection (1:1:8 DMSO, Tween 80, and 0.9% saline) and their body temperature was taken at 30, 60, 90 and 240-min post administration.
Tandem mass spectrometry (LC-MS/MS)
Standard solutions and reagents
Both standard and deuterated internal standard (IS) stock solutions were purchased from Cerilliant (Round Rock, TX, USA). The standard solutions, including Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THC-COOH) were dissolved in acetonitrile at a concentration of 1.0 mg/mL. The IS stock solutions including THC-d3 and THC-COOH-d3 were dissolved in acetonitrile at 0.1 mg/mL. LC/MS grade acetonitrile, water and formic acid were purchased from Thermo Fisher Scientific (Edmonton, Canada). All compounds and their serial dilutions were stored at − 80 °C freezer until use.
Calibration curves
The stock solutions of each standard were mixed and diluted in 50% methanol/water to produce a set of standards ranging from 0.1 to 100 ng/mL (0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, 100). Internal standard (IS; d3 analytes) solution contains each compound at 10 ng/mL and was prepared in 50% methanol/water. Solutions used to establish calibration curves were prepared by mixing 20 µL of each standard solution and 20 µL of IS solution. The calibrators were analyzed in triplicate and the resulting calibration curves were fit by line of regression using a weight of 1/x2. R2 of each calibration curve was at least 0.999. Lower limit of quantitation (LLOQ) of each analyte was determined to be at 0.1 ng/mL.
Sample preparation
Glass tubes containing 2 mL of acetonitrile and 100 µL of IS were prepared to receive plasma and brain samples. Each plasma sample was thawed at room temperature and 500 µL was directly pipetted into the prepared tubes. Each brain tissue sample was weighed and the frozen piece placed into the prepared glass tubes for manual homogenization with a glass rod until resembling sand. All samples were then sonicated in an ice bath for 30-min before being stored overnight at − 20 °C to precipitate proteins. The next day samples were centrifuged at 1800 rpm at 4 °C for 3–4 min to remove particulates and the supernatant from each sample was transferred to a new glass tube. Tubes were then placed under nitrogen gas to evaporate. Following evaporation, the tube sidewalls were washed with 250 µL acetonitrile to recollect any adhering lipids and then again placed under nitrogen gas to evaporate. Following complete evaporation, the samples were re-suspended in 100 µL of 1:1 methanol and deionized water. Resuspended samples went through two rounds of centrifugation (15,000 rpm at 4 °C for 20 min) to remove particulates and the supernatant transferred to a glass vial with a glass insert. Samples were then stored at − 80 °C until analysis by LC-MS/Multiple Reaction Monitoring (MRM).
LC-MS/MS analysis
LC-MS/MS analysis was performed using an Eksigent Micro LC200 coupled to an AB Sciex QTRAP 5500 mass spectrometry (AB Sciex, Ontario, Canada) at the Southern Alberta Mass Spectrometry (SAMS) facility located at the University of Calgary. The LC system consisted of a CTC refrigerated autosampler (held at 10 °C), a six-port sample injection valve with a 5 µL sample loop as well as a column oven. Chromatographic separation of the analytes was carried out on an Eksigent Halo C18 column (2.7 µm, 0.5 × 50 mm, 90 Å, AB Sciex) at 40 °C. The mobile phase A was composed of 0.1% formic acid in water and the mobile phase B of 0.1% formic acid in acetonitrile. The analytes (2 µL injection) were eluted at 30 µL/min using a gradient from 25 to 95% B in 2.5 min. The column was then cleaned and regenerated using the following program: 95% B for 2 min, 95 to 25% B in 0.2 min and 25% B for 2.3 min. Before each injection, the column was equilibrated at initial LC condition for 1 min. Carryover was checked by injection of a blank in between samples. The data were acquired in positive electrospray ionization (ESI) and MRM mode. MRM transitions and collision energies (CE) of the different compounds are listed in Table 1. Each compound was acquired with two transitions. The first one was used to quantify the compound and the second one to discriminate isomers when necessary. Ion spray voltage was set at 5500 V. Nebulizer gas (GS 1), auxiliary gas (GS 2), curtain gas (CUR) were set at 30, 30, 35 (arbitrary units), respectively. Collision gas was set to Medium. Declustering potential (DP), entrance potential (EP) and cell exit potential (CXP) were set at 80, 7 and 14 V, respectively. LC-MS/MRM data were processed using Analyst 1.6 software (AB Sciex). Quantitation of each analyte was calculated using its extracted ion chromatogram (XIC; peak area) normalized by the peak area of its corresponding IS. Analyte concentration (in pmol/µL) were normalized to sample volume/weight and converted to ng/mL or ng/g for statistical analysis and graphing.
Statistical analysis
All data are expressed as mean ± SEM. Data were analyzed using IBM SPSS Statistics 26 and graphed using GraphPad Prism 8. Basal body temperature differed between males and females, so the two sexes were analyzed separately. Temperature data were analyzed by three-way ANOVA with drug group (THC and control), administration group (injection and inhalation), and timepoint (30, 60, 90, and 240-min) as between-subjects’ factors. Analyte data were analyzed by three-way ANOVA with sex (male and female), administration group (THC-INH or INJ), and timepoint (15, 30, 60, 90 and 240-min) as between-subjects’ factors. Post-hoc comparisons used Bonferroni post hoc tests and differences were considered significant at p ≤ 0.05.
