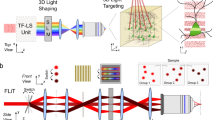
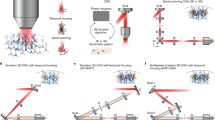
Abstract
Recent advances combining two-photon calcium imaging and two-photon optogenetics with computer-generated holography now allow us to read and write the activity of large populations of neurons in vivo at cellular resolution and with high temporal resolution. Such ‘all-optical’ techniques enable experimenters to probe the effects of functionally defined neurons on neural circuit function and behavioral output with new levels of precision. This greatly increases flexibility, resolution, targeting specificity and throughput compared with alternative approaches based on electrophysiology and/or one-photon optogenetics and can interrogate larger and more densely labeled populations of neurons than current voltage imaging-based implementations. This protocol describes the experimental workflow for all-optical interrogation experiments in awake, behaving head-fixed mice. We describe modular procedures for the setup and calibration of an all-optical system (~3 h), the preparation of an indicator and opsin-expressing and task-performing animal (~3–6 weeks), the characterization of functional and photostimulation responses (~2 h per field of view) and the design and implementation of an all-optical experiment (achievable within the timescale of a normal behavioral experiment; ~3–5 h per field of view). We discuss optimizations for efficiently selecting and targeting neuronal ensembles for photostimulation sequences, as well as generating photostimulation response maps from the imaging data that can be used to examine the impact of photostimulation on the local circuit. We demonstrate the utility of this strategy in three brain areas by using different experimental setups. This approach can in principle be adapted to any brain area to probe functional connectivity in neural circuits and investigate the relationship between neural circuit activity and behavior.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Code availability
Custom codes used for experimental control, data acquisition, behavioral training and analysis have been deposited at Naparm (https://github.com/llerussell/Naparm), PyBehaviour (https://github.com/llerussell/PyBehaviour), 3D SLM calibration (https://github.com/llerussell/SLMTransformMaker3D), STAMovieMaker (https://github.com/llerussell/STAMovieMaker), RawDataStream (https://github.com/llerussell/Bruker_PrairieLink), Objective rotation (https://github.com/llerussell/MONPangle) and TPBS (https://github.com/hwpdalgleish/TPBS). The code in this protocol has been peer reviewed.
References
Jacobs, A. L. et al. Ruling out and ruling in neural codes. Proc. Natl Acad. Sci. USA. 106, 5936–5941 (2009).
Panzeri, S., Harvey, C. D., Piasini, E., Latham, P. E. & Fellin, T. Cracking the neural code for sensory perception by combining statistics, intervention and behavior. Neuron 93, 491–507 (2017).
London, M., Roth, A., Beeren, L., Häusser, M. & Latham, P. E. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature 466, 123–127 (2010).
Histed, M. H. & Maunsell, J. H. R. Cortical neural populations can guide behavior by integrating inputs linearly, independent of synchrony. Proc. Natl Acad. Sci. USA. 111, E178–E187 (2014).
Gollisch, T. & Meister, M. Rapid neural coding in the retina with relative spike latencies. Science 319, 1108–1112 (2008).
Panzeri, S., Petersen, R. S., Schultz, S. R., Lebedev, M. & Diamond, M. E. The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron 29, 769–777 (2001).
Doron, G., von Heimendahl, M., Schlattmann, P., Houweling, A. R. & Brecht, M. Spiking irregularity and frequency modulate the behavioral report of single-neuron stimulation. Neuron 81, 653–663 (2014).
Shusterman, R., Smear, M. C., Koulakov, A. A. & Rinberg, D. Precise olfactory responses tile the sniff cycle. Nat. Neurosci. 14, 1039–1044 (2011).
Huber, D. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64 (2008).
Harris, K. D. & Mrsic-Flogel, T. D. Cortical connectivity and sensory coding. Nature 503, 51–58 (2013).
Pinto, L. & Dan, Y. Cell-type-specific activity in prefrontal cortex during goal-directed behavior. Neuron 87, 437–450 (2015).
Emiliani, V., Cohen, A. E., Deisseroth, K. & Hausser, M. All-optical interrogation of neural circuits. J. Neurosci. 35, 13917–13926 (2015).
Rickgauer, J. P., Deisseroth, K. & Tank, D. W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 17, 1816–1824 (2014).
