Abstract
Historically, osteoporosis has been viewed as a disease of women, with research, trials of interventions and guidelines predominantly focused as such. It is apparent, however, that this condition causes a substantial health burden in men also, and that its assessment and management must ultimately be addressed across both sexes. In this article, an international multidisciplinary working group of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases presents GRADE-assessed recommendations for the diagnosis, monitoring and treatment of osteoporosis in men. The recommendations are based on a comprehensive review of the latest research related to diagnostic and screening approaches for osteoporosis and its associated high fracture risk in men, covering disease burden, appropriate interpretation of bone densitometry (including the use of a female reference database for densitometric diagnosis in men) and absolute fracture risk, thresholds for treatment, and interventions that can be used therapeutically and their health economic evaluation. Future work should specifically address the efficacy of anti-osteoporosis medications, including denosumab and bone-forming therapies.
Similar content being viewed by others
Introduction
Osteoporosis is a condition characterized by loss of bone mass and impaired bone microarchitecture that leads to a substantially increased risk of fracture. It is a highly prevalent, though often neglected, condition, which primarily affects women1. However, since the operational definition of osteoporosis was established in the 1990s2 there has been relative uncertainty regarding the disease in men3, which has led to underdiagnosis of the condition and consequent undertreatment of this population4. This underdiagnosis persists, despite a backdrop of a considerable5,6 and increasing7 global burden of osteoporosis in men, which is associated with not only substantial morbidity but also excessive mortality compared with women with osteoporosis8.
It is estimated that in many populations one in five men over the age of 50 years will experience an osteoporotic fracture in their remaining lifetime5,6. As global populations age and expand, the number of fractures is expected to rise by 310% between 1990 and 2050 (ref. 7). The risk of hip fractures is greater in women than in men, but the gap lessens with increasing age9. In the Dubbo Osteoporosis Epidemiology Study the ratio of hip fracture incidence rates between men and women was 1:4.5 (95% CI 1.3–15.7) at age 60–69 years, 1:1.5 (95% CI 0.9–2.5) at age 70–79 years and 1:1.9 (95% CI 1.2–2.8) at age ≥80 years10. The prevalence of forearm fractures (using EU27 data from 2010) was approximately four times higher in women than in men (0.4% versus 0.1% of the population at risk)11, with a risk ratio of 4.5 between the sexes at the age of 50 years5.
Mortality rates also differ between the sexes, with men being at a substantially higher risk of death following a fracture than women, a difference thought to be attributable to excess comorbidity and infection rates12. In a group of older adults ≥60 years of age, inpatient mortality (median length of stay for survivors was 8 days with an interquartile range of 6–13 days) following a hip fracture was 10.2% in men compared with 4.7% in women, and 1-year mortality was 37.5% in men compared with 28.2% in women13. This elevated risk might persist for over 10 years14.
Age-related alterations to bone microarchitecture are differentially distributed across bone compartments in men and women. With increasing age, men experience trabecular bone loss driven largely by loss of trabecular thickness15,16 but with connectivity intact, whereas women lose trabecular connectivity17,18. Skeletal ageing in men is also associated with reductions in cortical bone mineral density (BMD) with increasing, and encroaching, trabecularization of the cortex and periosteal apposition19.
Although the majority of guidelines pertaining to the management of osteoporosis focus on women, specific guidelines for osteoporosis in men do exist, such as the Endocrine Society 2012 clinical practice guideline20, 2021 French recommendations from the Groupe de Recherche et D’Information sur les Ostéoporoses (GRIO) in collaboration with La Société Française de Rheumatologie (SFR)21, and the Danish Endocrine Society–National Board of Health 2020 recommendations22. The Endocrine Society guideline recommends treatment for men ‘at high risk of fracture’, including (but not limited to) those with a history of fragility fracture of the hip or vertebra, men with a BMD 2.5 (or more) standard deviations below the mean value for normal young white men (using young white men as the reference population) or those in the USA with BMD within the osteopenic range and a 20% 10-year risk of major osteoporotic fracture or 10% risk of hip fracture20. SFR–GRIO recommends a ‘1,2,3 approach’ with an intervention threshold T-score <−1 for a man with a severe osteoporotic fracture and a T-score <−2 for a man with any fracture, and recommend treatment for any man if the T-score is <−3 (ref. 21). In terms of treatment choices, bisphosphonates are widely advocated20,22, with teriparatide recommended for men with multiple vertebral fractures and denosumab for men with prostate cancer21.
The management of osteoporosis in men is also included in general osteoporosis guidelines23,24,25,26 but there is no clearly defined timetable for periodic revision of these recommendations and no consensus on the approach to management, leading to vast variation in the treatment of men with osteoporosis across the globe. This state of affairs clearly highlights the need for a new guideline informed by the latest developments in research and up-to-date expert opinion.
This Evidence-Based Guideline article documents the development and presentation of recommendations for the diagnosis, monitoring and treatment of osteoporosis in men, undertaken by an international working group.
Methods
In February 2023, the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) convened a working group to address the issue of ‘Osteoporosis in Men’. The working group included clinicians (rheumatologists, endocrinologists, orthopaedic surgeons), epidemiologists, public health and regulatory experts from at least 18 countries across three continents. At the meeting, the latest evidence regarding osteoporosis in men was reviewed and was synthesized with expert opinion to inform a GRADE (Grading of Recommendations Assessment, Development and Evaluation)27 assessment of statements for the diagnosis, monitoring and treatment of osteoporosis in men. A session was held specifically for patient representation.
Literature search
Literature searches were performed (by B.A., C.B., O.B., N.R.F., N.C.H., M.H., J.A.K., J.M.K., J.Y.R. and R.R.) and the results were presented to the working group in a series of sessions, including sessions on screening and diagnosis, health economic aspects, patient perspectives (from patient representatives) and therapeutic interventions (a systematic review of the latter has been published28). This evidence, together with expert opinion, was used to inform the GRADE assessment.
Grading of recommendations
After reviewing the evidence, the working group undertook a GRADE assessment to determine recommendations for the diagnosis, screening, treatment and monitoring of men with osteoporosis. For clarity, this GRADE assessment is not that used in the assessment of studies for meta-analysis, but has been modified for producing consensus around recommendations (and has been previously described29).
The GRADE process involved expert members of the working group (n = 28) grading a list of statements (which had been formulated by the core writing group (J.Y.R., R.R., O.B., C.B., N.C.H. and N.R.F.) on the basis of a preliminary review of the evidence) (Supplementary Table 1) with a level of agreement (‘agree’ or ‘disagree’) and a strength of recommendation (‘recommended’ or ‘not recommended’, rated as ‘strong’ or ‘weak’ depending on the extent to which the member agreed with the statement) based on the considered quality of evidence, magnitude of effect, risk-to-benefit ratio, health economic data, values and preferences. Working group members were allowed to choose the most appropriate category and there was one round of voting. If members did not feel that the statement fell within their area of expertise it was graded ‘Not qualified’ and if a response was not provided the statement was graded ‘Not recorded’.
Statements addressed the appropriateness of using a reference database of women in the context of densitometric diagnosis of osteoporosis in men, using FRAX to assess fracture risk and in the setting of intervention thresholds (and whether these thresholds should be age-dependent), and the role of previous fracture in determining the decision to treat.
Treatment decisions were addressed in statements referring to the need for vitamin D and calcium repletion, first-line use of oral bisphosphonates, second-line use of zoledronate or denosumab and sequential therapy with bone-forming agents (or first-line use of these agents in accordance with regulatory authorities). The recommendation of physical activity and balanced diet was also covered.
Monitoring was addressed in statements regarding the use of bone turnover markers to monitor adherence and measurement of serum testosterone in pre-treatment assessment (together with the appropriateness of hormone replacement therapy).