Results
Body temperature
Body temperature measures were compared to controls and analyzed separately by sex (female > male, main effect of sex [F(1,136) = 23.219 at p < 0.00001]). THC exposure resulted in hypothermia in male rats differentially depending on administration group (interaction effect of group and timepoint: F(9,52) = 5.831 at p < 0.0001, Fig. 2A). In particular, THC-INH resulted in immediate hypothermia at 30- and 60-min (30-min: THC-INH < CON-INH, CON-INJ, and THC-INJ at p < 0.05; 60-min: THC-INH < CON-INJ and THC-INJ at p < 0.05), whereas THC-INJ resulted in delayed hypothermia at 90- and 240-min (90-min: THC-INJ < CON-INJ and THC-INH at p < 0.05; 240-min: THC-INJ < THC-INJ, CON-INH, and THC-INH at p < 0.01). Along these lines, THC-INH resulted in lower body temperature at 30-min compared to 90- and 240-min (p < 0.01), as well as remained lower at 60-min compared to 90-min (p < 0.05). THC-INJ resulted in lower body temperature at 90-min compared to 30- and 60-min (p < 0.05), as well as remained lower at 240-min than all other timepoints (p < 0.01).
Body temperature. (A) Male temperature: Data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) with a solid and dashed green line indicates that THC-INH and THC-INJ respectively differ from one or more other groups. THC-INH resulted in immediate hypothermia at 30-min (THC-INH < all groups at *p < 0.05) and at 60-min (THC-INH < CON-INJ and THC-INJ at *p < 0.05), whereas THC-INJ resulted in delayed hypothermia at 90-min (THC-INJ < CON-INJ and THC-INH at *p < 0.05) and at 240-min (THC-INJ < all groups at **p < 0.01). (B) Female temperature: data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) with a solid and dashed green line indicates that THC-INH and THC-INJ respectively differ from one or more other groups. THC-INH resulted in immediate hypothermia at 30-min (THC-INH < all groups at **p < 0.01) and at 60-min (THC-INH < all groups at *p < 0.05), whereas THC-INJ resulted in delayed hypothermia at 90-min (THC-INJ < all groups at *p < 0.05) and at 240-min (THC-INJ < CON-INJ and THC-INH at **p < 0.01).
Females had an extremely similar pattern where THC exposure resulted in hypothermia in female rats differentially depending on administration group (interaction effect of group and time: F(9,56) = 7.097 at p < 0.00001, Fig. 2B). In particular, THC-INH resulted in immediate hypothermia at 30- and 60-min (30-min: THC-INH < CON-INH, CON-INJ, and THC-INJ at p < 0.001; 60-min: THC-INH < CON-INH, CON-INJ and THC-INJ at p < 0.05), whereas THC-INJ resulted in delayed hypothermia at 90- and 240-min (90-min: THC-INJ < CON-INJ, CON-INH and THC-INH at p < 0.01; 240-min: THC-INJ < CON-INJ and THC-INH at p < 0.001). Along these lines, THC-INH resulted in lower body temperature at 30-min compared to all other timepoints (p < 0.05). THC-INJ resulted in lower body temperature at 90- and 240-min compared to 30 and 60-min (p < 0.001).
THC
Control values serve as assay controls and were undetectable. Analyte concentrations were compared across administration groups and sex. THC chromatogram illustrates THC (black line) and THC-d3 (grey line) with an overlapping peak at 2.7 min (Fig. 3A).
THC Chromatogram and levels in blood and brain. (A) LC-MS Chromatogram: THC (black line) and THC-d3 (grey line) overlapping peaks at 2.7 min. (B) Blood levels: data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) indicates an administration difference with INH > INJ at 15-min at ****p < 0.0001. Presence of (#) indicates a timepoint difference with green and purple lines indicating an INH and INJ difference respectively; specifically, INH-15 > all timepoints and INH-30 > INH-60/90/240 at #p < 0.05, whereas INJ-30 > all timepoints and INJ-240 < all timepoints at ##p < 0.01. Presence of ($) indicates a sex difference; specifically, female-INH > female-INJ at $$$p < 0.001 and male-INJ > female-INJ at $$$p < 0.001). (C) Brain levels: data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) indicates an administration difference with INH > INJ at 15, 30, and 60-min at **p < 0.01. Presence of (#) indicates a timepoint difference with green and purple lines indicating an INH and INJ difference respectively; specifically, INH-15, 30, and 60 > INH-90 and 240 at ##p < 0.01, whereas INJ-90 > INJ-15 and 240 at ##p < 0.01. Presence of ($) indicates a sex difference with males > females at $$p < 0.01.
