Abstract
Type 2 diabetes (T2D) is associated with increased risk of cardiovascular disease (CVD). As disturbed angiogenesis and endothelial dysfunction are strongly implicated in T2D and CVD, we aimed to investigate the association between a novel anti-angiogenic protein, FK506-binding protein like (FKBPL), and these diseases. Plasma FKBPL was quantified by ELISA cross-sectionally in 353 adults, consisting of 234 T2D and 119 non–diabetic subjects with/without CVD, matched for age, BMI and gender. FKBPL levels were higher in T2D (adjusted mean: 2.03 ng/ml ± 0.90 SD) vs. non-diabetic subjects (adjusted mean: 1.79 ng/ml ± 0.89 SD, p = 0.02), but only after adjustment for CVD status. In T2D, FKBPL was negatively correlated with fasting blood glucose, HbA1c and diastolic blood pressure (DBP), and positively correlated with age, known diabetes duration, waist/hip ratio, urinary albumin/creatinine ratio (ACR) and fasting C-peptide. FKBPL plasma concentrations were increased in the presence of CVD, but only in the non-diabetic group (CVD: 2.02 ng/ml ± 0.75 SD vs. no CVD: 1.68 ng/ml ± 0.79 SD, p = 0.02). In non-diabetic subjects, FKBPL was positively correlated with an established biomarker for CVD, B-type Natriuretic Peptide (BNP), and echocardiographic parameters of diastolic dysfunction. FKBPL was a determinant of CVD in the non-diabetic group in addition to age, gender, total-cholesterol and systolic blood pressure (SBP). FKBPL may be a useful anti-angiogenic biomarker in CVD in the absence of diabetes and could represent a novel CVD mechanism.
Similar content being viewed by others
Introduction
Diabetes is becoming an epidemic disease of global proportion with over 460 million people living with the condition in 2019; type 2 diabetes mellitus (T2D) accounts for the majority1. People with diabetes have up to three-fold higher2 incidence of cardiovascular disease (CVD), the leading cause of death globally3. Despite several long-term clinical trials, such as UKPDS, showing that glucose lowering strategies are effective in reducing the incidence of CVD in diabetes, this is still significantly higher even in optimally treated patients4. Therefore, it is clear that better stratification of patients and more effective personalised treatment strategies are needed. Natriuretic Peptides (NPs), including B-type Natriuretic Peptide (BNP) and N-terminal pro BNP (NT-proBNP), are the most reliable biomarkers for identifying people with heart failure (HF), both in heart failure with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF), previously known as diastolic and systolic HF, respectively5. NPs have also been shown to predict future major adverse cardiovascular events in patients with CVD risk factors6. Inflammation appears to be one of the main underlying mechanisms leading to CVD in diabetes, whereas in the absence of diabetes other mechanisms are also implicated including angiogenesis, remodelling and metabolism7. Identifying novel mechanisms, which can be explored as biomarkers or therapeutic targets of both T2D and/or CVD, is important for the prevention of associated complications.
FK506 binding-protein like (FKBPL) is a novel angiogenesis-related protein, which inhibits endothelial cell migration through disruption of actin/tubulin dynamics via the CD44 pathway8,9, and regulates glucocorticoid receptor activity10. Recently, FKBPL has also been shown to target inflammatory the STAT3 pathway11. A first-in-class pre-clinical candidate peptide, AD-01, based on the active anti-angiogenic domain of FKBPL, was developed and has been extensively tested pre-clinically8,12,13. A clinical therapeutic peptide, ALM201, has completed a Phase I clinical trial for the treatment of solid tumours (EudraCT No: 2014-001175-31)14. As anticipated, FKBPL haploinsufficient (Fkbpl+/-) mice demonstrated that FKBPL has a critical role in physiological, developmental and pathological angiogenesis; blood vessel development was also impaired when FKBPL was knocked down in zebrafish15. Interestingly, homozygous knockout of the FKBPL gene led to embryonic lethality, highlighting its important role in developmental angiogenesis. Notably, Fkbpl+/− embryos were viable, however they showed signs of early endothelial dysfunction15, but developed normally with some level of vascular dysfunction and leakiness15.