Approaches to fracture risk assessment in men
BMD and absolute fracture risk
The original WHO consensus definition set the densitometric threshold for osteoporosis as an areal BMD of 2.5 standard deviations or more below the mean value for healthy young women, derived from the NHANES reference range30. This definitional approach has led to questions regarding whether the densitometric threshold for osteoporosis should be the same for men as for women31,32,33. Given that absolute BMD is on average higher in men than in women, it follows that using the same threshold and reference range in both sexes will lead to a lower prevalence of osteoporosis in men than in women. This approach seems appropriate, given the lower risk of fracture, on average, in men than in women. Furthermore, evidence suggests that the risk of hip fracture is very similar between sexes for a given absolute BMD, supporting the use of a common threshold and reference range31,33. Indeed, the gradient of risk (hazard ratio for fracture per unit decrease in BMD) is again very similar between men and women, and, in both sexes, the relative increase in fracture risk associated with a T-score of −2.5 declines with increasing age, because at older ages, a greater proportion of the population has a low T-score34. Finally, fracture risk varies substantially across the globe35, but BMD varies much less, suggesting that BMD contributes only part of the overall fracture risk. For example, the same T-score is associated with a different risk in Romania compared with that in Sweden36.
Thus, overall, the evidence supports the use of a common T-score threshold and the female NHANES reference range for both men and women. The question then arises of how best to incorporate BMD and other measures of risk into approaches to clinical risk assessment.
Although BMD is a reasonably specific marker of high fracture risk, it is not very sensitive. That is, people with a low BMD are individually at a high risk of fracture, but the majority of fractures happen in the population with a BMD above the T-score threshold of −2.5, simply because although they are at a lower risk of fracture individually, there are many more people in this population34. Thus, although BMD provides the definition of osteoporosis, it is only one of many risk factors for fracture. Improved capture of the risk associated with non-BMD risk factors enables improved risk prediction.
FRAX is a computer-based algorithm developed by the Sheffield WHO Collaborating Centre for Metabolic Bone Diseases and was first released in 2008 (ref. 37). The algorithm, intended for use in primary care, calculates fracture probability from easily obtainable clinical risk factors in men and women38. The output of FRAX is the 10-year probability of a major fracture (hip, clinical spine, humerus or wrist fracture) and the 10-year probability of hip fracture. Probability is calculated from the risk of fracture and death according to age, BMI and dichotomous risk factors comprising prior fragility fracture, parental history of hip fracture, current tobacco smoking, long-term use of oral glucocorticoids, rheumatoid arthritis, other causes of secondary osteoporosis and excessive alcohol consumption. Femoral neck BMD can be optionally inputted to enhance fracture-risk prediction38. The clinical risk factors considered in FRAX represent risk that is at least partly independent of BMD, and that, as with BMD, could be partly reversible with anti-osteoporosis treatment.
FRAX probability, which can be calculated with or without BMD (and potentially supplemented with trabecular bone score, which pertains to a measure of bone microarchitecture39,40), thus presents a highly practicable metric with which to assess absolute fracture risk in an individual man or woman. However, the measure of risk by itself is of no use, unless it is linked to a decision regarding subsequent treatment with anti-osteoporosis medications. An intervention threshold is therefore required. Approaches to determining an intervention threshold are as much based on philosophy as on science, and necessarily encompass consideration of equity, health economics, willingness to pay, availability and cost of medicines, burden of disease and health care provision34. Broadly speaking, intervention thresholds have generally been based on BMD and/or fracture probability, with the presence of a prior fragility fracture in an older person generally being viewed as an indication for assessment and treatment. A detailed exposition of the merits and demerits of the different approaches has been recently reviewed (in 2023) and is beyond the scope of this article34. At the fundamental level, a fixed BMD T-score threshold of −2.5 is associated with a progressively lower effect on relative fracture risk with increasing age, as average population T-score declines with age and in the oldest decades may be lower than −2.5 (ref. 34), and is clearly hampered by the issues of poor sensitivity described above. A fixed fracture probability threshold risks undertreatment of younger individuals and overtreatment of older individuals. In European guidance on the management of osteoporosis in postmenopausal women issued by ESCEO and the International Osteoporosis Foundation (IOF)41, which is supported by other guidelines including the UK National Osteoporosis Guideline Group (NOGG)23, the intervention threshold at a particular age is set at the age-specific probability of a future major osteoporotic or hip fracture within the next 10 years, conveyed by the presence of a prior fragility fracture, without consideration of BMD, other clinical risk factors being absent, and BMI being average. This method leads to an intervention threshold that rises with age. Importantly, as life expectancy might be <10 years at older ages, and because FRAX probability integrates risk of fracture with risk of death, the metric represents the lifetime probability of fracture. For reasons of equity in relation to individuals who have experienced a prior fragility fracture, the UK NOGG guidance incorporates a hybrid intervention threshold, which rises with age until the age of 70 years and levels off thereafter. Given the marked variation in average fracture probability by country around the world, the age-dependent threshold approach, using specific country-calibrated FRAX models, ensures, together with health economic analyses, appropriateness for probability distributions in individual countries34.
The next question to arise is whether intervention thresholds should be the same in men and women. This approach has indeed been taken in the European (ESCEO–IOF) guidelines41, on the basis of equity (given that the metric represents absolute fracture risk), and that health economic analysis suggests that such an approach is similarly cost-effective in men and women.
Stratified targeting of anti-osteoporosis medications
Studies of both teriparatide and romosozumab have demonstrated greater and more rapid therapeutic effects with these anabolic agents than with oral antiresorptives42,43. Recognizing this development, and given the urgent need for therapeutic interventions in those at a very high risk of fracture, the ESCEO–IOF has recommended that individuals eligible for treatment be dichotomized into those at a ‘high risk’ and those at a ‘very high risk’ of fracture44,45. In this way, patients at a very high risk of fracture can be directed to the more expensive, but more efficacious, anabolic therapy first42,43,46,47, whereas those at a ‘high risk’ can be directed to an antiresorptive agent such as a bisphosphonate or denosumab45.
Consistent with the age-dependent approach to the intervention threshold, in the ESCEO–IOF approach a ‘very high risk’ can be defined as a fracture probability that lies above the upper assessment threshold (1.2 times the intervention threshold) after a FRAX assessment, with or without the inclusion of BMD (if BMD testing is unavailable)44,48. A similar, but hybrid, approach has been applied nationally in the UK NOGG recommendations49, in which the threshold is adapted to incorporate the constant probability threshold above the age of 70 years50. The next question to address is what attributes and clinical risk factors are associated with FRAX probabilities in the ‘low’, ‘high’ and ‘very high’ fracture risk categories. In this setting, it is apparent that the presence of a single clinical risk factor rarely leads to a categorization of very high fracture risk, but a combination of risk factors, particularly older age, recent fracture and glucocorticoid use49.
There are several routes to an individual being categorized as being at a very high risk of fracture on the basis of their FRAX probability score. A key contributor is prior fragility fracture. Thus, studies have demonstrated that fracture risk is acutely elevated immediately after an index fracture and that this risk wanes over the following 2 years but does not return to baseline and subsequently increases with age51,52,53,54. Although a fracture at any time in the past is associated with an increased risk of an incident fracture, when this prior fragility fracture has occurred within the past 24 months the excess risk is even greater55,56. This pattern has been most comprehensively assessed in the Iceland Reykjavík cohort51,56, and further data from the Reykjavik Study have shown that, in individuals who sustained a recurrent fracture, 31–45% of these fractures occurred within 1 year of the first (sentinel) fracture, depending on the fracture site56. Importantly, the transient increase in risk following an index fracture is of sufficient magnitude to materially alter the subsequent 10-year probability of fracture56. Multipliers specific to age, sex and fracture site have been generated to modify FRAX probability, enabling the physician to accommodate the excess risk associated with recency and particular fracture types57. A platform enabling the easy incorporation of the multiplier as a modifier of the FRAX calculator has been developed and is available online as FRAXplus58. A key advantage of this approach is that recency and site of fracture, along with other modifiers of FRAX probability, for example, dose of glucocorticoids or trabecular bone score59, can be used to modify FRAX probability in a way that is immediately interpretable in the context of current national guidelines that are based on 10-year FRAX probability45.