In females, but not males, plasma THC concentrations were higher following INH than INJ regardless of timepoint (interaction effect of sex and group: F(1,94) = 11.164 at p < 0.01, post hoc female-INH > female-INJ at p < 0.001, Fig. 3B). Following injection, but not inhalation, males had higher plasma THC concentrations than females regardless of timepoint (male-INJ > female-INJ at p < 0.001). Further, regardless of group, males and females differed in plasma THC concentrations across timepoints (interaction effect of sex and timepoint: F(4,94) = 4.107 at p < 0.01, post hoc male-30 > all timepoints at p < 0.01, male-15 > male-90 at p < 0.05, male 240 < male-15, -30, -60 at p < 0.01, female-15 > all timepoints at p < 0.05, female-30 > female-60, -90, -240 at p < 0.05 Fig. 3B). Males also had higher plasma THC concentrations than females at 30- and 60-min (post hoc male-30 > female-30 at p < 0.001 and male-60 > female-60 at p < 0.05). Finally, regardless of sex, plasma THC concentrations were higher following inhalation than injection at 15-min but not different at any other timepoint (interaction effect of group and timepoint: F(4,94) = 5.301 at p < 0.01, post hoc INH-15 > INJ-15 at p < 0.0001, Fig. 3B). Plasma THC concentrations also differed by group across timepoint, such that following inhalation, plasma THC was higher at 15-min than all other timepoints and higher at 30-min than later timepoints (post hoc INH-15 > all timepoint at p < 0.01 and INH-30 > INH-60, -90, -240 at p < 0.05, Fig. 3B), whereas following injection, plasma THC was higher at 30-min compared to all other timepoints and lower at 240-min compared to all other timepoints (post hoc INJ-30 > all timepoints at p < 0.01 and INJ-240 < all timepoints at p < 0.01).
Brain THC concentrations were higher following INH than INJ at 15-, 30- and 60-min regardless of sex (interaction effect of group and timepoint: F(4,95) = 7.791 at p < 0.0001; post-hoc INH-15 > INJ-15 at p < 0.00001, INH-30 > INJ-30 at p < 0.0001, and INH-60 > INJ-60 at p < 0.01, Fig. 3C). Further, brain THC concentrations were higher at 15-, 30- and 60-min compared to 90- and 240-min following inhalation (post-hoc INH-15 > INH-90 and 240 at p < 0.01; INH-30 > INH-90 and 240 at p < 0.001; INH-60 > INH-90 and 240 at p < 0.01). Alternatively, brain THC concentrations were higher at 90-min than 15- and 240-min, and trending higher compared to 30-min, following injection (post-hoc INJ-90 > INJ-15 at p < 0.01; INJ-90 > INJ-30 at p = 0.07; INJ-90 > INJ-240 at p < 0.01). Brain THC concentrations were higher in males than females, regardless of group or timepoint (main effect of sex: F(1,95) = 9.482 at p < 0.01, Fig. 3C).
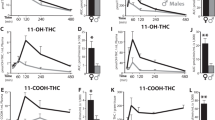
11-OH-THC
11-OH-THC chromatogram (Fig. 4A) illustrates 11-OH-THC (black line) and 11-OH-THC-d3 (grey line) with an overlapping peak at 2.0 min. Plasma 11-OH-THC concentrations were higher following INJ than INH at all timepoints regardless of sex (interaction effect of group and timepoint: F(4,95) = 4.412 at p = 0.003; post-hoc INH-15 < INJ-15 at p = 0.03, INH-30 < INJ-30 at p < 0.00001, INH-60 < INJ-60 at p < 0.00001, INH-90 < INJ-90 at p < 0.00001, INH-240 < INJ-240 at p = 0.037). Further, while plasma 11-OH-THC concentrations did not differ across timepoint following inhalation, following injection concentrations were lower at 15-min compared to 30-, 60-, and 90-min (post-hoc INJ-15 < INJ-30 at p = 0.0002, INJ-15 < INJ-60 at p = 0.038, INJ-15 < INJ-90 at p = 0.032), as well as lower at 240-min compared to 30-, 60- and 90-min (post-hoc INJ-240 < INJ-30 at p < 0.00001, INJ-240 < INJ-60 at p = 0.0005, INJ-240 < INJ-90 at p = 0.0005). Plasma 11-OH-THC concentrations were higher in females than males, regardless of group or timepoint (main effect of sex: F(1,95) = 4.613 at p = 0.034, Fig. 4B).
11-OH-THC chromatogram and levels in blood and brain. (A) LC-MS Chromatogram: 11-OH-THC (black line) and 11-OH-THC-d3 (grey line) peaks at 2.0 min. (B) Blood levels: data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) indicates an administration difference with INH < INJ at all timepoints at *p < 0.05. Presence of (#) indicates a timepoint difference with purple lines indicating an INJ difference where INJ-15 < INJ-30, 60, and 90 at #p < 0.05 and INJ-240 < INJ-30, 60, and 90 at ###p < 0.001. Presence of ($) indicates a sex difference; specifically, females > males at $p < 0.05. (C) Brain levels: data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) indicates an administration difference with INH < INJ at 30, 60, and 90-min at ***p < 0.001. Presence of (#) indicates a timepoint difference with purple lines indicating an INJ difference where INJ-15 and 240 < INJ-30, 60, and 90 at ###p < 0.001. Presence of ($) indicates a sex difference; specifically, male-INH < male-INJ at $$$p < 0.001, female-INH < female-INJ at $$$$p < 0.0001, and male-INJ < female-INJ at $$$$p < 0.0001.
In both sexes, brain 11-OH-THC concentrations were higher following INJ than INH regardless of timepoint (interaction effect of sex and group: F(1,95) = 5.356 at p = 0.023; post hoc male-INH < INJ at p = 0.0002 and female-INH < INJ at p < 0.00001, Fig. 4C). Further, following injection, brain 11-OH-THC concentration were higher in females than males (post hoc male-INJ < female-INJ at p < 0.00001). Regardless of sex, brain 11-OH-THC concentrations were higher following INJ than INH at 30-, 60- and 90-min (interaction effect of group and timepoint: F(4,95) = 6.411 at p = 0.0001; post hoc INH-30 < INJ-30 at p = 0.0002, INH-60 < INJ-60 at p < 0.00001, INH-90 < INJ-90 at p < 0.00001, Fig. 4C). Further, while brain 11-OH-THC concentrations did not differ across timepoint following inhalation, following injection brain 11-OH-THC concentration were lower at 15-min compared to 30-, 60-, and 90-min (post-hoc INJ-15 < INJ-30 at p = 0.0009, INJ-15 < INJ-60 at p < 0.00001, INJ-15 < INJ-90 at p < 0.00001), as well as lower at 30-min compared to 60-min (post hoc INJ-30 < INJ-60 at p = 0.023) and at 240-min compared to 30-, 60- and 90-min (post-hoc INJ-240 < INJ-30 at p = 0.001, INJ-240 < INJ-60 at p < 0.00001, INJ-240 < INJ-90 at p < 0.00001).