Packer, A. M., Russell, L. E., Dalgleish, H. W. P. & Hausser, M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Methods 12, 140–146 (2015).
Carrillo-Reid, L., Yang, W., Bando, Y., Peterka, D. S. & Yuste, R. Imprinting and recalling cortical ensembles. Science 353, 691–694 (2016).
Mardinly, A. R. et al. Precise multimodal optical control of neural ensemble activity. Nat. Neurosci. 21, 881–893 (2018).
Forli, A. et al. Two-photon bidirectional control and imaging of neuronal excitability with high spatial resolution in vivo. Cell Rep. 22, 3087–3098 (2018).
dal Maschio, M., Donovan, J. C., Helmbrecht, T. O. & Baier, H. Linking neurons to network function and behavior by two-photon holographic optogenetics and volumetric imaging. Neuron 94, 774–789 (2017).
Chettih, S. N. & Harvey, C. D. Single-neuron perturbations reveal feature-specific competition in V1. Nature 567, 334–340 (2019).
Jennings, J. H. et al. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565, 645–649 (2019).
Gill, J. V. et al. Precise holographic manipulation of olfactory circuits reveals coding features determining perceptual detection. Neuron 108, 1–12 (2020).
Grienberger, C. & Konnerth, A. Imaging calcium in neurons. Neuron 73, 862–885 (2012).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Denk, W. et al. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J. Neurosci. Methods 54, 151–162 (1994).
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Rickgauer, J. P. & Tank, D. W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl Acad. Sci. USA. 106, 15025–15030 (2009).
Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods 9, 5–7 (2012).
Packer, A. M. et al. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods 9, 1202–1208 (2012).
Nikolenko, V. et al. SLM microscopy: scanless two-photon imaging and photostimulation with spatial light modulators. Front. Neural Circuits 2, 1–14 (2008).
Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010).
Scanziani, M. & Häusser, M. Electrophysiology in the age of light. Nature 461, 930–939 (2009).
Pégard, N. C. et al. Three-dimensional scanless holographic optogenetics with temporal focusing (3D-SHOT). Nat. Commun. 8, 1–14 (2017).
Zhang, Z., Russell, L. E., Packer, A. M., Gauld, O. M. & Häusser, M. Closed-loop all-optical interrogation of neural circuits in vivo. Nat. Methods 15, 1037–1040 (2018).
Marshel, J. H. et al. Cortical layer–specific critical dynamics triggering perception. Science 365, 1–12 (2019).
Szabo, V., Ventalon, C., DeSars, V., Bradley, J. & Emiliani, V. Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fiberscope. Neuron 84, 1157–1169 (2014).
Shemesh, O. A. et al. Temporally precise single-cell-resolution optogenetics. Nat. Neurosci. 20, 1796–1806 (2017).
Dalgleish, H. W. P. et al. How many neurons are sufficient for perception of cortical activity? Elife 9, e58889 (2020).
Russell, L. E. et al. The influence of visual cortex on perception is modulated by behavioural state. Preprint at https://www.biorxiv.org/content/10.1101/706010v2 (2019).
Daie, K., Svoboda, K. & Druckmann, S. Targeted photostimulation uncovers circuit motifs supporting short-term memory. Nat. Neurosci. 24, 259–265 (2021).
Robinson, N. T. M. et al. Targeted activation of hippocampal place cells drives memory-guided spatial behavior. Cell 183, 1586–1599.e10 (2020).
Carrillo-Reid, L. et al. Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178, 1–11 (2019).
Yang, W., Carrillo-Reid, L., Bando, Y., Peterka, D. S. & Yuste, R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. Elife 7, e32671 (2018).
Cohen, M. R. & Newsome, W. T. What electrical microstimulation has revealed about the neural basis of cognition. Curr. Opin. Neurobiol. 14, 169–177 (2004).
Murasugi, C. M., Salzman, C. D. & Newsome, W. T. Microstimulation in visual area MT: effects of varying pulse amplitude and frequency. J. Neurosci. 13, 1719–1729 (1993).
Salzman, C. D., Britten, K. H. & Newsome, W. T. Cortical microstimulation influences perceptual judgements of motion direction. Nature 346, 174 (1990).