Biochemical assessment
Biochemical assessments in men with osteoporosis can be helpful in diagnosis and also in fracture risk assessment, providing information complementary to that from FRAX and BMD. In addition to renal function, bone profile (including phosphate) and screening for secondary causes of osteoporosis, bone turnover markers and serum (free) testosterone can also assist in the management of men with osteoporosis48.
Bone turnover markers, including procollagen type I N-propeptide (P1NP) and C-terminal telopeptide (CTX) can be measured prior to treatment and again at 3 months to ascertain whether the suppression of bone turnover has been adequately achieved and medication adherence has been satisfactory48,60.
Increased levels of bone turnover markers have been shown to be associated with a greater extent of bone loss and periosteal expansion in men61, thus justifying their inclusion in the algorithm of clinical treatment for osteoporosis in both men and women48.
Serum total testosterone concentration, complemented by free testosterone concentration if serum values are borderline and/or in a clinical situation in which testosterone binding might be altered (for example, by obesity or glucocorticoid use), can also be measured to identify those men who are hypogonadal and who might therefore benefit from testosterone supplementation62,63.
Therapeutic interventions for men with osteoporosis
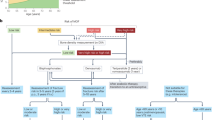
Many studies of anti-osteoporosis medications have been performed in post-menopausal women; however, some trials have specifically sought to address the issue of efficacy and safety of these agents in the male population. One issue is that the majority of these studies are limited in their ability to address fracture incidence as an outcome and so regulatory authorities have sanctioned the use of ‘bridging’ studies64 that look at the similarity of BMD response between men and women with a similar fracture risk. In this case, the primary outcome is improvement in BMD rather than fracture risk, and thresholds for benefit (surrogate threshold effect) have been proposed65 with differences between the change in BMD in the intervention group versus the placebo group of 1.83% for any fracture, 1.42% for vertebral fracture, 3.18% for hip fracture and 2.13% for non-vertebral fracture65,66 (Fig. 1). These thresholds are derived from a large series of trials in women with changes in fracture risk in relation to changes in total hip BMD. We can therefore use this approach when examining historical trials of therapeutic interventions for osteoporosis in men28.
The figure provides a representation of the percentage improvement of total hip bone mineral density (BMD) with use of anti-osteoporosis medications including alendronate, risedronate, zoledronate, ibandronate, denosumab, abaloparatide and romosozumab28. Thresholds for benefit (surrogate threshold effect (STE))65,66 for fracture, depicted as horizontal lines on the graph, are 3.18% for hip fracture, 2.13% for non-vertebral fracture, 1.83% for all fractures and 1.42% for vertebral fractures. These thresholds are derived from a large series of placebo-controlled trials evaluating various anti-osteoporosis agents in women with osteoporosis.
Antiresorptive agents (bisphosphonates and denosumab)
A rich body of literature supports the use of oral bisphosphonates for osteoporosis in men, with studies ranging in duration from 6 months67 to 3 years68 having demonstrated significant improvements in femoral neck BMD. These benefits were documented in a 2023 systematic review and meta-analysis: alendronate monotherapy improved BMD at the lumbar spine with a mean difference (MD) of 5.2% (95% CI 2.76–7.64), total hip with an MD of 2.34% (95% CI 1.66–3.03) and femoral neck with an MD of 2.53% (95% CI 1.76–3.31), and risedronate monotherapy improved BMD at the lumbar spine with an MD of 4.39% (95% CI 3.46–5.31), total hip with an MD of 2.46% (95% CI 1.71–3.22) and femoral neck with an MD of 1.95% (95% CI 0.62–3.27)28. Two studies of zoledronate69,70 reported significant improvements in lumbar spine BMD (MD 6.10%; 95% CI 4.99–7.21)70, femoral neck BMD (MD 3.1%; 95% CI 2.2–5.4) and total hip BMD (MD 3.8%; 95% CI 2.2–5.4). Benefits for vertebral fracture incidence were also observed following 12 months of zoledronate treatment and 24 months of follow-up in one of the studies (relative risk 0.33; 95% CI 0.16–0.7, P = 0.002)70, but no benefits for fracture outcomes were observed in the other study69. A head-to-head comparison of zoledronate and alendronate found that zoledronate was not inferior to alendronate, but did not demonstrate the superiority of zoledronate71. One study investigated the efficacy of ibandronate in 132 men and reported significant improvements in lumbar spine BMD (MD 2.58%; 95% CI 1.41–3.76) and total hip BMD (MD 2.13%; 95%CI 1.34–2.92) at 1 year72. Denosumab administered via 6-monthly subcutaneous injections seemed to provide benefits in BMD accrual compared with placebo in randomized controlled trials of 242 men73 and 47 men74 with osteoporosis over 2 years of follow-up. The results of a 2023 meta-analysis demonstrated the benefits of denosumab for BMD at the lumbar spine with an MD of 5.80% (95% CI 3.5–8.1), femoral neck BMD with an MD of 2.07% (95% CI 1.23–2.92) and total hip BMD with an MD of 2.28% (95% CI 1.51–3.04)28.
Thus, the evidence base strongly supports the use of bisphosphonates or denosumab in the treatment of osteoporosis in men, and suggests that oral bisphosphonates should be recommended as first-line therapy with intravenous bisphosphonates as second-line therapy (similar to the approach taken for the treatment of post-menopausal women41).
Adherence is a substantial issue when considering any therapy75 but particularly so with oral bisphosphonates (owing to complexities of dosing regimes and adverse effects)76,77,78. Adherence can be monitored by measurement of bone turnover markers at baseline and at 3 months to identify a decrease above the least significant change (that is, reductions of more than 38% for P1NP and 56% for CTX), an approach that is supported by international guidelines60.
Anabolic agents
Teriparatide has been compared with placebo in two studies, one with 309 men with osteoporosis followed over 11 months79 and the other involving 23 men for 18 months80. Meta-analysis of these studies showed that teriparatide treatment significantly improved BMD at the lumbar spine (MD 8.19%; 95% CI 1.14–15.25) and femoral neck (MD 1.33; 95% CI 0.39–2.27)28. Teriparatide has been compared with alendronate in two head-to-head studies, in which teriparatide treatment led to significantly greater increases in BMD at the lumbar spine81,82 and femoral neck82. Comparisons with risedronate indicated a lack of superiority of teriparatide unless teriparatide was followed by risedronate83, supporting the drive towards sequential therapy when bone-forming agents are employed. Two studies published in 2022 investigated the effect of abaloparatide versus placebo in a total of 248 men with osteoporosis over a period of 12 months in a trial in the USA, Poland and Italy84 and over 18 months in a Japanese population85. Meta-analysis of the two studies demonstrated a significant improvement in abaloparatide-treated patients in BMD at the lumbar spine (MD 11.29%; 95% CI 1.80–20.8), femoral neck (MD 3.98%; 95% CI 1.10–6.85), and total hip (MD 3.91%; 95% CI 0.34–7.49)28. Only one study has compared romosozumab with placebo, in 245 men over a 12-month period. This study found significantly greater improvements in the romosozumab group than in the placebo group (P < 0.001 for all comparisons) in percentage change in BMD at the lumbar spine (12.1% versus 1.2%), femoral neck (2.2% versus −0.2%) and total hip (2.5% versus −0.5%)86. The possibly increased risk of cardiovascular adverse events should be considered when using romosozumab in men87.