THC-COOH
THC-COOH chromatogram (Fig. 5A) illustrates THC-COOH (black line) and THC-COOH-d3 (grey line) with an overlapping peak at 2.0 min. In both sexes, plasma THC-COOH concentrations were higher following INJ than INH regardless of timepoint (interaction effect of sex and group: F(1,93) = 12.241 at p = 0.0007; post hoc male-INH < INJ at p = 0.00006 and female-INH < INJ at p < 0.00001, Fig. 5B). Further, following injection, plasma THC-COOH concentration were higher in females than males (post hoc male-INJ < female-INJ at p < 0.00001). Regardless of sex, plasma THC-COOH concentrations were higher following INJ than INH at 30-, 60-, 90- and 240-min (interaction effect of group and timepoint: F(4,93) = 3.0 at p = 0.022; post hoc INH-30 < INJ-30 at p < 0.00001, INH-60 < INJ-60 at p < 0.00001, INH-90 < INJ-90 at p < 0.00001, INH-240 < INJ-240 at p = 0.00003, Fig. 5B). Further, while plasma THC-COOH concentrations did not differ across timepoint following inhalation, following injection plasma THC-COOH concentration were lower at 15-min compared to all other timepoints (post-hoc INJ-15 < all timepoints at p < 0.003).
THC-COOH chromatogram and levels in blood and brain. (A) LC-MS Chromatogram: THC-COOH (black line) and THC-COOH-d3 (grey line) peaks at 2.0 min. (B) Blood levels: Data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) indicates an administration difference with INH < INJ at 30, 60, 90 and 240-min at *p < 0.05. Presence of (#) indicates a timepoint difference with purple lines indicating an INJ difference where INJ-15 < all timepoints at ##p < 0.01. Presence of ($) indicates a sex difference; specifically, male-INH < male-INJ and female-INH < female-INJ at $$$$p < 0.0001, and male-INJ < female-INJ at $$$$p < 0.0001. (C) Brain levels: Data are presented as mean ± SEM; n = 5–6 for each group. Presence of (*) indicates an administration difference with INH > INJ at ****p < 0.0001. Presence of ($) indicates a sex difference with males < females at $$$p < 0.001.
Irrespective of group or timepoint, brain THC-COOH concentrations were higher in females than males (main effect of sex: F(1,95) = 15.442 at p = 0.0002, Fig. 5C). Further, irrespective of sex or timepoint, brain THC-COOH concentrations were higher following inhalation than injection (main effect of group: F(1,95) = 56.656 at p < 0.00001, Fig. 5C).
Discussion
Given the increased usage, demand, and potency of cannabis over the last few years1,39,40, establishing an animal model that closely reflects human consumption is critical to study the impact of cannabis on brain and behaviour. Most animal studies to date examine the effects of cannabis through IP injection of THC, which produces markedly different effects than inhalation3. In efforts to make THC injection studies possess more face validity for translatability to humans, these previous studies aimed to produce peak plasma THC concentrations that are comparable to concentrations in human cannabis smokers. Utilizing ‘comparable’ dosages established in previous literature4,25, as well as through pilot work in our laboratory, we sought to produce similar peak plasma THC concentrations following injection and inhalation that fell within the range produced in humans from cannabis17,18,19,20,21,23 in order to determine if route of administration influenced metabolism or central accumulation of THC. Our data provide clear evidence on the different physiological response and pharmacokinetic profiles of THC and metabolites following comparable dosing of inhaled versus injected THC, which could have significant impacts for data interpretation and generalizability. Importantly, we found that inhalation led to immediate hypothermia and an initial higher plasma and brain THC concentration, while injection led to delayed hypothermia, dramatically higher 11-OH-THC concentrations in both plasma and brain and higher THC-COOH concentration in the brain. Males in general had higher THC levels while females had higher metabolite levels, supporting previous findings of robust sex differences in the pharmacokinetics of THC.
Body temperature
It is well established that THC exposure induces a hypothermic response in both humans and animals12,25,29. Hypothermia was found in both males and females following inhalation and injection of THC. In accordance with previous literature25,29, THC inhalation led to immediate hypothermia with lower temperatures at 30- and 60-min which normalized by 90- and 240-min, whereas THC injection results in delayed hypothermia with lower temperatures at 90- and 240-min compared to 30- and 60-min. This hypothermic difference based on route of administration is not surprising given that inhaled THC quickly enters the bloodstream and is taken up by the brain, whereas injected THC undergoes metabolism by the liver before reaching systemic circulation10. Along these lines, the onset of the hypothermic response seen in this study occurs in conjunction with peak brain THC levels following both inhalation and injection, at 30- and 90-min respectively, indicating that this is a good physiological proxy readout of central accumulation of cannabinoids. The impact of route of administration on the temporal kinetics of hypothermia was not influenced by sex. Notably, body temperature was also decreased at 240-min in rats exposed to CON inhalation. The delayed hypothermic effect in control animals is likely attributed to removal from their home cage at 30-, 60-, 90, and 240-min to record body temperature, compared to THC exposed animals in which body temperature was recorded separately for each timepoint due to euthanasia for mass spectrometry analysis. Alternately, as stress is known to produce mild hyperthermic responses41, it is also possible that exposure to the control vapor itself was a mildly stressful experience that produced a transient hyperthermic response that waned over time.