Vascular and endothelial dysfunction precipitated by hyperglycaemia are well-studied aberrant mechanisms in diabetes and CVD16,17,18. Microangiopathy, as a result of endothelial dysfunction and angiogenic imbalance, is implicated in the development of CVD in diabetes19. Therefore, since FKBPL regulates angiogenesis and glucocorticoid receptor signalling, we hypothesised that it could also have a role in the pathogenesis of vascular damage in T2D as well as CVD. The main aims of our study were to investigate the relationship between circulating FKBPL levels and the presence of T2D and/or CVD and determine the correlations with metabolic and cardiac function parameters. This report investigates circulating FKBPL levels in diabetes and/or CVD for the first time.
Methods
Subjects and samples
Plasma samples used in this study were collected from 234 subjects with T2D aged 50–75 years as part of the prospective, randomised, double-blind, placebo controlled Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, from well-characterised patients who were recruited and screened but not randomised thereafter to receive fenofibrate or placebo. Participants in the FIELD study were statin-naïve and had the following lipid profile: total-cholesterol concentration of 3.0–6.5 mmol/L and a total-cholesterol/HDL-cholesterol ratio of 4.0 or more or plasma triglyceride of 1.0–5.0 mmol/L. Further details on the criteria for the recruitment into the FIELD study were described previously20 (ClinicalTrials.gov identifier: ISRCTN64783481). These subjects were matched for age, body mass index (BMI) and gender to non-diabetic controls, at an approximately 2:1 ratio (234 T2D plasma samples vs. 130 non-diabetes plasma samples), drawn from the STOP-HF study21 (ClinicalTrials.gov identifier: NCT00921960). The STOP-HF study is a prospective, randomized, controlled trial, which recruited patients with at least one risk factor for ventricular dysfunction, including hypertension, hypercholesterolaemia, obesity, coronary artery disease or diabetes mellitus22,23. For this study, we only selected participants without diabetes. Non-diabetic patients from the STOP-HF cohort were confirmed by measuring fasting blood glucose levels. This is part of the usual STOP-HF annual review programme. Patients with readings outside the normal range were referred for follow up. Individual glucose readings are not available for the analysis in this study. Study participant characteristics from both study groups are presented in Table 1. All subjects provided written informed consent to participate in the relevant parent FIELD or STOP-HF study, inclusive of biomarker studies, as per the principles outlined in the Declaration of Helsinki. The STOP-HF study was approved by the research ethics committee of St. Vincent’s University Hospital, Dublin. The FIELD study was approved by the University of Sydney Human Research Ethics Committee. The current analysis was approved as part of both studies.
FKBPL analysis
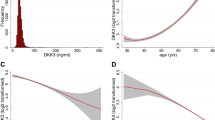
Plasma FKBPL concentrations were measured in 364 patients using a validated FKBPL ELISA assay (Cloud-Clone, China) as per manufacturer’s instructions. Samples were stored at − 80 °C until analysis. Average intra- and inter-assay CV for control samples, were 5% and 19%, respectively. Percentages of samples from T2D, non-diabetic control and those with or without CVD loaded onto each plate were kept constant. Plasma samples were analysed in duplicate, and FKBPL concentration for each sample was calculated using a 4-parameter fit based on the standard curve for each plate. Any duplicate samples with CV (%) > 15% were reanalysed and samples were only included in the analysis if the CV was < 15%. For 11 non-diabetic subjects there was inadequate sample volume for reanalysis. Demographics of subjects that we did not include were not significantly different from those who were included (Supplementary Table 1). BNP was measured at the point-of-care in the STOP-HF cohort only as previously reported21.