Hormone replacement therapy
Reductions in sex steroid production and increases in levels of sex hormone binding globulin (SHBG) reduce the availability of free testosterone in men as they age63,88.Testosterone is released from the testes in response to luteinizing hormone stimulation and is converted to oestradiol via aromatase (CYP19A1). Oestradiol is thought to mediate the major downstream effects on bone homeostasis as it acts on osteoclasts, osteoblasts and osteocytes via binding to α and β oestrogen receptors. Indeed, oestradiol was shown to mediate the main effects of testosterone on bone homeostasis in healthy men 20–50 years of age treated with a gonadotropin hormone-releasing hormone (GnRH) analogue to suppress endogenous testosterone and receiving testosterone replacement with or without an aromatase inhibitor89,90,91. Profound hypogonadism, such as in androgen deprivation therapy for prostate cancer, is a well known cause of osteoporosis and increased fracture risk. As for endogenous sex steroid levels in community-dwelling older men, most data suggest a role of low oestradiol levels in increased bone loss and fracture risk and, with some exceptions90, no clear association of testosterone levels with bone loss and fracture incidence91.
One of the important initiatives in understanding the role of testosterone replacement in men was the series of ‘T-trials’ emanating from the National Institute on Aging. The T-trial specifically for bone demonstrated a significant increase (7%) in lumbar spine trabecular volumetric BMD after 1 year of testosterone replacement92. Bone microarchitectural benefits were also observed after 2 years of testosterone replacement (compared with placebo), with significant increases in cortical volumetric BMD (3%) and significant increases in areal BMD at the lumbar spine and hip93.
However, consistently robust benefits of testosterone replacement therapy have not been demonstrated. In a systematic review and meta-analysis of testosterone therapy, benefit was only observed in lumbar spine BMD and only in a hypogonadal population62.
Answers might come from the TRAVERSE (Testosterone Replacement therapy for Assessment of long-term Vascular Events and efficacy ResponSE in hypogonadal men) trial of testosterone supplementation versus placebo (in men with hypogonadal symptoms, low serum testosterone levels and high cardiovascular risk), which demonstrated cardiovascular safety of supplementation as the primary end point; the supplementary material in this paper states that clinical fracture outcomes will be discussed in a future publication94.
The potential for hormone replacement therapy to benefit BMD in hypogonadal men supports the assessment of serum total or free testosterone levels in men undergoing investigation for osteoporosis, and in those with established osteoporosis. However, there is a lack of controlled data on fracture incidence in response to testosterone therapy, and owing to the specificity of this treatment for men, it is not possible to ‘bridge’ from the effects on fracture in studies in women.
For the above reasons, serum free or total testosterone levels should be measured as a facet of the investigatory ‘work-up’ for osteoporosis in men. Testosterone therapy might be indicated in the case of symptomatic deficiency, with the decision to recommend testosterone therapy made on the basis of a holistic assessment of the patient across bone, cardiometabolic and sexual function, ideally in conjunction with endocrinology expertise. Furthermore, hypogonadal men with osteoporosis should usually be treated with an established anti-osteoporosis medication, regardless of whether testosterone therapy is instituted, in order to most effectively reduce fracture risk.
Efficacy summary
From the studies reported above, it is clear that anti-osteoporosis medications can substantially benefit the male skeleton in the case of osteoporosis via the accrual of BMD, but also via demonstrated improvements in fracture outcomes70. First-line treatment with oral bisphosphonates followed by second-line deployment of intravenous bisphosphonates and denosumab is supported as an approach to anti-resorptive therapy (driven by the relative ease and cost of administration of these oral agents, compared with health care professional-delivered intravenous and subcutaneous preparations). For those men who would benefit from initial bone-forming therapy, the available data on BMD supports the utility of abaloparatide (Fig. 1), although further studies and collection of data on teriparatide should be a research priority.
Health economic aspects
In addition to their substantial effects on morbidity and mortality, there are considerable costs associated with osteoporosis and fragility fractures in men. For payers and policy-makers to develop a robust strategy to combat osteoporosis in men, they require evidence not only of clinical efficacy but also of economic value. This need is set within a competitive landscape of rising demands for health care but limited resources, and so the case for resource and funding for osteoporosis in men needs to be made.
Studies in the USA have shown that men account for a quarter of the overall cost of fractures95 and a claims database study found that the average cost of a fracture is notably greater in men than in women ($52,000 versus $17,000)96. The authors hypothesized that the reason for this discrepancy could be the fact that fractures in men are associated with greater co-morbidity.
The cost-effectiveness of osteoporosis interventions in men was investigated in a 2023 systematic review of relevant studies, including those concerning anti-osteoporosis medications (8 studies), nutritional interventions (4 studies), screening strategies (6 studies), intervention thresholds (5 studies) and post-fracture care programmes (2 studies)97. The systematic review found that there were fewer studies in men than in women and that those that were published were largely from the USA or Europe and only two had been published in the preceding 5 years, highlighting the need for up-to-date research in this area97. The quality of the studies was fair, with a score of 18.8 out of 25 (range 13–23.5)97. This systematic review also examined the breadth of input data used in economic models and, interestingly, found that although there was men-specific data for hip fracture incidence, hip fracture cost, baseline mortality and excess mortality, the vast majority of inputs (for example, ‘treatment effects’) used combined or even women-only inputs for economic modelling, potentially introducing inaccuracy into the model and, again, highlighting the need for more high-quality research into osteoporosis in men.
In terms of the cost-effectiveness of medications, oral bisphosphonates have been proven cost-effective in men 55 years of age or older with a history of fracture, low bone mass, rheumatoid arthritis or use of high-dose glucocorticoids98,99,100,101,102, although there are no studies comparing the cost-effectiveness of different bisphosphonate formulations. Denosumab was shown to be cost-effective in comparison with bisphosphonates and teriparatide in high-risk populations103,104, and calcium and vitamin D supplementation (or vitamin D-fortified dairy products) were cost-effective in all men >80 years of age and in men >60 years of age with osteoporosis105,106,107. With regard to medications for men with osteoporosis, there are important gaps in the literature that need to be closed, for example, demonstration of the cost-effectiveness of denosumab for treating glucocorticoid-induced osteoporosis.
Screening for osteoporosis, using BMD as measured by dual-energy X-ray absorptiometry (DXA), was cost-effective in a cohort of men who had sustained a fall108 and a study in US men suggested that a fracture risk assessment strategy using age, femoral neck BMD and vertebral fracture assessment (with DXA) would be cost-effective for men 50–60 years of age109. However, in Switzerland, a DXA-based population screening approach (followed by treatment with alendronate for cases of osteopenia with fracture or defined osteoporosis) was not found to be cost-effective in men (although it was in women)110, reflecting geographic variation in factors that influence cost-effectiveness, as well as in the screening approaches taken.
Post-fracture care services (centred on secondary fracture prevention and models including fracture liaison services or orthogeriatric services) were universally cost-effective in Sweden (with zero net costs and 35 quality-adjusted life years gained compared with a ‘do nothing’ approach)111 and in the UK (at £14,525 per quality-adjusted life years for orthogeriatric services aimed at men 83 years of age)112.
The aforementioned systematic review found no significant difference in the cost-effectiveness of intervention thresholds between men and women97. However, when dissecting studies that included men and women, the incremental cost-effectiveness ratio (calculated as the difference in the cost of an intervention divided by the difference in the effect of an intervention) was numerically superior for men than for women in 75% of the studies examined and was inferior for men in 25% of those studies97. It should be noted that 73% of studies concluded that, although numerically different, there was no statistically significant difference in cost-effectiveness between men and women97.
An important consideration is the use of sequential therapies (that is, the use of a bone-forming agent followed by an anti-resorptive agent), and in this area a regimen of abaloparatide followed by alendronate was dominant over alendronate monotherapy or biosimilar teriparatide followed by alendronate in a cohort of US men with a BMD T-score ≤−2.5 and a history of fracture113, in terms of cost-effectiveness. This finding, however, remains to be demonstrated in other populations.