THC concentrations in plasma and brain
Utilizing ‘comparable’ doses of THC inhalation and injection, as determined by previous literature and our pilot work4,25, we show altered plasma THC levels across route of administration and timepoint. Specifically, inhalation led to higher plasma THC concentrations than injection at 15-min, but the two routes of administration did not differ at any other timepoint. This is not surprising as inhalation results in much more rapid uptake into the bloodstream than injection. In fact, it is known that inhalation produces peak plasma THC levels 10–15-min after initial administration in humans and rodents17,19,20,21,30, whereas peak levels are found at a slightly later timepoint following injection in rodents4,25,26,28. Along these lines, plasma THC concentrations were highest following inhalation at 15- and 30-min compared to 60-, 90-, and 240-min. Alternatively, plasma THC concentrations were higher following injection at 30-min compared to all other timepoints. As anticipated, there was no difference between peak plasma THC levels between the two routes of administration (peak THC following inhalation: 73 ng/mL vs. peak THC following injection: 72 ng/mL), and importantly these levels fall within the range found in human studies (60–200 ng/mL)17,18,19,20,21,23.
Despite the similar peak plasma THC concentrations, inhalation led to higher brain THC concentrations at 15-, 30-, and 60-min compared to injection. This is not surprising as cannabis inhalation provides rapid delivery into the blood stream, bypasses initial liver metabolism, and results in more immediate uptake by highly perfused tissues, such as the brain10,11,12. Further, in accordance with previous literature showing earlier peak brain THC concentrations following inhalation than injection30, we found brain THC levels peaked at 15- and 30-min following inhalation, while peak brain THC levels did not occur until 90-min following injection. Interestingly, the peak subjective “high” in humans following cannabis inhalation is ~ 30-min following onset of administration32, which corresponds to the higher brain THC concentrations found following inhalation.
11-OH-THC levels in plasma and brain
Overall, injection administration yielded significantly higher plasma and brain concentrations of 11-OH-THC compared to inhalation. Specifically, both plasma and brain concentrations were relatively low (~ 13 and ~ 22 ng/mL respectively) following inhalation and did not differ across timepoints. Low concentrations of 11-OH-THC following inhalation are common as concentrations are recirculated through the enterohepatic pathway (liver to bile to small intestine back to liver) and quickly metabolized to THC-COOH32. Alternatively, plasma concentrations of 11-OH-THC were highest at 30-, 60-, and 90-min compared to 15- and 240-min following injection, reaching average peak levels of ~ 88 ng/mL, which is about 8 times higher than inhalation levels. Further, brain concentrations of 11-OH-THC were also highest at 30-, 60-, and 90-min compared to 15- and 240-min following injection, reaching average peak levels of ~ 98 ng/g, which is about 4.5 times higher than inhalation levels. This striking difference in the production of 11-OH-THC is not trivial because 11-OH-THC is an agonist at CB1R, is psychoactive, is believed to pass into the brain more readily than THC, and is as, or more, potent than THC in its ability to produce behavioural and physiological effects32,33,34. Recognizing that it is ultimately the activation of CB1R in the brain, which is readily achieved by both THC and possibly more so by 11-OH-THC, the overall impact administration of THC will have on central CB1R activation must take both THC and 11-OH-THC levels into account. Following injection, extremely high concentrations of 11-OH-THC in the brain will produce different physiological, psychological, and behavioural effects as compared to inhalation. In fact, given the dramatic accumulation of 11-OH-THC in the brain following injection, as well as its significant potency at the CB1R, it seems reasonable to hypothesize that injection of THC produces a much more robust and sustained activation of brain CB1R than THC administered via inhalation. As our data indicate that inhalation produces a rapid accumulation of THC in the brain, followed by relatively rapid clearance, this would suggest that the ability of inhaled THC to activate brain CB1R is likely a time-limited effect. This is consistent with our hypothermia data and the relatively rapid peak, and diminution, of psychological effects and intoxication produced by inhaled cannabis or THC. Alternatively, injected THC produces lower initial brain THC concentrations compared to inhaled with levels accumulating over time to reach peak THC levels at 90-min that are comparable to inhaled. However, injection administration also includes the addition of high 11-OH-THC levels, produced through hepatic metabolism, and sequestered into the brain. As such, injections of THC will potentially produce profoundly different biological effects since the magnitude of CB1R activation (through brain levels of both THC and 11-OH-THC) will be much greater than that following inhaled THC. Given that there is a notable discrepancy between many of the beneficial and adverse impacts of THC that have been documented in rodent studies using injection-based approaches relative to human studies examining cannabis users, one must consider that the injection-based approach for THC has limitations for translational research. As the cellular impacts of CB1R activation will be influenced by the magnitude and duration of its activation, the impacts of accumulating 11-OH-THC in the brain following injections of THC must be considered in future rodent studies.