Salzman, C. D., Murasugi, C. M., Britten, K. H. & Newsome, W. T. Microstimulation in visual area MT: effects on direction discrimination performance. J. Neurosci. 12, 2331–2355 (1992).
Tanke, N., Borst, J. G. G. & Houweling, A. R. Single-cell stimulation in barrel cortex influences psychophysical detection performance. J. Neurosci. 38, 2057–2068 (2018).
Ceballo, S. et al. Cortical recruitment determines learning dynamics and strategy. Nat. Commun. 10, 1–12 (2019).
Ceballo, S., Piwkowska, Z., Bourg, J., Daret, A. & Bathellier, B. Targeted cortical manipulation of auditory perception. Neuron 104, 1168–1179 (2019).
Znamenskiy, P. & Zador, A. M. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 (2013).
Anselmi, F., Ventalon, C., Bègue, A., Ogden, D. & Emiliani, V. Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning. Proc. Natl Acad. Sci. USA. 108, 19504–19509 (2011).
Dal Maschio, M. et al. Simultaneous two-photon imaging and photo-stimulation with structured light illumination. Opt. Express 18, 18720 (2010).
Lechasseur, Y. et al. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat. Methods 8, 319–325 (2011).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2012).
Royer, S. et al. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur. J. Neurosci. 31, 2279–2291 (2010).
Lima, S. Q., Hromádka, T., Znamenskiy, P. & Zador, A. M. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One 4, e6099 (2009).
Kravitz, A. V., Owen, S. F. & Kreitzer, A. C. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 1511, 21–32 (2013).
Hossein Eybposh, M., Caira, N. W., Atisa, M., Chakravarthula, P. & Pégard, N. C. DeepCGH: 3D computer-generated holography using deep learning. Opt. Express 28, 26636 (2020).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Ouzounov, D. G. et al. In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nat. Methods 14, 388–390 (2017).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Wang, T. et al. Three-photon imaging of mouse brain structure and function through the intact skull. Nat. Methods 15, 789–792 (2018).
Yildirim, M., Sugihara, H., So, P. T. C. & Sur, M. Functional imaging of visual cortical layers and subplate in awake mice with optimized three-photon microscopy. Nat. Commun. 10, 1–12 (2019).
Weisenburger, S. et al. Volumetric Ca2+ imaging in the mouse brain using hybrid multiplexed sculpted light microscopy. Cell 177, 1050–1066 (2019).
Inoue, M. et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat. Methods 12, 64–70 (2015).
Zhao, Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Cell 557, 1888–1891 (2011).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. Elife 5, e12727 (2016).
Wang, K. et al. Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue. Nat. Commun. 6, 1–6 (2015).
Sun, W., Tan, Z., Mensh, B. D. & Ji, N. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315 (2016).
Levene, M. J., Dombeck, D. A., Kasischke, K. A., Molloy, R. P. & Webb, W. W. In vivo multiphoton microscopy of deep brain tissue. J. Neurophysiol. 91, 1908–1912 (2004).
Demas, J. et al. High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy. Nat. Methods 18, 1103–1111 (2021).
Sofroniew, N. J., Flickinger, D., King, J. & Svoboda, K. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. Elife 5, e14472 (2016).
Stirman, J. N., Smith, I. T., Kudenov, M. W. & Smith, S. L. Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nat. Biotechnol. 34, 865–870 (2016).
Tsai, P. S. et al. Ultra-large field-of-view two-photon microscopy. Opt. Express 23, 13833–13847 (2015).
Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at https://www.biorxiv.org/content/10.1101/061507v2 (2016).
Zhang, T. et al. Kilohertz two-photon brain imaging in awake mice. Nat. Methods 16, 1119–1122 (2019).
Wu, J. et al. Kilohertz two-photon fluorescence microscopy imaging of neural activity in vivo. Nat. Methods 17, 287–290 (2020).
Kazemipour, A. et al. Kilohertz frame-rate two-photon tomography. Nat. Methods 16, 778–786 (2019).
Lu, R. et al. Video-rate volumetric functional imaging of the brain at synaptic resolution. Nat. Neurosci. 20, 620–628 (2017).
Yu, J. et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 704, 699–704 (2019).
Adam, Y. et al. Voltage imaging and optogenetics reveal behaviour dependent changes in hippocampal dynamics. Nature 569, 413–417 (2019).