As well as the direct costs of fracture care and prevention, there are also the notable consequences of impacts on social care, increased mortality and the increased risk of subsequent fractures.
In summary, osteoporosis in men gives rise to a substantial health economic burden. Cost-effective interventions for osteoporosis in men exist, including medications, screening approaches, post-fracture care and sequential therapy, and, overall, the cost-effectiveness of these interventions is similar between men and women. However, further research focused on male populations is required to close knowledge gaps concerning epidemiology, costs and model inputs, in order to tailor health economic outputs in this population. These health economic factors were considered by the working group when grading recommendations.
Physical exercise and a balanced diet
The skeletal benefits of physical activity, a balanced diet and calcium and vitamin D supplementation have been well-documented.
Exercise
Aside from cardiometabolic benefits, evidence supports the skeletal benefits of exercise, including weight-bearing exercise, resistance exercise and multi-modal approaches114. This literature is further fortified by a 2021 trial, focused on men, which demonstrated that a multi-component exercise approach had significant benefits for BMD in middle-aged and older men115. Although the benefits of exercise interventions have not demonstrated a reduction in fracture (as an end point) in a public health realm116, on the basis of BMD associations and adjunctive benefits across organ systems, it is usually appropriate to recommend exercise to men with osteoporosis.
Falls
A 2020 systematic review that served as an update of a 2019 Cochrane review of evidence for the effect of exercise on prevention of falls found that exercise reduces the risk of falls by 23% (ref. 117), again emphasizing the potential benefits of exercise on musculoskeletal health. The effect of falls on fracture risk has also been included in FRAXplus58, with the option to adjust the 10-year fracture probability according to the number of falls occurring in the past year (0, 1, 2 and 3 or more falls).
Balanced diet and supplementation
Advocating a balanced diet is recommended for men with osteoporosis, echoing similar guidelines in women41. Adequate protein intake is important and consumption at levels that are higher than the recommended daily allowance might be of benefit to skeletal health118,119.
The above should be considered in light of particular diets, with vegetarian and vegan diets seeming to potentially reduce BMD120 and caloric restriction (although not intermittent fasting) being associated with lower BMD120.
In general, 800–1200 mg of calcium should be consumed via the diet on a daily basis; calcium supplementation can be considered if the daily intake is below 800 mg and vitamin D supplementation (800 IU) should be considered for those at an increased risk of fracture and those in whom vitamin D levels are insufficient41.
Patient perspectives of osteoporosis in men
Patient preference and perspectives are important to consider in the development of recommendations, to ensure that a patient-centred approach is adopted121. In the development of the recommendations presented herein, the working group included patient input, which highlighted patients’ expectations of a clinical approach to osteoporosis.
With regard to the treatment of osteoporosis in men, it was emphasized by the patient representation that anti-osteoporosis therapy was desired as quickly as possible as patients want to ‘stop the clock of bone metabolism’ in order to ‘have no further fractures’.
In our qualitative work examining patient preferences, no concern was forthcoming from the patients that osteoporosis was considered a ‘female’ condition and so associated with a stigma. However, a review of wider experience (that is, beyond the working group) suggests that clinicians should consider this potential issue when managing men with osteoporosis. For example, a 72-year-old patient in another study was quoted as saying “When I was first diagnosed my first thought was ‘why have I got it, isn’t this just for old women?’”122.
Also, no issues with adherence were reported to the working group, although previous estimates have suggested that up to 64% of men are non-adherent to bisphosphonate therapy by 12 months123, highlighting the need for patient education, counselling and, potentially, adherence monitoring78.
Summary of recommendations and guidelines
Here we summarize the recommendations of the working group for the diagnosis, screening, assessment and treatment of men with osteoporosis. The statements supported by the working group are itemised below and detailed ratings are presented in Supplementary Table 1. Statements were graded ‘weak recommendations’ if 75% of voters selected either ‘strong do’ or ‘weak do’, and were graded as ‘strong recommendations’ if 75% of voters selected ‘strong do’.
-
A female reference database should be used for the densitometric diagnosis of osteoporosis in men (strong recommendation).
-
FRAX is the appropriate tool for the assessment of fracture risk and as the basis for setting intervention thresholds in men with osteoporosis (strong recommendation).
-
FRAX-based intervention thresholds should be age dependent in men with osteoporosis (strong recommendation).
-
Trabecular bone score, used with BMD and FRAX probability, provides useful information for fracture risk assessment in men (weak recommendation).
-
All men with a prior fragility fracture should be considered for treatment with anti-osteoporosis medications (strong recommendation).
-
The anti-osteoporosis treatment regimen in men should be adapted to an individual’s baseline fracture risk (strong recommendation).
-
Vitamin D and calcium repletion should be ensured in all men above the age of 65 years (strong recommendation).
-
Oral bisphosphonates (alendronate or risedronate) are first-line treatments for men at a high risk of fracture (strong recommendation).
-
Denosumab or zoledronate are second-line treatments for men at a high risk of fracture (strong recommendation).
-
A sequential therapy starting with a bone-forming agent followed by an anti-resorptive agent should be considered for men at a very high risk of fracture (strong recommendation).
-
Biochemical markers of bone turnover are the appropriate tool to assess adherence to anti-resorptive therapy in men (weak recommendation).
-
Bone-forming agents, when given as first-line treatment in men at a very high risk of fracture, should be used in accordance with the recommendations of the regulatory authorities (strong recommendation).
-
Physical exercise and a balanced diet should be recommended to all men with osteoporosis (strong recommendation).
-
Serum total testosterone should be assessed, as part of the pre-treatment assessment of men with osteoporosis (weak recommendation).
-
Appropriate hormone replacement therapy should be considered in men with low levels of total or free serum testosterone (weak recommendation).
-
Based on available BMD data, abaloparatide is considered an appropriate first-line treatment for men with osteoporosis at a very high risk of osteoporotic fracture (weak recommendation).
It should be noted that these recommendations should be taken as a whole, and not in isolation. For example, the recommendation “All men with a prior fragility fracture should be considered for treatment with anti-osteoporosis medications” should be interpreted in the context of those recommendations recommending the assessment of fracture risk using FRAX.
In addition, given that prior fracture is such a strong predictor of future fracture, and in line with other national23 and international41 guidelines, treatment should be strongly considered in those who have sustained a fracture. In the UK NOGG guideline, it is suggested that FRAX can be used to adjudicate the type of anti-osteoporosis treatment used in those with a prior fragility fracture23, and in European guidelines, it is recommended that all women over 65 years of age be considered for treatment if they have sustained a prior fracture, without the need for further assessment41.
Unlike previous guidelines20,21,22, these recommendations address the particular issue of the sex of the reference population used in the densitometric diagnosis of osteoporosis in men, provide guidance for the usage of FRAX in this population and delineate the use of pharmacological approaches for the treatment of osteoporosis in men based on the latest, systematically evaluated evidence28. This review provides a particularly timely perspective, as anabolic therapies become increasingly available in clinical practice.
Limitations and research outlook
Although the method of producing the above guidelines is robust, the recommendations are constrained by the current limitations in terms of the scope of research regarding osteoporosis in men. Future research should be particularly aimed at addressing treatment efficacy, focusing on denosumab, abaloparatide, teriparatide and romosozumab, and further studies are required to determine the role of testosterone in the treatment of osteoporosis in men.
Conclusions
In conclusion, osteoporosis in men continues to be a substantial clinical and health economic concern for health care workers, policy-makers and, most importantly, patients. Medications for reducing fracture risk exist and evidence of their efficacy is presented above, as are robust recommendations to enable clinicians to navigate this potentially difficult area of clinical practice. In terms of treatment, these guidelines advocate the use of oral anti-resorptive agents as first-line agents in men at a high risk of fracture and the use of bone-forming agents followed sequentially by anti-resorptive agents in men at a very high risk of fracture (with abaloparatide supported by the strongest data with respect to BMD changes), following an approach similar to that advocated for women with osteoporosis41.