Statistical analysis
Analysis was performed on 119 Controls and 234 T2D cases. Group comparisons were analysed using independent samples t-tests (for continuous variables) or Mann–Whitney U-test, depending on the normality of the continuous data distribution, or using the χ2-test (with Yates’ continuity correction) for categorical variables. Analysis of variance (ANOVA) was used to compare FKBPL levels across 3 smoking categories. Penalised logistic regression was used to derive an efficient model assessing associations of clinical characteristics and FKBPL levels with the presence of clinically evident CVD. The correlation between two continuous variables was assessed based on the Pearson’s correlation coefficient. Statistical significance was defined as p < 0.05 (two-sided). Statistical analyses were performed using SPSS software, version 24 (IBM Corp, Armonk, NY, USA).
Results
Subject demographics
Study participants without diabetes (n = 119) and with T2D (n = 234) were well matched for age, gender and BMI (Table 1). The percentage of subjects with known CVD was twice as high in the non-diabetic group, however both groups represent only a small subgroup from each of the relevant studies. Smoking status and diastolic blood pressure (DBP) were similar in both groups, whereas systolic blood pressure (SBP) and total cholesterol were higher in patients with T2D. High-density lipoprotein cholesterol (HDL-C) levels were lower in subjects with T2D.
FKBPL plasma concentration in T2D and/or CVD
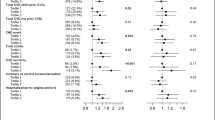
There was no difference in FKBPL plasma concentration between those with T2D and non-diabetic subjects. However, after adjustment for CVD status, FKBPL levels were higher in T2D (adjusted mean: 2.03 ng/ml ± 0.90 SD) vs. non-diabetic subjects (adjusted mean: 1.79 ng/ml ± 0.89 SD, p = 0.02) (Table 1, Supplementary Fig. 1A). When analysing associations of FKBPL levels with CVD, in a univariate analysis, no differences were observed between subjects with vs. without CVD irrespective of diabetes (without CVD: 1.89 ng/ml ± 0.91 SD vs. CVD: 2.09 ng/ml ± 0.82 SD, p = 0.07). However, in the non-diabetic group, plasma FKBPL concentrations were higher in those with than without CVD (CVD: 2.02 ng/ml ± 0.75 SD vs without CVD: 1.68 ng/ml ± 0.79 SD, p = 0.02). In diabetes only group, no difference in FKBPL levels was observed between subjects with vs without CVD (control: 1.97 ng/ml ± 0.93 SD vs. CVD: 2.16 ng/ml ± 0.9 SD, p = 0.21; Supplementary Table 2).
FKBPL as a potential diagnostic biomarker of CVD in the absence of diabetes
FKBPL plasma concentration was modestly associated with CVD status in non-diabetic adults, with an area under the curve (AUC) on the receiver operating characteristic (ROC) curve of 0.62 (p = 0.02; Supplementary Fig. 1B). When clinical parameters including age, SBP, cholesterol and gender were included in addition to FKBPL, the AUC increased to 0.72 (p = 0.01; Supplementary Fig. 1C). Using both diabetes and non-diabetes samples together (n = 351), FKBPL, age, SBP, cholesterol, gender and the presence of diabetes, the AUC was 0.73 (p < 0.001; Supplementary Fig. 1D). A well-established biomarker of CVD, BNP24, in the non-diabetic group provided an AUC of 0.70 (p = 0.001; Supplementary Fig. 2A). FKBPL and BNP were positively correlated (rs = 0.299, p = 0.001, n = 119). BNP together with clinical parameters (age, SBP, cholesterol and gender) showed an AUC of 0.77 (p = 0.001; Supplementary Fig. 2B). Furthermore, using penalised logistic regression, we identified a number of determinants of CVD in patients without diabetes and only one clinical determinant i.e. age in the T2D group (Table 2). In the non-diabetic group, FKBPL, age, SBP, total cholesterol and gender were associated with CVD (Table 2). Interestingly, total cholesterol was negatively correlated with the incidence of CVD, however, this could be related to the use of statins, considering this was a high-CVD risk non-diabetic group from the STOP-HF trial. Interestingly, FKBPL plasma levels were higher in men compared to women (2.19 ng/ml ± 0.89 SD vs. 1.66 ng/ml ± 0.79 SD, p˂0.001, Supplementary Table 3). Also, a difference in FKBPL levels was observed between smokers (2.43 ng/ml ± 1.05 SD), ex-smokers (2.13 ng/ml ± 0.84 SD) and non-smokers (1.68 ng/ml ± 0.81 SD) in the aggregated groups (ANOVA p ˂0.001; Supplementary Table 4).