Piatkevich, K. D. et al. Population imaging of neural activity in awake behaving mice. Nature 574, 413–417 (2019).
Gong, Y. et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 350, 1361–1366 (2015).
Fan, L. Z. et al. All-optical electrophysiology reveals the role of lateral inhibition in sensory processing in cortical layer 1. Cell 180, 1–15 (2020).
Lou, S. et al. Genetically targeted all-optical electrophysiology with a transgenic cre-dependent optopatch mouse. J. Neurosci. 36, 11059–11073 (2016).
Adam, Y. All-optical electrophysiology in behaving animals. J. Neurosci. Methods 353, 109101 (2021).
Steinmetz, N. A. et al. Aberrant cortical activity in multiple GCaMP6-expressing transgenic mouse lines. eNeuro 4, 1–15 (2017).
Podgorski, K. & Ranganathan, G. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 116, 1012–1023 (2016).
Picot, A. et al. Temperature rise under two-photon optogenetic brain stimulation. Cell Rep. 24, 1243–1253.e5 (2018).
Peron, S. & Svoboda, K. From cudgel to scalpel: toward precise neural control with optogenetics. Nat. Methods 8, 30–34 (2011).
Packer, A. M., Roska, B. & Häusser, M. Targeting neurons and photons for optogenetics. Nat. Neurosci. 16, 805–815 (2013).
Papagiakoumou, E., Ronzitti, E. & Emiliani, V. Scanless two-photon excitation with temporal focusing. Nat. Methods 17, 571–581 (2020).
Arrizón, V., Carreón, E. & Testorf, M. Implementation of Fourier array illuminators using pixelated SLM: efficiency limitations. Opt. Commun. 160, 207–213 (1999).
Hernandez, O., Guillon, M., Papagiakoumou, E. & Emiliani, V. Zero-order suppression for two-photon holographic excitation. Opt. Lett. 39, 5953–5956 (2014).
Andermann, M. L., Kerlin, ΑM. & Reid, R. C. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front Cell. Νeurosci. 4, 3 (2010).
Mank, M. et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat. Methods 5, 805–811 (2008).
Margolis, D. J. et al. Reorganization of cortical population activity imaged throughout long-term sensory deprivation. Nat. Neurosci. 15, 1539–1546 (2012).
Peron, S., Freeman, J., Iyer, V., Guo, C. & Svoboda, K. A cellular resolution map of barrel cortex activity during tactile behavior. Neuron 86, 783–799 (2015).
Poort, J. et al. Learning enhances sensory and multiple non-sensory representations in primary visual cortex. Neuron 86, 1478–1490 (2015).
Rose, T., Jaepel, J., Hübener, M. & Bonhoeffer, T. Cell-specific restoration of stimulus preference after monocular deprivation in the visual cortex. Science 352, 1319–1322 (2016).
Schoonover, C. E., Ohashi, S. N., Axel, R. & Fink, A. J. P. Representational drift in primary olfactory cortex. Nature 594, 541–546 (2021).
Ziv, Y. et al. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 16, 264–266 (2013).
Driscoll, L. N., Pettit, N. L., Minderer, M., Chettih, S. N. & Harvey, C. D. Dynamic reorganization of neuronal activity patterns in parietal cortex. Cell 170, 986–999.e16 (2017).
Kentros, C. G., Agnihotri, N. T., Streater, S., Hawkins, R. D. & Kandel, E. R. Increased attention to spatial context increases both place field stability and spatial memory. Neuron 42, 283–295 (2004).
Huber, D. et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature 484, 473–478 (2012).
Gonzalez, W. G., Zhang, H., Harutyunyan, A. & Lois, C. Persistence of neuronal representations through time and damage in the hippocampus. Science 365, 821–825 (2019).
Marks, T. D. & Goard, M. J. Stimulus-dependent representational drift in primary visual cortex. Nat. Commun. 12, 1–16 (2021).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. Elife 8, e38173 (2019).
Grosenick, L., Marshel, J. H. & Deisseroth, K. Closed-loop and activity-guided optogenetic control. Neuron 86, 106–139 (2015).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Venkatachalam, V. & Cohen, A. E. Imaging GFP-based reporters in neurons with multiwavelength optogenetic control. Biophys. J. 107, 1554–1563 (2014).