We finish by emphasizing that osteoporosis in men is an area of relative neglect, and that further research, investment and focus is required to address some fundamental areas of the disease in this patient group.
References
Curtis, E. M. et al. Epidemiology of fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone 87, 19–26 (2016).
Kanis, J. A., Melton, L. J. III, Christiansen, C., Johnston, C. C. & Khaltaev, N. The diagnosis of osteoporosis. J. Bone Min. Res. 9, 1137–1141 (1994).
Orwoll, E. S. & Bliziotes, M. Heterogeneity in osteoporosis. Men versus women. Rheum. Dis. Clin. North. Am. 20, 671–689 (1994).
Rinonapoli, G. et al. Osteoporosis in men: a review of an underestimated bone condition. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22042105 (2021).
Kanis, J. A. et al. Long-term risk of osteoporotic fracture in Malmö. Osteoporos. Int. 11, 669–674 (2000).
Melton, L. J. III, Atkinson, E. J., O’Connor, M. K., O’Fallon, W. M. & Riggs, B. L. Bone density and fracture risk in men. J. Bone Min. Res. 13, 1915–1923 (1998).
Gullberg, B., Johnell, O. & Kanis, J. A. World-wide projections for hip fracture. Osteoporos. Int. 7, 407–413 (1997).
Kannegaard, P. N., van der Mark, S., Eiken, P. & Abrahamsen, B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing 39, 203–209 (2010).
Borgström, F. et al. Fragility fractures in Europe: burden, management and opportunities. Arch. Osteoporos. 15, 59 (2020).
Chang, K. P., Center, J. R., Nguyen, T. V. & Eisman, J. A. Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo osteoporosis epidemiology study. J. Bone Min. Res. 19, 532–536 (2004).
Hernlund, E. et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 8, 136 (2013).
Wehren, L. E. et al. Gender differences in mortality after hip fracture: the role of infection. J. Bone Min. Res. 18, 2231–2237 (2003).
Jiang, H. X. et al. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J. Bone Min. Res. 20, 494–500 (2005).
Katsoulis, M. et al. Excess mortality after hip fracture in elderly persons from Europe and the USA: the CHANCES project. J. Intern. Med. 281, 300–310 (2017).
Shanbhogue, V. V., Brixen, K. & Hansen, S. Age- and sex-related changes in bone microarchitecture and estimated strength: a three-year prospective study using HRpQCT. J. Bone Min. Res. 31, 1541–1549 (2016).
Wagner, P., Chapurlat, R., Ecochard, R. & Szulc, P. Low muscle strength and mass is associated with the accelerated decline of bone microarchitecture at the distal radius in older men: the prospective STRAMBO study. J. Bone Min. Res. 33, 1630–1640 (2018).
Seeman, E. The growth and age-related origins of bone fragility in men. Calcif. Tissue Int. 75, 100–109 (2004).
Seeman, E. et al. Osteoporosis in men–consensus is premature. Calcif. Tissue Int. 75, 120–122 (2004).
Chaitou, A. et al. Association between bone turnover rate and bone microarchitecture in men: the STRAMBO study. J. Bone Min. Res. 25, 2313–2323 (2010).
Watts, N. B. et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 97, 1802–1822 (2012).
Bouvard, B. et al. Recommandations françaises de la prise en charge et du traitement de l’ostéoporose masculine. Rev. du Rhum. 88, 173–182 (2021).
Danish Endocrine Society. Treatment of male osteoporosis. https://endocrinology.dk/nbv/calcium-og-knoglemetabolisme/behandling-af-mandlig-osteoporose/ (2022).
Gregson, C. L. et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 17, 58 (2022).
Swedish Osteoporosis Society. Guideline. http://svos.se (2021).
DVO Dachverband Osteologie. Osteoporosis Guideline. https://www.dv-osteologie.org/ (2017).
Italian Medicines Agency. Osteoporosis Guideline. http://Aifa.gov.it (2016).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br. Med. J. 336, 924–926 (2008).
Beaudart, C. et al. Efficacy of osteoporosis pharmacological treatments in men: a systematic review and meta-analysis. Aging Clin. Exp. Res. 35, 1789–1806 (2023).
Honvo, G. et al. Recommendations for the reporting of harms in manuscripts on clinical trials assessing osteoarthritis drugs: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Drugs Aging 36, 145–159 (2019).
World Health Organization Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. (1994).
Kanis, J. A. et al. Towards a diagnostic and therapeutic consensus in male osteoporosis. Osteoporos. Int. 22, 2789–2798 (2011).
De Laet, C. E. et al. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J. Bone Min. Res. 13, 1587–1593 (1998).
Binkley, N., Adler, R. & Bilezikian, J. P. Osteoporosis diagnosis in men: the T-score controversy revisited. Curr. Osteoporos. Rep. 12, 403–409 (2014).
Kanis, J. A. et al. The need to distinguish intervention thresholds and diagnostic thresholds in the management of osteoporosis. Osteoporos. Int. 34, 1–9 (2023).
Kanis, J. A. et al. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 23, 2239–2256 (2012).
Kanis, J. A. et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch. Osteoporos. 16, 82–82 (2021).
Kanis, J. A. et al. A decade of FRAX: how has it changed the management of osteoporosis? Aging Clin. Exp. Res. 32, 187–196 (2020).
Kanis, J. A. et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos. Int. 18, 1033–1046 (2007).
Harvey, N. C. et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 78, 216–224 (2015).
McCloskey, E. V. et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Min. Res. 31, 940–948 (2016).
Kanis, J. A., Cooper, C., Rizzoli, R. & Reginster, J. Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 30, 3–44 (2019).
Kendler, D. L. et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391, 230–240 (2018).
Saag, K. G. et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 377, 1417–1427 (2017).
Kanis, J. A. et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos. Int. 31, 1–12 (2019).
Curtis, E. M. et al. Management of patients at very high risk of osteoporotic fractures through sequential treatments. Aging Clin. Exp. Res. 34, 695–714 (2022).
Barrionuevo, P. et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J. Clin. Endocrinol. Metab. 104, 1623–1630 (2019).
Díez-Pérez, A. et al. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone 120, 1–8 (2019).
Lorentzon, M. et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv. Ther. 36, 2811–2824 (2019).
Kanis, J. A. et al. An assessment of intervention thresholds for very high fracture risk applied to the NOGG guidelines : a report for the National Osteoporosis Guideline Group (NOGG). Osteoporos. Int. 32, 1951–1960 (2021).
McCloskey, E. et al. FRAX-based assessment and intervention thresholds–an exploration of thresholds in women aged 50 years and older in the UK. Osteoporos. Int. 26, 2091–2099 (2015).
Johansson, H. et al. Imminent risk of fracture after fracture. Osteoporos. Int. 28, 775–780 (2017).
Johnell, O. et al. Fracture risk following an osteoporotic fracture. Osteoporos. Int. 15, 175–179 (2004).
Ahmed, L. A. et al. Progressively increasing fracture risk with advancing age after initial incident fragility fracture: the Tromsø study. J. Bone Miner. Res. 28, 2214–2221 (2013).
van Geel, T. A., van Helden, S., Geusens, P. P., Winkens, B. & Dinant, G. J. Clinical subsequent fractures cluster in time after first fractures. Ann. Rheum. Dis. 68, 99–102 (2009).
McCloskey, E. V. et al. Short time horizons for fracture prediction tools: time for a rethink. Osteoporos. Int. 32, 1019–1025 (2021).
Kanis, J. A. et al. Characteristics of recurrent fractures. Osteoporos. Int. 29, 1747–1757 (2018).