Furthermore, FKBPL demonstrated strong correlations with several clinical characteristics and traditional risk factors in the T2D group. As shown in Table 3, FKBPL was positively correlated with age, known duration of diabetes, C-peptide level, urinary albumin to creatinine ratio (ACR), and waist to hip ratio. Negative correlations were observed between FKBPL and blood glucose, HbA1c, and DBP. In the non-diabetic control group, FKBPL showed correlation with measurements of cardiac structure and function based on a number of echocardiography parameters. Notably, parameters that are important features in diastolic dysfunction were correlated with FKBPL levels, including positive FKBPL correlations with left atrium volume (p = 0.001) and size (p = 0.003), interventricular septal thickness at end of diastole (IVSd; p = 0.049), and deceleration time (DT; p <0.001), and negative correlations with peak E (p = 0.049) and E/A ratio (p = 0.048; Table 4).
Discussion
In this cross-sectional study, we investigated the associations between circulating FKBPL in T2D and CVD using age-, BMI- and gender-matched participant samples from two major studies; T2D samples were obtained from the FIELD study20 and non-diabetic high CVD risk samples from the STOP-HF study21. We demonstrated that FKBPL plasma concentrations were higher in patients with T2D than in controls, when adjusted for the presence of CVD. Furthermore, FKBPL levels were negatively correlated with characteristic metabolic parameters for T2D, glucose and HbA1c, and positively correlated with C-peptide (reflecting endogenous insulin secretion) and the duration of diabetes, in the FIELD study samples. Given that FKBPL is involved in glucocorticoid receptor signalling, and we have demonstrated correlations with glycaemia and insulin secretion, FKBPL may be implicated in the complex mechanisms of glucose control; specifically we observed that better glucose control is associated with high levels of FKBPL10. The role of glucocorticoids via glucocorticoid receptors is well established in glucose production and metabolism by stimulating gluconeogenesis, and reducing insulin secretion and glucose uptake25. It is possible that this represents a compensatory mechanism in diabetes, which may be mediated e.g. via miRNAs involved in insulin signalling and glucose transport via the PI3K/Akt/m-TOR signalling pathway. Whilst this is speculative, other members of the immunophilin family, which include FKBPL, regulate this pathway and are also implicated in T2D phenotype and associated vascular complications26. Inhibition of the PI3K/Akt/mTOR pathway has been shown to lead to cardiomyocyte autophagy triggered by reactive advance glycation end products in diabetes27. The mTOR pathway has been shown to regulate insulin secretion and signalling as well as endothelial function thus it is also implicated in cardiovascular complications of diabetes28. Nevertheless, this needs to be investigated in future basic science and clinical studies.