Ronzitti, E. et al. Submillisecond optogenetic control of neuronal firing with two-photon holographic photoactivation of chronos. J. Neurosci. 37, 10679–10689 (2017).
Chaigneau, E. et al. Two-photon holographic stimulation of ReaChR. Front. Cell. Neurosci. 10, 234 (2016).
Inoue, M. et al. Rational engineering of XCaMPs, a multicolor GECI suite for in vivo imaging of complex brain circuit dynamics. Cell 177, 1346–1360.e24 (2019).
Forli, A., Pisoni, M., Printz, Y., Yizhar, O. & Fellin, T. Optogenetic strategies for high-efficiency all-optical interrogation using blue light-sensitive opsins. Elife 10, e63359 (2021).
Baker, C. A., Elyada, Y. M., Parra, A. & Bolton, M. Cellular resolution circuit mapping with temporal-focused excitation of soma-targeted channelrhodopsin. Elife 5, 1–15 (2016).
Lim, S. T., Antonucci, D. E., Scannevin, R. H. & Trimmer, J. S. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron 25, 385–397 (2000).
Sridharan, S. et al. High performance microbial opsins for spatially and temporally precise perturbations of large neuronal networks. Neuron 110, 1–17 (2021).
Antinucci, P. et al. A calibrated optogenetic toolbox of stable zebrafish opsin lines. Elife 9, 1–31 (2020).
Wiegert, J. S., Mahn, M., Prigge, M., Printz, Y. & Yizhar, O. Silencing neurons: tools, applications, and experimental constraints. Neuron 95, 504–529 (2017).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2012).
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Bounds, H. A. et al. Multifunctional Cre-dependent transgenic mice for high-precision all-optical interrogation of neural circuits. Preprint at https://www.biorxiv.org/content/10.1101/2021.10.05.463223v2.article-info (2021).
Koester, H. J., Baur, D., Uhl, R. & Hell, S. W. Ca2+ fluorescence imaging with pico- and femtosecond two-photon excitation: signal and photodamage. Biophys. J. 77, 2226–2236 (1999).
Hopt, A. & Neher, E. Highly nonlinear photodamage in two-photon fluorescence microscopy. Biophys. J. 80, 2029–2036 (2001).
Zhang, Z., You, Z. & Chu, D. Fundamentals of phase-only liquid crystal on silicon (LCOS) devices. Light Sci. Appl. 3, 1–10 (2014).
Ahderom, S., Raisi, M., Lo, K., Alameh, K. E. & Mavaddat, R. Applications of liquid crystal spatial light modulators in optical communications. 5th IEEE International Conference on High Speed Networks and Multimedia Communication. 239–242 (2002).
Gerchberg, R. W. & Saxton, W. O. A practical algorithm for the determination of phase from image and diffraction plane pictures. Opt. (Stuttg.). 35, 237–246 (1972).
Yang, W. et al. Simultaneous multi-plane imaging of neural circuits. Neuron 89, 269–284 (2016).
Thalhammer, G., Bowman, R. W., Love, G. D., Padgett, M. J. & Ritsch-Marte, M. Speeding up liquid crystal SLMs using overdrive with phase change reduction. Opt. Express 21, 1779–1797 (2013).
Love, G. D., Major, J. V. & Purvis, A. Liquid-crystal prisms for tip-tilt adaptive optics. Opt. Lett. 19, 1170 (1994).
Wu, S.-T. & Wu, C.-S. High-speed liquid-crystal modulators using transient nematic effect. J. Appl. Phys. 65, 527–532 (1989).
Xun, X., Cho, D. J. & Cohn, R. W. Spiking voltages for faster switching of nematic liquid-crystal light modulators. Appl. Opt. 45, 3136–3143 (2006).
Hu, H. et al. Advanced single-frame overdriving for liquid-crystal spatial light modulators. Opt. Lett. 37, 3324–3326 (2012).
Bhatia, A., Moza, S. & Bhalla, U. S. Patterned optogenetic stimulation using a DMD projector. In Methods in Molecular Biology. Vol. 2191 173–188 (Humana Press Inc., 2021).
Dombeck, D. A., Harvey, C. D., Tian, L., Looger, L. L. & Tank, D. W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440 (2010).