THC-COOH levels in plasma and brain
Injection administration yielded higher levels of plasma THC-COOH but lower levels of brain THC-COOH compared to inhalation. More specifically, plasma concentrations of THC-COOH were relatively low (~ 7 ng/mL) following inhalation and did not differ across timepoints. Whereas plasma concentrations of THC-COOH were highest at 30-, 60-, 90- and 240-min compared to 15-min, reaching average peak levels of ~ 60 ng/mL, about 9 times higher than inhalation levels. Along these lines, previous studies have also shown peak THC-COOH concentrations at later timepoints (60–120 min) following injection in rats28 and inhalation in humans18. Further, given that plasma 11-OH-THC concentrations were higher following injection, and 11-OH-THC is the metabolic precursor of THC-COOH, it is not surprising that THC-COOH follows the same pattern. Low levels of THC-COOH in the brain are anticipated as it is the primary metabolite for urinary elimination11.
Sex differences
Females exhibited significantly higher levels of 11-OH-THC and THC-COOH in both brain and blood, indicating that females metabolize THC at a faster rate than males do, which is consistent with previous work in rats27,31,42 and humans43. Interestingly, many studies in humans report that females are more sensitive to the effects of THC relative to males, particularly in the context of adverse effects of THC or subjective ratings of “drug effect”43,44. Further, rodent studies also show sex differences in hypothermia, motor effects, anti-nociception, anxiety-like behaviour, and feeding behaviour with generally greater effects in females than males27,29,45. The sex-differences in the subjective experience and behavioural effects of cannabis are likely attributed to higher levels of 11-OH-THC in females. In line with accelerated metabolism of THC and increased concentrations of THC metabolites in females in our study, female rats were found to have lower levels of THC itself relative to males, particularly in the brain. Previous animal studies have shown no differences between male and female THC blood and brain levels25,27,29,31. This sex difference in the production of 11-OH-THC, particularly following injections, has relevance for interpreting preclinical studies. Our data suggests that while females achieve slightly lower brain THC levels than males, they appear to acquire brain 11-OH-THC levels that are double that of males following injection of THC. Given the bioactivity and potency of 11-OH-THC, this suggests that injection-based studies of THC may show sex differences in some outcomes, but this effect may be an artifact of the elevated levels of 11-OH-THC produced by injection; an effect which does not occur following inhalation where brain 11-OH-THC levels are quite low and comparable between males and females. These sex differences in THC metabolism may also have implications for human THC consumption, especially when it is consumed via an oral route as entero-hepatic metabolism will impact THC metabolism.
Limitations and conclusions
Of note, the current studies do not include oral or edible intake of cannabis, another popular form of consumption. This is an area of future work in our group and has recently been successfully executed by others46. Oral consumption would also result in an increase in 11-OH-THC production due to first pass metabolism; however, given that an intoxicating dose of oral cannabis in humans produces peak blood levels of THC in the range of 1–5 ng/mL47, which is about 1/20–1/100 of the current level produced by injection of THC, one can anticipate that the levels of 11-OH-THC produced would be substantially lower than what we see following injection. Thus, despite the potential similarities in pharmacokinetic trajectories, injections would not be a suitable comparison for oral routes of administration of cannabis or THC. Along these lines, passive inhalation approaches, such as those utilized in the current experiments, expose the entire animal to cannabis vapor resulting in potential exposure through skin and/or through oral exposure by grooming of fur. However, peak THC levels occur roughly 2–4 h following oral consumption48 and our inhalation exposure groups show a steady decline in blood and brain THC levels at these timepoints, suggesting any exposure through skin or grooming is very limited. Finally, our current comparison of injection and inhalation routes utilized different vehicle solvents. Both solvents were used at levels much lower than concentrations leading to toxicity, however it remains possible these solvents contributed slightly to the different THC and metabolite levels in blood and brain. Nevertheless, these solvents are some of the most commonly used diluents for these routes of administration, which further represents the dissimilarities between injection and inhalation approaches and supports the importance of modeling human consumption in translational research.
In conclusion, our data demonstrate significant and biologically relevant differences in the pharmacokinetics and accumulation of THC and metabolites following injection versus inhalation. The current study generally supports previous findings but provides the first direct comparison of both sex and route of administration of THC to reveal an accurate picture of how these variables are impacting THC metabolism and central accumulation. These findings should be considered for translational preclinical studies attempting to model the impacts of cannabis or THC on the brain and behavioural processes. IP injections are the most frequent route of administration for animal models examining the effects of cannabis (THC) and many previous studies claim the translatability and relevance to human consumption by aiming to produce peak plasma THC concentrations that are comparable to concentrations in human cannabis smokers. Utilizing doses that produced comparable peak plasma THC concentrations, our study illustrates robust differences in the pharmacokinetics and central accumulation of THC and its bioactive metabolites when administered via injection versus inhalation. These differences likely underlie the inconsistency of reproducible behavioural findings between THC injections and inhalation and support the importance of appropriately modeling the route of administration in preclinical studies. This is not uncommon in the drug research field, and in fact studies utilizing injections of ethanol have long been abandoned over the appropriate use of ethanol vapor or drinking as this produces comparable effects to humans and allows for the study of volitional administration. Based on the data generated herein, we suggest that researchers conducting translational work in the realm of THC and cannabis strongly consider utilizing inhalation models, or oral routes of administration, to ensure that any biological effects they see from THC or cannabis extract administration are not artifacts produced by the accumulation of 11-OH-THC in the brain and activating CB1R in a temporal manner that is likely quite distinct from what is occurring with humans during typical cannabis use.