In our T2D group, positive correlations were also observed between FKBPL levels and age, urinary ACR, waist/hip ratio whereas FKBPL was negatively correlated with DBP. As age was the only determinant of CVD in the T2D group it is possible that FKBPL is indirectly involved in cardiovascular complications of T2D, which merits further investigation into the FKBPL mechanism rather than its biomarker potential in diabetes. Previously published work on FKBPL demonstrated that FKBPL has a key role in physiological and pathological angiogenesis and that whilst Fkbpl+/− haploinsufficient mice displayed a pro-angiogenic phenotype, early signs of vascular dysfunction were also observed15. There is a plethora of evidence to suggest that in the presence of diabetes, endothelial dysfunction leads to increased vascular permeability and irregular angiogenesis by inducing inflammation, which can lead to atherosclerosis and CVD29,30. Nevertheless, in our T2D group there was no difference in FKBPL plasma concentrations between subjects with and without CVD, though low subject numbers may limit statistical power. Interestingly, in the non-diabetic group, the plasma concentration of FKBPL was higher in the presence of CVD. This might suggest that in the presence of diabetes there is a compensatory vascular mechanism, which attenuates this increase in FKBPL levels. In terms of the strength of association of FKBPL level with CVD, in the non-diabetic group, the AUC was comparable to that of an established biomarker for CVD, BNP, when adjusted for important clinical parameters. A similar AUC was observed when both groups were combined together and adjusted for clinical parameters and the presence of diabetes. Previous reports on the biomarker role of BNP in asymptomatic (Stage-B) heart failure demonstrated similar AUC for both diabetic and non-diabetic patients31. In the same group, BNP was found to be positively correlated with age, female gender and DBP32, whereas, in this study, FKBPL was positively correlated with age and BNP and negatively correlated with DBP in the diabetic group (Table 3). FKBPL plasma concentration in female participants from both groups were lower than male participants (Supplementary Table 3). Previous reports from the STOP-HF trial demonstrated that screening with BNP in primary care and combined with a collaborative care intervention reduced cardiovascular complications, including LV systolic dysfunction, diastolic dysfunction and heart failure21. Based on our results and the AUC measurements from the ROC curve, in a subgroup of patients from the STOP-HF trial, both FKBPL and BNP demonstrate similar biomarker potential. Using penalised logistic regression, FKBPL was identified to be associated with CVD only in the non-diabetic patient group together with age, SBP, total cholesterol and gender. These clinical parameters have been associated with CVD in a number of studies previously33,34. Furthermore, in the non-diabetic group, FKBPL was correlated with echocardiographic parameters indicative of diastolic dysfunction. Disturbed angiogenesis has been previously implicated in diastolic dysfunction35 and in HFpEF36. Even though we did not have echocardiogram data available in the diabetic group to explore for correlations with FKBPL, previous reports in diabetic rats demonstrated that increased cardiac angiogenesis is associated with reduced diastolic dysfunction37, which could suggest that reduction in FKBPL might be beneficial in these settings.
Strengths and limitations
This is the first cross-sectional study that shows associations between plasma FKBPL levels and T2D and CVD status using samples from two clinical studies with well-characterised subjects. Study limitations include its cross-sectional nature, and modest sample sizes. In the non-diabetic group, the number of people with CVD was overrepresented in comparison to the diabetic group because this study recruited subjects with CVD risk factors; the selected subgroup excluded people with diabetes. Nevertheless, a strength was that the two groups were well matched in terms of age, BMI and gender. We did not have echocardiographic parameters and BNP measurements for the diabetic group, which limited correlations between FKBPL and echocardiographic parameters and BNP only to non-diabetic group of patients. Whilst the current commercially available FKBPL ELISA kit had modest CVs, each sample was analysed in duplicate on the same plate and only those with a CV under 15% were included. The biomarker potential of FKBPL was investigated in combination with other established clinical risk factors for CVD and the levels correlated with characteristic metabolic and cardiac parameters.
Conclusions
In this cross-sectional study, we identified associations between the novel anti-angiogenic protein, FKBPL, and T2D parameters and CVD. FKBPL could be involved in the pathogenesis of cardiometabolic diseases including T2D, CVD and cardiac dysfunction. The biomarker role of FKBPL in CVD should be investigated further in larger studies, including cohorts of non-diabetic patients at low-risk for CVD. The therapeutic and diagnostic role of FKBPL in this setting may warrant further investigation, particularly in the absence of diabetes.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- ACR:
-
Albumin/Creatinine ratio
- BNP:
-
B-type natriuretic peptide
- T2D:
-
Type 2 diabetes mellitus
- CVD:
-
Cardiovascular disease
- FKBPL:
-
FK506 binding protein like
- BMI:
-
Body mass index
References
International Diabetes Federation | About Diabetes. https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (2020).