Kanis, J. A. et al. Adjusting conventional FRAX estimates of fracture probability according to the recency of sentinel fractures. Osteoporos. Int. 31, 1817–1828 (2020).
Schini, M. et al. An overview of the use of the fracture risk assessment tool (FRAX) in osteoporosis. J. Endocrinol. Invest. 47, 501–511 (2024).
Shevroja, E. et al. Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos. Int. 34, 1501–1529 (2023).
Diez-Perez, A. et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos. Int. 28, 767–774 (2017).
Marques, E. A. et al. Association of bone turnover markers with volumetric bone loss, periosteal apposition, and fracture risk in older men and women: the AGES-Reykjavik longitudinal study. Osteoporos. Int. 27, 3485–3494 (2016).
Corona, G. et al. Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J. Endocrinol. Invest. 45, 911–926 (2022).
Kaufman, J. M. Diagnosis of hypogonadism in ageing men. Rev. Endocr. Metab. Disord. 23, 1139–1150 (2022).
Kehoe, T., Blind, E. & Janssen, H. Regulatory aspects of the development of drugs for metabolic bone diseases — FDA and EMA perspective. Br. J. Clin. Pharmacol. 85, 1208–1212 (2019).
Black, D. M. et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 8, 672–682 (2020).
Eastell, R. et al. Validation of the surrogate threshold effect for change in bone mineral density as a surrogate endpoint for fracture outcomes: the FNIH-ASBMR SABRE project. J. Bone Min. Res. 37, 29–35 (2022).
Hwang, J. S. et al. The effects of weekly alendronate therapy in Taiwanese males with osteoporosis. J. Bone Min. Metab. 28, 328–333 (2010).
Gonnelli, S. et al. Alendronate treatment in men with primary osteoporosis: a three-year longitudinal study. Calcif. Tissue Int. 73, 133–139 (2003).
Boonen, S. et al. Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J. Am. Geriatr. Soc. 59, 2084–2090 (2011).
Boonen, S. et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N. Engl. J. Med. 367, 1714–1723 (2012).
Orwoll, E. S. et al. Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J. Bone Min. Res. 25, 2239–2250 (2010).
Orwoll, E. S. et al. Efficacy and safety of monthly ibandronate in men with low bone density. Bone 46, 970–976 (2010).
Orwoll, E. et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J. Clin. Endocrinol. Metab. 97, 3161–3169 (2012).
Nakamura, T. et al. Clinical trials express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J. Clin. Endocrinol. Metab. 99, 2599–2607 (2014).
Fischer, M. A. et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J. Gen. Intern. Med. 25, 284–290 (2010).
Rabenda, V. et al. Low incidence of anti-osteoporosis treatment after hip fracture. J. Bone Jt. Surg. Am. 90, 2142–2148 (2008).
Fatoye, F., Smith, P., Gebrye, T. & Yeowell, G. Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open. 9, e027049 (2019).
Hiligsmann, M. et al. Determinants, consequences and potential solutions to poor adherence to anti-osteoporosis treatment: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Osteoporosis Foundation (IOF). Osteoporos. Int. 30, 2155–2165 (2019).
Orwoll, E. S. et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J. Bone Min. Res. 18, 9–17 (2003).
Kurland, E. S. et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J. Clin. Endocrinol. Metab. 85, 3069–3076 (2000).
Qi, Y., Wang, W., Sun, W. L. & Pan, Q.-Y. Comparative efficacy and safety of alendronate and teriparatide in bone loss reduction and prevention of vertebral fracture in osteoporotic Chinese patients. Trop. J. Pharm. Res. https://doi.org/10.4314/tjpr.v20i10.26 (2021).
Finkelstein, J. S. et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N. Engl. J. Med. 349, 1216–1226 (2003).
Walker, M. D. et al. Combination therapy with risedronate and teriparatide in male osteoporosis. Endocrine 44, 237–246 (2013).
Czerwinski, E. et al. The efficacy and safety of abaloparatide-SC in men with osteoporosis: a randomized clinical trial. J. Bone Min. Res. 37, 2435–2442 (2022).
Matsumoto, T. et al. Abaloparatide increases lumbar spine and hip BMD in Japanese patients with osteoporosis: the phase 3 ACTIVE-J study. J. Clin. Endocrinol. Metab. 107, e4222–e4231 (2022).
Lewiecki, E. M. et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J. Clin. Endocrinol. Metab. 103, 3183–3193 (2018).
Fuggle, N. R. et al. Assessment of cardiovascular safety of anti-osteoporosis drugs. Drugs 80, 1537–1552 (2020).
Feldman, H. A. et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 87, 589–598 (2002).
Finkelstein, J. S. et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J. Clin. Invest. 126, 1114–1125 (2016).
Meier, C. et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch. Intern. Med. 168, 47–54 (2008).
Kaufman, J. M. Management of osteoporosis in older men. Aging Clin. Exp. Res. 33, 1439–1452 (2021).
Snyder, P. J. et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern. Med. 177, 471–479 (2017).
Ng Tang Fui, M. et al. Effect of testosterone treatment on bone microarchitecture and bone mineral density in men: a 2-year RCT. J. Clin. Endocrinol. Metab. 106, e3143–e3158 (2021).
Lincoff, A. M. et al. Cardiovascular safety of testosterone-replacement therapy. N. Engl. J. Med. 389, 107–117 (2023).
Burge, R. et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J. Bone Min. Res. 22, 465–475 (2007).
Williams, S. A. et al. Economic burden of osteoporosis-related fractures in the US Medicare population. Ann. Pharmacother. 55, 821–829 (2021).
Li, N. et al. Cost effectiveness analyses of interventions for osteoporosis in men: a systematic literature review. Pharmacoeconomics 41, 363–391 (2023).
Kreck, S. et al. Cost effectiveness of ibandronate for the prevention of fractures in inflammatory bowel disease-related osteoporosis: cost-utility analysis using a Markov model. Pharmacoeconomics 26, 311–328 (2008).
van Staa, T. P. et al. Individual fracture risk and the cost-effectiveness of bisphosphonates in patients using oral glucocorticoids. Rheumatology 46, 460–466 (2007).
Schousboe, J. T. et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. J. Am. Med. Assoc. 298, 629–637 (2007).
Ito, K., Elkin, E. B., Girotra, M. & Morris, M. J. Cost-effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann. Intern. Med. 152, 621–629 (2010).
Borgström, F., Johnell, O., Jönsson, B., Zethraeus, N. & Sen, S. S. Cost effectiveness of alendronate for the treatment of male osteoporosis in Sweden. Bone 34, 1064–1071 (2004).
Silverman, S., Agodoa, I., Kruse, M., Parthan, A. & Orwoll, E. Denosumab for elderly men with osteoporosis: a cost-effectiveness analysis from the US payer perspective. J. Osteoporos. 2015, 627631 (2015).
Parthan, A., Kruse, M., Agodoa, I., Silverman, S. & Orwoll, E. Denosumab: a cost-effective alternative for older men with osteoporosis from a Swedish payer perspective. Bone 59, 105–113 (2014).
Ethgen, O., Hiligsmann, M., Burlet, N. & Reginster, J. Y. Public health impact and cost-effectiveness of dairy products supplemented with vitamin D in prevention of osteoporotic fractures. Arch. Public. Health 73, 48 (2015).
Hiligsmann, M., Burlet, N., Fardellone, P., Al-Daghri, N. & Reginster, J. Y. Public health impact and economic evaluation of vitamin D-fortified dairy products for fracture prevention in France. Osteoporos. Int. 28, 833–840 (2017).
Hiligsmann, M. et al. Cost-effectiveness of vitamin D and calcium supplementation in the treatment of elderly women and men with osteoporosis. Eur. J. Public. Health 25, 20–25 (2015).
Ito, K. Cost-effectiveness of screening for osteoporosis in older men with a history of falls. JAMA Netw. Open 3, e2027584 (2020).