References
Goodman, S., Wadsworth, E., Leos-Toro, C. & Hammond, D. Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int. J. Drug Policy 76, 102658 (2020).
Cuttler, C., Mischley, L. K. & Sexton, M. Sex differences in cannabis use and affects: A cross-sectional survey of cannabis users. Cannabis Cannabinoid Res. 1, 166–175 (2016).
Manwell, L. A., Ford, B., Matthews, B. A., Heipel, H. & Mallet, P. E. A vaporized δ9-tetrahydrocannabinol (δ9-THC) delivery system part II: Comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J. Pharmacol. Toxicol. Methods 70, 112–119 (2014).
Nguyen, J. D. et al. Inhaled delivery of Δ9-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109, 112–120 (2016).
Freels, T. G. et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. J. Neurosci. 40, 1897–1908 (2020).
Ashton, C. H. Pharmacology and effects of cannabis a brief review. Br. J. Psychiatry 178, 101–106 (2001).
Kirkham, T. C. & Williams, C. M. Endogenous cannabinoids and appetite. Nutr. Res. Rev. 14, 65–86 (2005).
Sharma, P., Murthy, P. & Bharath, M. M. S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 7, 149–156 (2012).
Lukas, G., Brindle, S. D. & Greengard, P. The route of absorption of intraperitoneally administered compounds. J. Pharmacol. Exp. Ther. 178, 562–566 (1971).
Turner, P. V., Brabb, T., Pekow, C. & Vasbinder, M. A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 50, 600–613 (2011).
Huestis, M. A. Pharmacokinetics and metabolism of the plant cannabinoids, ∆9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb. Exp. Pharmacol. 168, 657–690 (2005).
Huestis, M. A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 4, 1770–1804 (2007).
Lee, D. C., Crosier, B. S., Borodovsky, J. T., Sargent, J. D. & Budney, A. J. Online survey characterizing vaporizer use among cannabis users. Drug Alcohol Depend. 159, 227–233 (2016).
Morean, M. E., Kong, G., Camenga, D. R., Cavallo, D. A. & Krishnan-Sarin, S. High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics 136, 611–616 (2015).
Spindle, T. R. et al. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: A crossover trial. JAMA Netw. Open 1, e184841 (2018).
Loflin, M. & Earleywine, M. A new method of cannabis ingestion: The dangers of dabs?. Addict. Behav. 39, 1430–1433 (2014).
Hartman, R. L. et al. Controlled cannabis vaporizer administration: Blood and plasma cannabinoids with and without alcohol. Clin. Chem. 61, 850–869 (2015).
Huestis, M. A., Henningfield, J. E. & Cone, E. J. Blood cannabinoids. ii. Models for the prediction of time of marijuana exposure from plasma concentrations of ∆9-tetrahydrocannabinol (thc)and 11-nor-9-carboxy-∆9- tetrahydrocannabinol (thccooh). J. Anal. Toxicol. 16, 283–290 (1992).
Huestis, M. A. & Cone, E. J. Relationship of Δ9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J. Anal. Toxicol. 28, 394–399 (2004).
Newmeyer, M. N. et al. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: Identification of recent cannabis intake. Clin. Chem. 62, 1579–1592 (2016).
Schwope, D. M., Karschner, E. L., Gorelick, D. A. & Huestis, M. A. Identification of recent cannabis use: whole-blood and plasma free glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis adminsitration. Clin. Chem. 57, 1406–1414 (2011).
Spindle, T. R. et al. Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J. Anal. Toxicol. 43, 233–258 (2019).
Abrams, D. I. et al. Vaporization as a smokeless cannabis delivery system: A pilot study. Clin. Pharmacol. Ther. 82, 572–578 (2007).
Bidwell, L. C. et al. Association of naturalistic administration of cannabis flower and concentrates with intoxication and impairment. JAMA Psychiat. 9, 1–10 (2020).
Javadi-Paydar, M. et al. Effects of Δ9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology 235, 2541–2557 (2017).
Taffe, M. A., Creehan, K. M., Vandewater, S. A., Kerr, T. M. & Cole, M. Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague–Dawley and wistar rats. Exp. Clin. Psychopharmacol. https://doi.org/10.1037/1064-1297.11.3.c2 (2020).
Ruiz, C. M. et al. Pharmacokinetic, behavioral, and brain activity effects of ?9-tetrahydrocannabinol in adolescent male and female rats. Neuropsychopharmacology 46, 959–969 (2021).
Torrens, A. et al. Comparative pharmacokinetics of Δ9-tetrahydrocannabinol in adolescent and adult male mice. J. Pharmacol. Exp. Ther. https://doi.org/10.1124/jpet.120.265892 (2020).
Nguyen, J. D., Creehan, K. M., Kerr, T. M. & Taffe, M. A. Lasting effects of repeated ∆9-tetrahydrocannabinol vapor inhalation during adolescence in male and female rats. Br. J. Pharmacol. 177, 188–203 (2020).
Hložek, T. et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 27, 1223–1237 (2017).
Wiley, J. L. & Burston, J. J. Sex differences in δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci. Lett. 576, 51–55 (2014).
Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 42, 327–360 (2003).
Lemberger, L., Crabtree, R. E. & Rowe, H. M. 11-hydroxy-∆9-tetrahydrocannabinol: pharmacology, disposition, and metabolism of a major metabolite of marihuana in man. Science (80-) 177, 62–64 (1972).