Stamler, J., Vaccaro, O., Neaton, J. D. & Wentworth, D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16, 434–444 (1993).
WHO | Cardiovascular diseases (CVDs). WHO https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (2017).
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. W. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
Chen, H., Chhor, M., Rayner, B., McGrath, K., & McClements, L. Diagnostics and prognostic potential of current biomarkers in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Preprint at https://doi.org/10.1101/2020.04.18.20070482 (2020)
Gallagher, J. et al. B-type natriuretic peptide and ventricular dysfunction in the prediction of cardiovascular events and death in hypertension. Am. J. Hypertens. 31, 228–234 (2018).
Lazar, S., Rayner, B., Lopez Campos, G., McGrath, K. & McClements, L. Mechanisms of heart failure with preserved ejection fraction in the presence of diabetes mellitus. Transl. Metab. Syndr. Res. 3, 1–5 (2020).
Valentine, A. et al. FKBPL and peptide derivatives: novel biological agents that inhibit angiogenesis by a CD44-dependent mechanism. Clin. Cancer Res. 17, 1044–1056 (2011).
Yakkundi, A. et al. The anti-migratory effects of FKBPL and its peptide derivative, AD-01: regulation of CD44 and the cytoskeletal pathway. PLoS ONE 8, e55075. https://doi.org/10.1371/journal.pone.0055075 (2013).
McKeen, H. D. et al. A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling. Endocrinology 149, 5724–5734 (2008).
Annett, S. et al. FKBPL-based peptide, ALM201, targets angiogenesis and cancer stem cells in ovarian cancer. Br. J. Cancer 122, 361–371 (2020).
McClements, L. et al. Targeting treatment-resistant breast cancer stem cells with FKBPL and Its peptide derivative, AD-01, via the CD44 pathway. Clin. Cancer Res. 19, 3881–3893 (2013).
McClements, L. et al. FKBPL and its peptide derivatives inhibit endocrine therapy resistant cancer stem cells and breast cancer metastasis by downregulating DLL4 and Notch4. BMC Cancer 19, 351 (2019).
El-Helali, A. et al. A phase I dose-escalation study of the novel peptide ALM201 in patients (pts) with advanced solid tumours. Ann Oncol. 28 (Abstracts Developmental therapeutics), Supplement 5 (2017).
Yakkundi, A. et al. FKBPL is a critical antiangiogenic regulator of developmental and pathological angiogenesis. Arterioscler. Thromb. Vasc. Biol. 35, 845–854 (2015).
Versari, D., Daghini, E., Virdis, A., Ghiadoni, L. & Taddei, S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 32, S314–S321 (2009).
Schram, M. T. et al. Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 26, 2165–2173 (2003).
Isidori, A. M., Venneri, M. A. & Fiore, D. Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J. Endocrinol. Invest. 39, 1235–1246 (2016).
Rawal, S. et al. Down-regulation of proangiogenic microRNA-126 and microRNA-132 are early modulators of diabetic cardiac microangiopathy. Cardiovasc. Res. 113, 90–101 (2017).
Keech, A. et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366, 1849–1861 (2005).
Ledwidge, M. et al. Natriuretic peptide-based screening and collaborative care for heart failure. JAMA 310, 66 (2013).
McClelland, S. et al. New-onset heart failure in the STOP-HF programme. Natriuretic peptide defines and tracks risk and enables earlier diagnosis of heart failure. Eur. J. Heart Fail. 22, 378–380 (2020).
Collier, P. et al. Progression of left atrial volume index in a population at risk for heart failure: a substudy of the STOP-HF (St Vincent’s Screening TO Prevent Heart Failure) trial. Eur. J. Heart Fail. 14, 957–964 (2012).