Nayak, S. & Greenspan, S. L. Cost-effectiveness of osteoporosis screening strategies for men. J. Bone Min. Res. 31, 1189–1199 (2016).
Schwenkglenks, M. & Lippuner, K. Simulation-based cost-utility analysis of population screening-based alendronate use in Switzerland. Osteoporos. Int. 18, 1481–1491 (2007).
Johansson, P., Sadigh, S., Tillgren, P. & Rehnberg, C. Non-pharmaceutical prevention of hip fractures — a cost-effectiveness analysis of a community-based elderly safety promotion program in Sweden. Cost Eff. Resour. Alloc. 6, 11 (2008).
Leal, J. et al. Cost-effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: a population-based study. J. Bone Min. Res. 32, 203–211 (2017).
Hiligsmann, M. et al. Cost-effectiveness of sequential abaloparatide/alendronate in men at high risk of fractures in the United States. Pharmacoeconomics 41, 819–830 (2023).
Daly, R. M., Dalla Via, J., Duckham, R. L., Fraser, S. F. & Helge, E. W. Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz. J. Phys. Ther. 23, 170–180 (2019).
Daly, R. M., Dalla Via, J., Fyfe, J. J., Nikander, R. & Kukuljan, S. Effects of exercise frequency and training volume on bone changes following a multi-component exercise intervention in middle aged and older men: secondary analysis of an 18-month randomized controlled trial. Bone 148, 115944 (2021).
Lamb, S. E. et al. Screening and intervention to prevent falls and fractures in older people. N. Engl. J. Med. 383, 1848–1859 (2020).
Sherrington, C. et al. Evidence on physical activity and falls prevention for people aged 65+ years: systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 17, 144 (2020).
Rizzoli, R., Biver, E. & Brennan-Speranza, T. C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 9, 606–621 (2021).
Rizzoli, R. et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos. Int. 29, 1933–1948 (2018).
Veronese, N. & Reginster, J. Y. The effects of calorie restriction, intermittent fasting and vegetarian diets on bone health. Aging Clin. Exp. Res. 31, 753–758 (2019).
Beauvais, C. et al. Understanding patients’ perspectives and educational needs by type of osteoporosis in men and women and people with glucocorticosteroid-induced osteoporosis: a qualitative study to improve disease management. Calcif. Tissue Int. 105, 589–608 (2019).
Royal Osteoporosis Society. Osteoporosis and men: a message to my younger self. https://theros.org.uk/blog/osteoporosis-and-men-a-message-to-my-younger-self/ (14 June 2022).
Mikyas, Y., Agodoa, I. & Yurgin, N. A systematic review of osteoporosis medication adherence and osteoporosis-related fracture costs in men. Appl. Health Econ. Health Policy 12, 267–277 (2014).
Acknowledgements
The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) working group that produced this Evidence-Based Guideline was funded by the ESCEO. The ESCEO receives unrestricted educational grants to support its educational and scientific activities from non-governmental organisations, not-for-profit organisations, non-commercial or corporate partners. The choice of topics, participants, content and agenda of the working group as well as the writing, editing, submission and reviewing of the manuscript are the sole responsibility of the ESCEO, without any influence from third parties. The authors wish to thank and acknowledge their patient partners who provided expert patient input and insight.
Author information
Authors and Affiliations
Contributions
N.R.F., C.B., O.B., B.A., M.H., J.A.K., J.M.K., R.R., C.C., J.Y.R. and N.H. researched data for the article. N.R.F., C.B., O.B., B.A., N.B., M.C., M.R.R., B.C., W.D., P.H., M.H., J.A.K., J.M.K., A.K., O.L., A.L., S.M., R.M., E.M., A.M., M.C.P.Y, R.P.R., S.Si., N.V., R.R., C.C., J.Y.R. and N.H. contributed substantially to discussion of the content. N.R.F., C.B., O.B., M.C., M.R.R., B.C., W.D., M.H., J.A.K., J.M.K., O.L., S.Si., R.R., J.Y.R. and N.H. wrote the article; N.R.F. and C.B. are joint first authors. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.R.F. declares that he has received travel bursaries from Eli Lilly and Pfizer and speaker’s fees from Viatris outside the submitted work. B.A. declares that he has received speaker and/or consulting fees from UCB, Amgen, Kyowa-Kirin, and Pharmacosmos, and institutional research grants (with funds paid to the institution) from Novartis, Kyowa-Kirin and Pharmacosmos; he is a member of the Executive Committee of the European Calcified Tissue Society. O.B. declares that he has received consulting or lecture fees from Amgen, Aptissen, Biophytis, IBSA, Mylan, Novartis, Orifarm, Sanofi, UCB and Viatris outside the submitted work. B.C. declares that he has received personal fees, consultancy, lecture fees and/or honoraria from Alexion, Amgen, Expansience, Kyowa-Kirin, MSD, Novartis,Theramex, UCB, Viatris. M.C. declares that she has received honoraria from Kyowa Kirin and AMGEN for speaking and chairing engagements. W.D. declares that he is a shareholder and former employee of Amgen, and former board of director member of Radius Health. M.H. declares that he has received research grants (paid to his institution) from Radius Health and Amgen, consulting fees from UCB and lecture fees from Mylan Pharmaceuticals and IBSA (paid to his institution), outside this work. J.A.K. declares that he is a director of Osteoporosis Research Ltd, a member of the National Osteoporosis Guideline Group (UK), a member of the International Osteoporosis Foundation, and a member of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). J.M.K. declares that he is a board member (treasurer) of ESCEO. S.M. declares that she has received research grants and personal fees as an advisory board member and/or speaker fees from GSK, Pfizer, Merck, Sanofi, Takeda, Novavax, Viatris and Janssen outside the submitted work. R.M. declares that she has received personal fees from Amicus, ElPharma, Abela Pharm. A.K. declares that he has participated in advisory boards for Amgen, UCB, Agnovos, Alexion, Image Biopsy Lab, Theramex and speakers bureau for Amgen, Merit Medical, UCB, Agnovos, Alexion, Theramex, Stada; he is president of the Dachverband Osteologie e.V., Germany. M.M.R. declares that he is a Member of the Scientific Advice Working Party and Member of the Central Nervous System Working Party at the European Medicines Agency. S.Si. declares that he has received grant support from Amgen, consultancy fees from Amgen and Radius. N.V. declares that she has received personal fees from IBSA, Mylan, Viatris, Fidia, MSD, Bayer outside this work. R.R. declares that he has received fees as a speaker or consultant for Abiogen, Effryx, Nestlé, ObsEva and Theramex. C.C. declares that he has received personal fees from ABBH, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier and Takeda. J.Y.R. declares that he has received consulting fees or been on paid advisory boards for IBSA-Genevrier, Mylan, Radius Health, Pierre Fabre, Faes Pharma, Rejuvenate Biomed, Samumed, Teva, Theramex, Pfizer, Mithra Pharmaceuticals, received lecture fees when speaking at the invitation of the sponsor for IBSA-Genevrier, Mylan, Cniel, Dairy Research Council (DRC), Nutricia, Danone, Agnovos and received grant support from IBSA-Genevrier, Mylan, Cniel, Radius Health, TRB. N.C.H. declares that he has received personal fees, consultancy, lecture fees and/or honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, UCB, Kyowa Kirin, Servier, Shire, Consilient Healthcare, Theramex and Internis Pharma. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Julia Pasco and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FRAX: http://www.shef.ac.uk/FRAX
FRAXplus: https://www.fraxplus.org
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fuggle, N.R., Beaudart, C., Bruyère, O. et al. Evidence-Based Guideline for the management of osteoporosis in men. Nat Rev Rheumatol 20, 241–251 (2024). https://doi.org/10.1038/s41584-024-01094-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01094-9