Perez-Reyes, M., Timmons, M. C., Lipton, M. A., Davis, K. H. & Wall, M. E. Intravenous injection in man of Δ9 tetrahydrocannabinol and 11-OH-Δ9-tetrahydrocannabinol. Science (80-) 177, 633–635 (1972).
Burstein, S. H. The cannabinoid acids: Nonpsychoactive derivatives with therapeutic potential. Pharmacol. Ther. 82, 87–96 (1999).
Galvao, J. et al. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 28, 1317–1330 (2014).
Traboulsi, H. et al. Inhalation toxicology of vaping products and implications for pulmonary health. Int. J. Mol. Sci. 21, 3495 (2020).
CISCO. Safety Data Sheet: Polyethylene Glycol 400. CAS# 25322-68-3. http://www.ciscochem.com/assets/polyethylene-glycol-400-sds.pdf (2015).
ElSohly, M. A. et al. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biol. Psychiatry 79, 613–619 (2016).
Canadian Cannabis Survey. Government of Canada. http://publications.gc.ca/collections/collection_2019/sc-hc/H21-312-2019-2-eng.pdf (2019).
Dallmann, R., Steinlechner, S., Von Hörsten, S. & Karl, T. Stress-induced hyperthermia in the rat: Comparison of classical and novel recording methods. Lab. Anim. 40, 186–193 (2006).
Tseng, A. H., Harding, J. W. & Craft, R. M. Pharmacokinetic factors in sex differences in Δ9- tetrahydrocannabinol-induced behavioral effects in rats. Behav. Brain Res. 154, 77–83 (2004).
Sholler, D. J., Strickland, J. C., Spindle, T. R., Weerts, E. M. & Vandrey, R. Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addict. Biol. 26, 1–12 (2021).
Cuttler, C., Mischley, L. K. & Sexton, M. Sex differences in cannabis use and effects: A cross-sectional survey of cannabis users. Cannabis Cannabinoid Res. 1, 166–175 (2016).
Tseng, A. H. & Craft, R. M. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur. J. Pharmacol. 430, 41–47 (2001).
Kruse, L. C., Cao, J. K., Viray, K., Stella, N. & Clark, J. J. Voluntary oral consumption of Δ9-tetrahydrocannabinol by adolescent rats impairs reward-predictive cue behaviors in adulthood. Neuropsychopharmacology 44, 1406–1414 (2019).
Vandrey, R. et al. Pharmacokinetic profile of oral cannabis in humans: Blood and oral fluid disposition and relation to pharmacodynamic outcomes. J. Anal. Toxicol. 41, 83–99 (2017).
Karschner, E. L. et al. Implications of plasma {Delta}9-tetrahydrocannabinol, 11-hydroxy-{THC}, and. J Anal Toxicol 33, 469–477 (2009).
Acknowledgements
This study was supported by operating funds from the Canadian Institutes of Health Research (CIHR TCP-431510; MNH) and the Hotchkiss Brain Institute (MNH and SL Borgland). SL Baglot received salary support from a Vanier Scholarship from CIHR, CH received salary support from Eyes High Postdoctoral Scholarship and Harley Hotchkiss—Samuel Weiss Postdoctoral Fellowship, GNP received salary support from the Branch Out Neurological Foundation, RJA received salary support from the Mathison Centre for Mental Health Research & Education, SHML received salary support from the William H. Davies Medical Research Scholarship. All funding agencies had no influence on design, execution, or publishing of this work. Thank you to all current and former members of the Hill laboratory for their assistance and expertise. Thank you to Maury Cole and La Jolla Alcohol Research Inc. for technical assistance with the vapor chambers and Cece Hillard for input on the manuscript.
Author information
Authors and Affiliations
Contributions
The study was conceptualized by S.L.B and M.N.H. with assistance from L.P., S.L.B., and R.J.M.; S.L.B. setup the vapor chambers, carried out the experimental procedures, and completed data acquisition and analysis with help from C.H., G.N.P., R.J.A., S.H.M.L., and L.M.G. R.Z. and L.B. carried out mass spectrometry method development and sample execution. The original manuscript draft was prepared by S.L.B. and subsequently edited by R.Z., L.P., J.M.R., S.L.B., R.J.M., L.B., and M.N.H.. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.N.H. is a member of the scientific advisory board for Shoppers Drug Mart, Jazz Pharmaceuticals and Lundbeck; all other authors have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baglot, S.L., Hume, C., Petrie, G.N. et al. Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci Rep 11, 23990 (2021). https://doi.org/10.1038/s41598-021-03242-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03242-7
This article is cited by
-
Effects of prenatal THC vapor exposure on body weight, glucose metabolism, and feeding behaviors in chow and high-fat diet fed rats
International Journal of Obesity (2024)
-
Measurement of Δ9THC and metabolites in the brain and peripheral tissues after intranasal instillation of a nanoformulation
Journal of Cannabis Research (2023)
-
Adolescent Δ-9-tetrahydrocannabinol exposure induces differential acute and long-term neuronal and molecular disturbances in dorsal vs. ventral hippocampal subregions
Neuropsychopharmacology (2023)
-
Characterization of cannabinoid plasma concentration, maternal health, and cytokine levels in a rat model of prenatal Cannabis smoke exposure
Scientific Reports (2023)
-
Measuring the diversity gap of cannabis clinical trial participants compared to people who report using cannabis
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.