Di Angelantonio, E. et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation 120, 2177–2187 (2009).
Whirledge, S. & DeFranco, D. B. Glucocorticoid signaling in health and disease: insights from tissue-specific GR knockout mice. Endocrinology 159, 46–64 (2018).
McClements, L., Annett, S., Yakkundi, A. & Robson, T. The role of peptidyl prolyl isomerases in aging and vascular diseases. Curr. Mol. Pharmacol. 9, 165–179 (2015).
Hou, X. et al. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc. Diabetol. 13, 78 (2014).
Chong, Z. & Maiese, K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc. Diabetol. 11, 45 (2012).
Wierzbicki, A. S. et al. Cardiovascular risk factors and endothelial dysfunction. Clin. Sci. (Lond) 107, 609–615 (2004).
Weis, S. M. Vascular permeability in cardiovascular disease and cancer. Curr. Opin. Hematol. 15, 243–249 (2008).
Watson, C. et al. Influence of diabetes on natriuretic peptide thresholds in screening for Stage B heart failure. Biomarkers 21, 538–543 (2016).
Conlon, C. M. et al. B-type natriuretic peptide measurement in primary care; magnitude of associations with cardiovascular risk factors and their therapies. Observations from the STOP-HF (St. Vincent’s Screening TO Prevent Heart Failure) study. Clin. Chem. Lab. Med. 49, 719–728 (2011).
Cook, N. R. et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic women’s health initiative. Circulation 125, 1748–1756 (2012).
Thomas, M. R. & Lip, G. Y. H. Novel risk markers and risk assessments for cardiovascular disease. Circ. Res. 120, 133–149 (2017).
He, X. et al. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J. Mol. Cell. Cardiol. 112, 104–113 (2017).
Barroso, M. C. et al. Endostatin a potential biomarker for heart failure with preserved ejection fraction. Arq. Bras. Cardiol. 109, 448–456 (2017).
Lu, J. et al. Erythropoietin attenuates cardiac dysfunction by increasing myocardial angiogenesis and inhibiting interstitial fibrosis in diabetic rats. Cardiovasc. Diabetol. 11, 105 (2012).
Acknowledgements
We thank Dr Liping Li for helping us with the FIELD samples retrieval. We also thank all the participants for taking part in both FIELD and STOP-HF studies.
Funding
This work was supported by the Faculty Collaboration Seed funding, Faculty of Medicine, Health, Life Sciences, Queen’s University Belfast, Northern Ireland, United Kingdom (LM), the NHMRC Program grant to the NHMRC Clinical Trials Centre, and NHMRC Fellowships (ACK, AJJ), and the Health Research Board of Ireland, Grant number CSA-2012-36 (C.W, M.L, K.M).
Author information
Authors and Affiliations
Contributions
A.S.J. made significant contribution to data acquisition, data analysis, results interpretation and manuscript drafting. C.J.W. made significant contribution to experimental design, data interpretation and manuscript revision. V.O. analysed and interpreted the data. T.R., K.M., M.L. contributed to the conception, experimental design or data interpretation. A.J.J. and A.C.K. made significant contribution to experimental design, data interpretation, and drafting and revision the manuscript. L.M. conceptualised and designed the experiments, acquired, analysed and interpreted the data, and drafted manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Januszewski, A.S., Watson, C.J., O’Neill, V. et al. FKBPL is associated with metabolic parameters and is a novel determinant of cardiovascular disease. Sci Rep 10, 21655 (2020). https://doi.org/10.1038/s41598-020-78676-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78676-6
This article is cited by
-
A placenta-on-a-chip model to determine the regulation of FKBPL and galectin-3 in preeclampsia
Cellular and Molecular Life Sciences (2023)
-
The emerging importance of immunophilins in fibrosis development
Molecular and Cellular Biochemistry (2023)
-
Characterisation of cardiac health in the reduced uterine perfusion pressure model and a 3D cardiac spheroid model, of preeclampsia
Biology of Sex Differences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.