Miller, L. R. et al. Considering sex as a biological variable in preclinical research. FASEB J. 31, 29–34 (2017).
Guo, Z. V. et al. Procedures for behavioral experiments in head-fixed mice. PLoS One 9, e88678 (2014).
Di Leonardo, R., Ianni, F. & Ruocco, G. Computer generation of optimal holograms for optical trap arrays. Opt. Express 15, 1913 (2007).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1244 (2009).
Cetin, A., Komai, S., Eliava, M., Seeburg, P. H. & Osten, P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 1, 3166–3173 (2006).
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits. Neuron 57, 634–660 (2008).
Madisen, L. et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015).
Ehrengruber, M. U. et al. Gene transfer into neurons from hippocampal slices: comparison of recombinant Semliki Forest virus, adenovirus, adeno-associated virus, lentivirus, and measles virus. Mol. Cell. Neurosci. 17, 855–871 (2001).
Zhang, F., Wang, L. P., Boyden, E. S. & Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 3, 785–792 (2006).
Wekselblatt, J. B., Flister, E. D., Piscopo, D. M. & Niell, C. M. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 115, 2852–2866 (2016).
Carandini, M. & Churchland, A. K. Probing perceptual decisions in rodents. Nat. Neurosci. 16, 824–831 (2013).
Issa, J. B. et al. Multiscale optical Ca2+ imaging of tonal organization in mouse auditory cortex. Neuron 83, 944–959 (2014).
Marshel, J. H., Garrett, M. E., Nauhaus, I. & Callaway, E. M. Functional specialization of seven mouse visual cortical areas. Neuron 72, 1040–1054 (2011).
Couto, J. et al. Chronic, cortex-wide imaging of specific cell populations during behavior. Nat. Protoc. 16, 3241–3263 (2021).
Chen, J. L., Pfäffli, O. A., Voigt, F. F., Margolis, D. J. & Helmchen, F. Online correction of licking-induced brain motion during two-photon imaging with a tunable lens. J. Physiol. 19, 4689–4698 (2013).
Zhang, F. et al. The microbial opsin family of optogenetic tools. Cell 147, 1446–1457 (2011).
Peron, S. et al. Recurrent interactions in local cortical circuits. Nature 579, 256–259 (2020).
Acknowledgements
We thank Hillel Adesnik, Isaac Bianco, Spencer Brown, Jacques Carolan, Valentina Emiliani, Darcy Peterka, Evelyn Wong and Zihui Zhang for comments on the manuscript; Theshika Jeyaratnam for CA1/CA3 behavioral training and surgeries; Soyon Chun, Agnieszka Jucht and Olivia Houghton for mouse breeding; Selmaan Chettih and Christopher Harvey for developing and sharing the somatically restricted C1V1 opsin; and Bruker Corporation for technical support. This work was supported by grants from the Wellcome Trust, Gatsby Charitable Foundation, ERC, MRC and the BBSRC.
Author information
Authors and Affiliations
Contributions
L.E.R., H.W.P.D., R.N., O.M.G., D.H., M.F. and A.M.P. built experimental apparatus, wrote software, performed surgeries, trained animals, carried out experiments and analyzed data. L.E.R. and H.W.P.D. wrote the manuscript, with input from A.M.P. and M.H., and all authors jointly edited and revised the protocol.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Andrew Hires, Jinho Kim, Luis Carrillo-Reid, Weijian Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Dalgleish, H. W. et al. eLife 9, e58889 (2020): https://doi.org/10.7554/eLife.58889
Packer, A. M. et al. Nat. Methods 12, 140–146 (2015): https://doi.org/10.1038/nmeth.3217
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
About this article
Cite this article
Russell, L.E., Dalgleish, H.W.P., Nutbrown, R. et al. All-optical interrogation of neural circuits in behaving mice. Nat Protoc 17, 1579–1620 (2022). https://doi.org/10.1038/s41596-022-00691-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00691-w
This article is cited by
-
The influence of cortical activity on perception depends on behavioral state and sensory context
Nature Communications (2024)
-
Cortico-cortical feedback engages active dendrites in visual cortex
Nature (2023)
-
Behaviorally relevant decision coding in primary somatosensory cortex neurons
Nature Neuroscience (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.